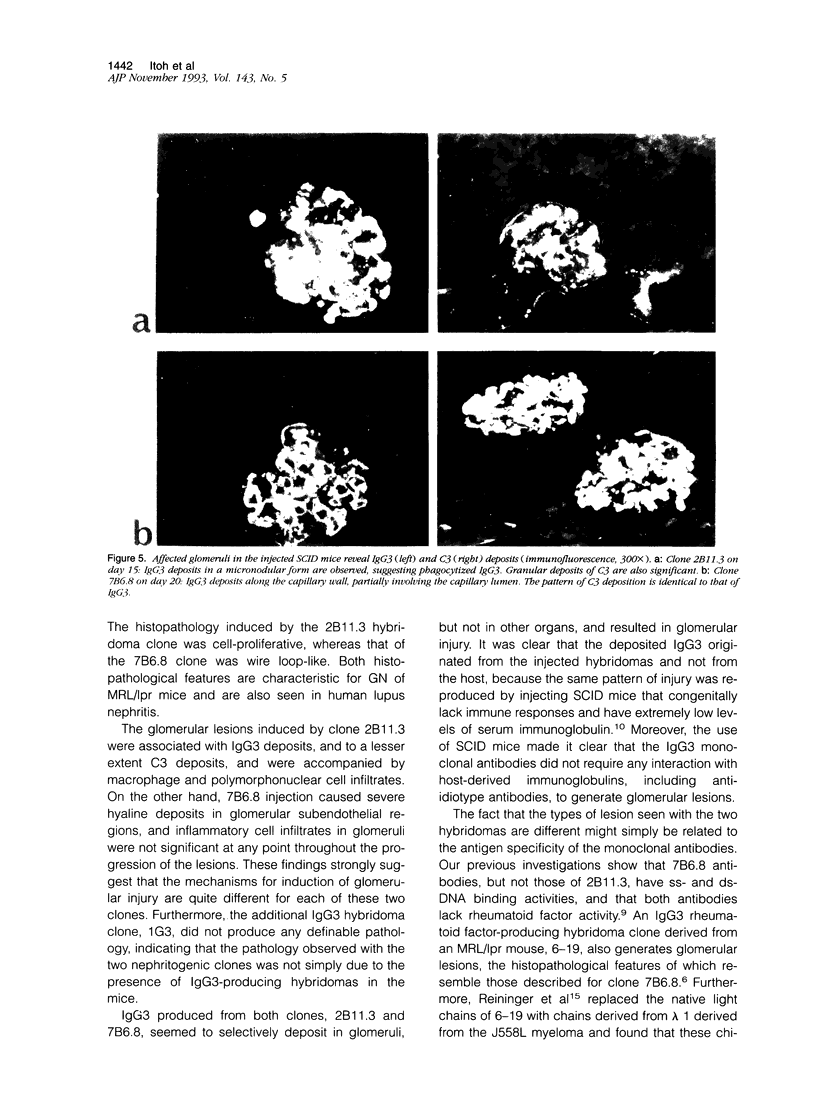
Abstract
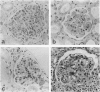
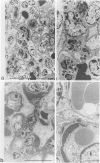
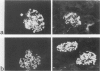
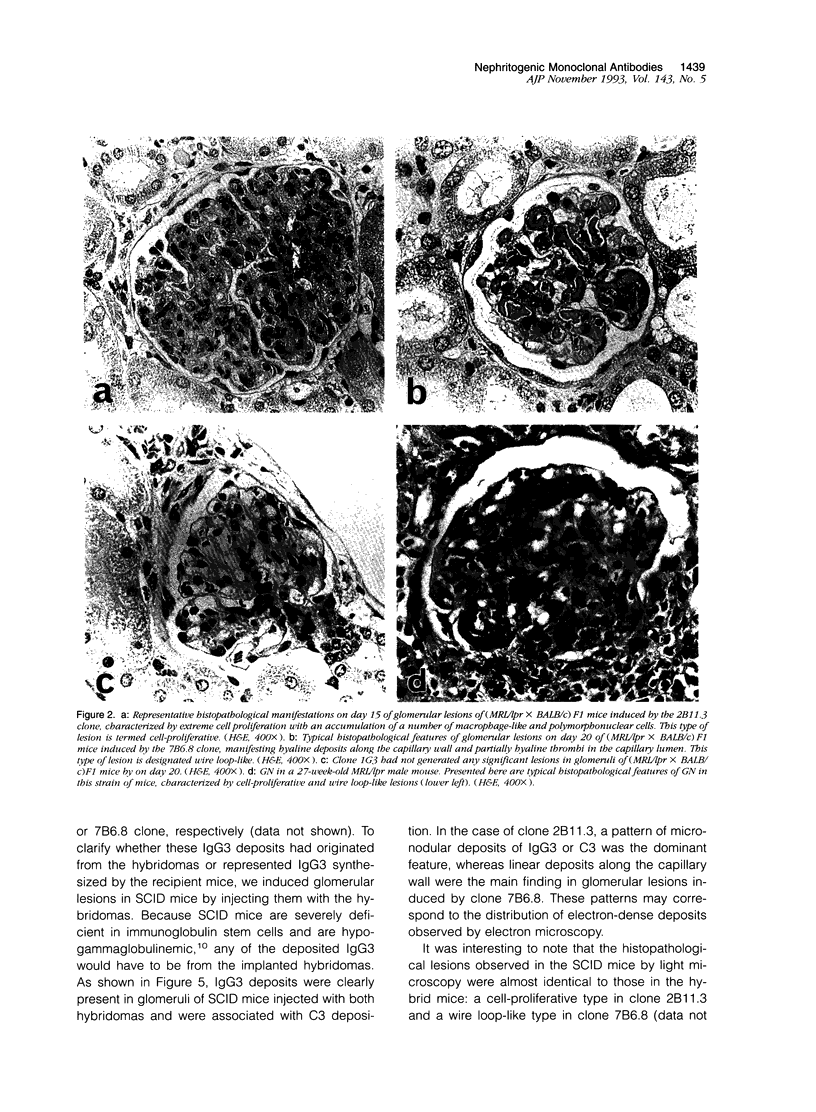
MRL/Mp-lpr/lpr(MRL/lpr) lupus mice develop glomerulonephritis in which the histopathological manifestations of the disease are characterized by diffuse cell-proliferative, crescentic, and/or wire loop-like lesions, resembling those of human lupus nephritis. Although these lesions are thought to be mediated by antibodies, little data is available to explain these regular variations in glomerular lesions induced by antibodies at the monoclonal level. We studied glomerular lesions of normal or severe combined immunodeficient mice injected with nephritogenic immunoglobulin G3-producing hybridoma clones (2B11.3 and 7B6.8), which we previously established from an unmanipulated MRL/lpr mouse. Both clones caused increased serum levels of immunoglobulin G3 with identical patterns over time and both induced glomerular deposits of immunoglobulin G3 and C3. However, 2B11.3 and 7B6.8 induced glomerular lesions that differed in their histopathological manifestations. The 2B11.3 clone generated cell-proliferative lesions associated with marked Mac-2-positive macrophage infiltrates, but the 7B6.8 clone induced lesions characterized by subendothelial hyaline deposits resembling wire loops. The latter was not associated with significant inflammatory cell infiltrates at any point throughout the progression of the lesion. Thus, our findings suggest that the histopathological variation in glomerulonephritis seen in MRL/lpr mice results from clonally expanded B cell clones that produce nephritogenic antibodies with different pathogenic potencies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Ebling F., Hahn B. H. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980 Apr;23(4):392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- Gyotoku Y., Abdelmoula M., Spertini F., Izui S., Lambert P. H. Cryoglobulinemia induced by monoclonal immunoglobulin G rheumatoid factors derived from autoimmune MRL/MpJ-lpr/lpr mice. J Immunol. 1987 Jun 1;138(11):3785–3792. [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Theofilopoulos A. N., Dixon F. J. Association of circulating retroviral gp70-anti-gp70 immune complexes with murine systemic lupus erythematosus. J Exp Med. 1979 May 1;149(5):1099–1116. doi: 10.1084/jem.149.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Nardella F. A., Teller D. C., Izui S., Mannik M. Self-associating IgG rheumatoid factors in MRL/l autoimmune mice. Arthritis Rheum. 1984 Oct;27(10):1165–1173. doi: 10.1002/art.1780271013. [DOI] [PubMed] [Google Scholar]
- Nose M., Katoh M., Okada N., Kyogoku M., Okada H. Tissue distribution of HRF20, a novel factor preventing the membrane attack of homologous complement, and its predominant expression on endothelial cells in vivo. Immunology. 1990 Jun;70(2):145–149. [PMC free article] [PubMed] [Google Scholar]
- Nose M., Nishimura M., Kyogoku M. Analysis of granulomatous arteritis in MRL/Mp autoimmune disease mice bearing lymphoproliferative genes. The use of mouse genetics to dissociate the development of arteritis and glomerulonephritis. Am J Pathol. 1989 Aug;135(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Reininger L., Berney T., Shibata T., Spertini F., Merino R., Izui S. Cryoglobulinemia induced by a murine IgG3 rheumatoid factor: skin vasculitis and glomerulonephritis arise from distinct pathogenic mechanisms. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10038–10042. doi: 10.1073/pnas.87.24.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Itoh J., Nose M., Ono M., Yamamoto T., Kyogoku M. Cloning and cDNA sequence analysis of nephritogenic monoclonal antibodies derived from an MRL/lpr lupus mouse. Mol Immunol. 1993 Feb;30(2):177–182. doi: 10.1016/0161-5890(93)90089-t. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nose M., Sasaki J., Yamamoto T., Kyogoku M. IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J Immunol. 1991 Jul 15;147(2):515–519. [PubMed] [Google Scholar]