Abstract
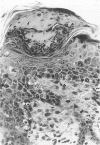
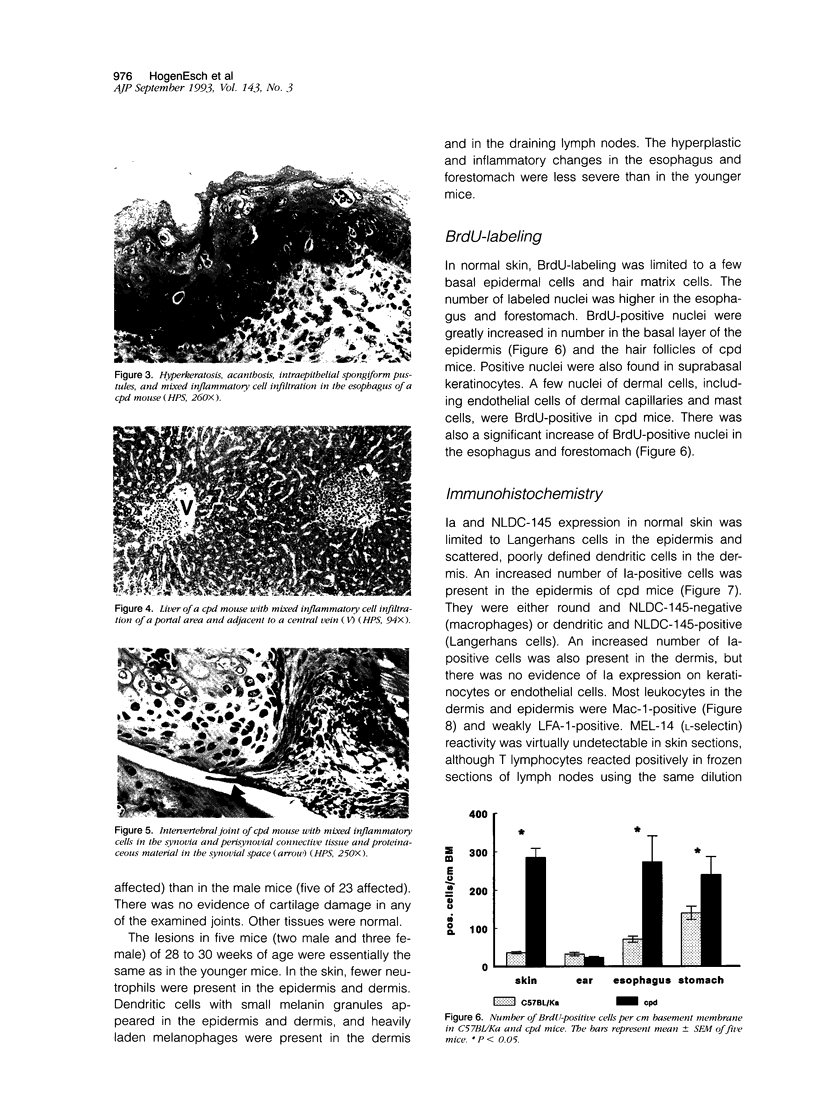
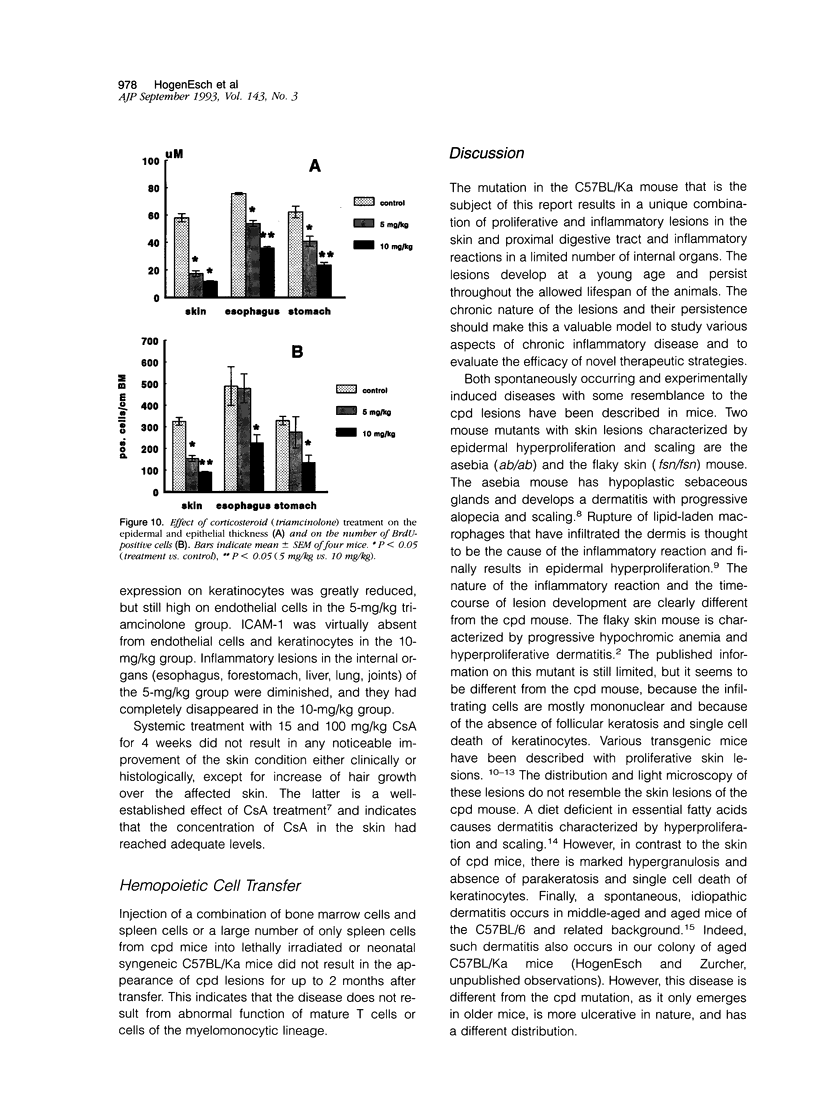
Chronic proliferative dermatitis is a new spontaneous mutation in C57BL/Ka mice. Breeding results suggest an autosomal recessive mode of inheritance. Mutant mice develop skin lesions at the age of 5 to 6 weeks. The lesions occur in the ventral and dorsal skin of the body, whereas ears, footpads, and tail are not involved. The lesions are characterized by epidermal hyperplasia, hyper- and parakeratosis, and single cell necrosis of keratinocytes. The dermis and epidermis are infiltrated by granulocytes and macrophages, and occasionally subcorneal and intracorneal microabscesses are formed. The number of mast cells in the dermis progressively increases with age. There is dilatation and proliferation of dermal capillaries. Similar lesions develop in the mouth, esophagus, and forestomach, which, in the mouse, are all lined by orthokeratinizing stratified squamous cell epithelium. Studies with bromodeoxyuridine confirm the increased rate of epithelial cell proliferation. Most inflammatory cells in the affected skin express Mac-1, and few express the T lymphocyte marker CD3. There is increased expression of intracellular adhesion molecule-1 on keratinocytes and endothelial cells. Infiltration of neutrophils and macrophages are also seen in the liver, lung, and several joints. The disease could not be transferred by bone marrow or spleen transplants into irradiated normal syngeneic hosts. Treatment of the mice with triamcinolone, a long-acting corticosteroid, resulted in nearly complete regression of the lesions over a period of 4 weeks, whereas systemic cyclosporin A treatment was ineffective.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baadsgaard O., Gupta A. K., Taylor R. S., Ellis C. N., Voorhees J. J., Cooper K. D. Psoriatic epidermal cells demonstrate increased numbers and function of non-Langerhans antigen-presenting cells. J Invest Dermatol. 1989 Feb;92(2):190–195. doi: 10.1111/1523-1747.ep12276718. [DOI] [PubMed] [Google Scholar]
- Bailleul B., Surani M. A., White S., Barton S. C., Brown K., Blessing M., Jorcano J., Balmain A. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990 Aug 24;62(4):697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Fry L. The immunology of psoriasis. Br J Dermatol. 1992 Jan;126(1):1–9. doi: 10.1111/j.1365-2133.1992.tb08394.x. [DOI] [PubMed] [Google Scholar]
- Barker J. N., Mitra R. S., Griffiths C. E., Dixit V. M., Nickoloff B. J. Keratinocytes as initiators of inflammation. Lancet. 1991 Jan 26;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Barker J. N., Sarma V., Mitra R. S., Dixit V. M., Nickoloff B. J. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest. 1990 Feb;85(2):605–608. doi: 10.1172/JCI114481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham T. Y., Nickoloff B. J., Merigan T. C., Morhenn V. B. Recombinant gamma interferon induces HLA-DR expression on cultured human keratinocytes. J Invest Dermatol. 1984 Aug;83(2):88–90. doi: 10.1111/1523-1747.ep12262597. [DOI] [PubMed] [Google Scholar]
- Bieber T., Braun-Falco O. Distribution of CD1a-positive cells in psoriatic skin during the evolution of the lesions. Acta Derm Venereol. 1989;69(2):175–178. [PubMed] [Google Scholar]
- Brown W. R., Hardy M. H. A hypothesis on the cause of chronic epidermal hyperproliferation in asebia mice. Clin Exp Dermatol. 1988 Mar;13(2):74–77. doi: 10.1111/j.1365-2230.1988.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Darling S. M., Abbott C. M. Mouse models of human single gene disorders. I: Nontransgenic mice. Bioessays. 1992 Jun;14(6):359–366. doi: 10.1002/bies.950140602. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enk A. H., Katz S. I. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J., Arizono N., Murakami T., Dvorak A. M., Fox J. G. Development of large numbers of mast cells at sites of idiopathic chronic dermatitis in genetically mast cell-deficient WBB6F1-W/Wv mice. Blood. 1987 Jun;69(6):1661–1666. [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990 Jul 19;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Nickoloff B. J. Keratinocyte intercellular adhesion molecule-1 (ICAM-1) expression precedes dermal T lymphocytic infiltration in allergic contact dermatitis (Rhus dermatitis). Am J Pathol. 1989 Dec;135(6):1045–1053. [PMC free article] [PubMed] [Google Scholar]
- Horley K. J., Carpenito C., Baker B., Takei F. Molecular cloning of murine intercellular adhesion molecule (ICAM-1). EMBO J. 1989 Oct;8(10):2889–2896. doi: 10.1002/j.1460-2075.1989.tb08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks C., Duncan J. I., Oliver A. M., Thomson A. W. Adhesion molecule expression in psoriatic skin lesions and the influence of cyclosporin A. Clin Exp Immunol. 1991 Apr;84(1):157–162. doi: 10.1111/j.1365-2249.1991.tb08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger G. G. A perspective of psoriasis as an aberration in skin modified to expression by the inflammatory/repair system. Immunol Ser. 1989;46:425–445. [PubMed] [Google Scholar]
- Leonard J. M., Abramczuk J. W., Pezen D. S., Rutledge R., Belcher J. H., Hakim F., Shearer G., Lamperth L., Travis W., Fredrickson T. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science. 1988 Dec 23;242(4886):1665–1670. doi: 10.1126/science.3201255. [DOI] [PubMed] [Google Scholar]
- Lowe N. J., Stoughton R. B. Essential fatty acid deficient hairless mouse: a model of chronic epidermal hyperproliferation. Br J Dermatol. 1977 Feb;96(2):155–162. doi: 10.1111/j.1365-2133.1977.tb12537.x. [DOI] [PubMed] [Google Scholar]
- Marks R., Pongsehirun D., Saylan T. A method for the assay of topical corticosteroids. Br J Dermatol. 1973 Jan;88(1):69–74. doi: 10.1111/j.1365-2133.1973.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J. The cytokine network in psoriasis. Arch Dermatol. 1991 Jun;127(6):871–884. [PubMed] [Google Scholar]
- Paus R., Stenn K. S., Link R. E. The induction of anagen hair growth in telogen mouse skin by cyclosporine A administration. Lab Invest. 1989 Mar;60(3):365–369. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Vassalli P. Subcutaneous perfusion of tumor necrosis factor induces local proliferation of fibroblasts, capillaries, and epidermal cells, or massive tissue necrosis. Am J Pathol. 1990 Jan;136(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiag P., Coulomb B., Lebreton C., Bell E., Dubertret L. Psoriatic fibroblasts induce hyperproliferation of normal keratinocytes in a skin equivalent model in vitro. Science. 1985 Nov 8;230(4726):669–672. doi: 10.1126/science.2413549. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe H. D., Wagner J. L., Pick J. R. A debilitating fatal murine dermatitis. Lab Anim Sci. 1971 Dec;21(6):892–897. [PubMed] [Google Scholar]
- Vassar R., Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991 May;5(5):714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Wilson J. B., Weinberg W., Johnson R., Yuspa S., Levine A. J. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell. 1990 Jun 29;61(7):1315–1327. doi: 10.1016/0092-8674(90)90695-b. [DOI] [PubMed] [Google Scholar]