Abstract
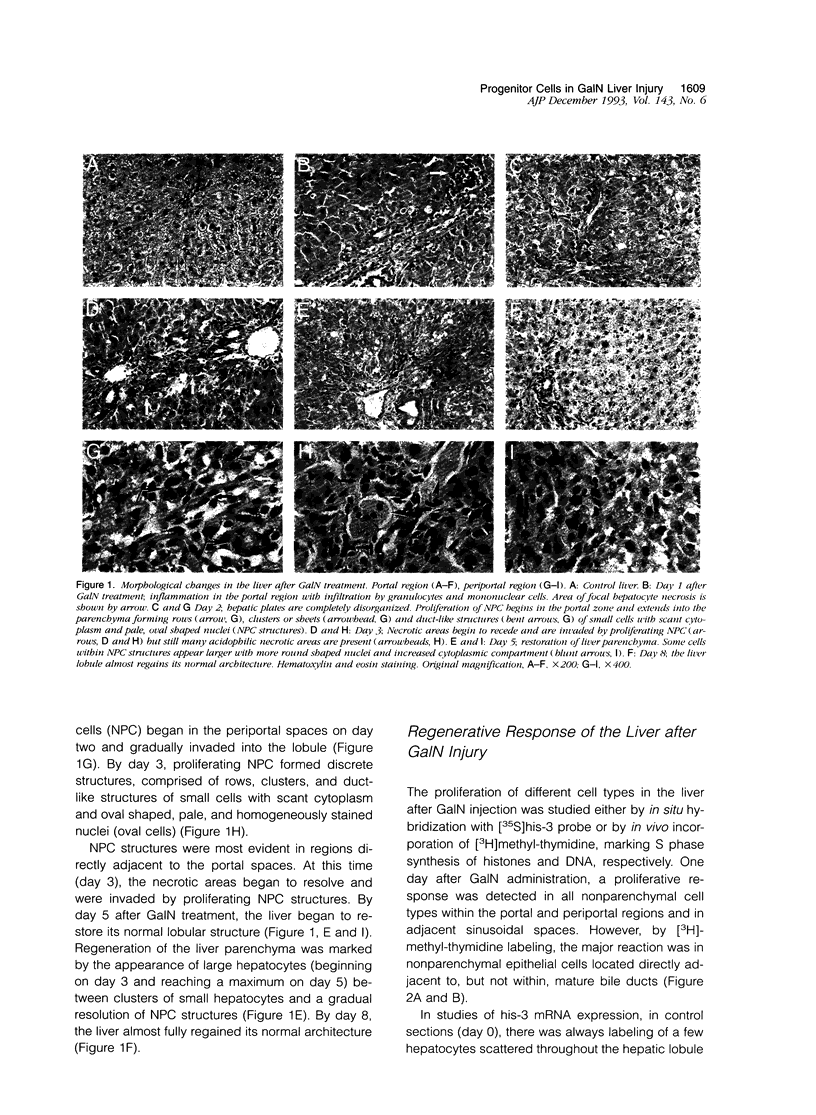
Rat liver regeneration was studied from 24 hours to 8 days after a single intraperitoneal injection of D-galactosamine (GalN). Morphological changes in the liver were analyzed in parallel with sequential changes in expression of histone-3 mRNA (a marker of cell proliferation), fetal alpha-fetoprotein (AFP) mRNA and gamma-glutamyl transpeptidase (GGT) (markers of fetal hepatocytes), and albumin mRNA and glucose-6-phosphatase (G6Pase) (markers of adult hepatocytes). Proliferation of nonparenchymal epithelial cells (NPC), detected in situ by [3H]thymidine labeling or histone-3 mRNA expression, began after 24 hours primarily in the portal area around the bile ducts. After 2 days, histone-3 labelling intensity increased in rows and clusters of NPC which expanded from the portal zone and invaded into the parenchyma. On days 3 and 5, NPC expressing his-3 mRNA expanded further, forming pseudo-ducts and islet-like structures (NPC structures). Proliferating NPC were positive for GGT. Some GGT positive cells were also positive for the fetal form of AFP mRNA, which lagged behind GGT by 24 hours and peaked on day 5. On day 3, some cells with the appearance of NPC expressed albumin mRNA. Double label in situ hybridization for fetal AFP and albumin mRNAs and dual histochemistry for GGT and G6Pase showed simultaneous expression of these markers in NPC on day 5. Other cells expressing fetal AFP mRNA or GGT on day 5 had a morphological appearance between NPC and hepatocytes (transitional cells). Proliferation of hepatocytes began on day 2, reached maximum on day 5 and then declined. Proliferating hepatocytes did not express fetal AFP mRNA or GGT. These findings indicate that after GalN injury, the liver responds by activation of progenitor cells that proliferate and then differentiate into mature hepatocytes. Adult hepatocytes can also proliferate after GAlN injury, but these hepatocytes do not undergo dedifferentiation/redifferentiation during regeneration of the hepatic lobule.
Full text
PDF




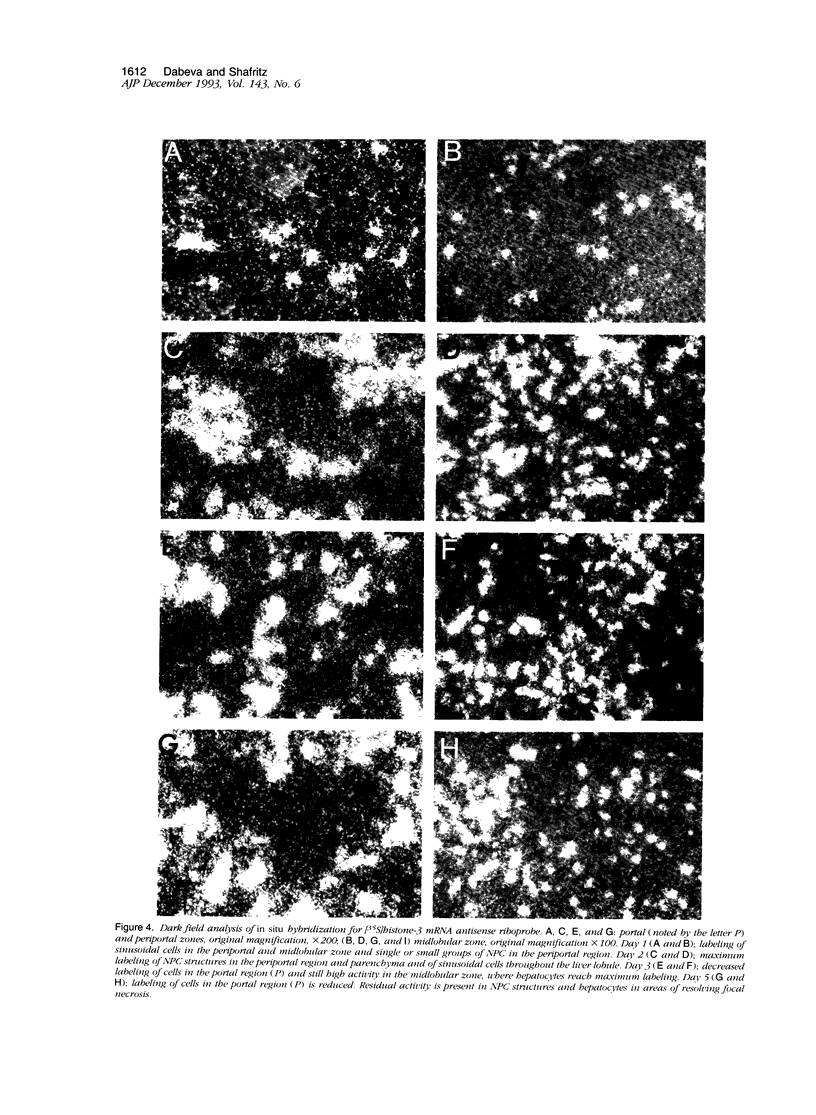
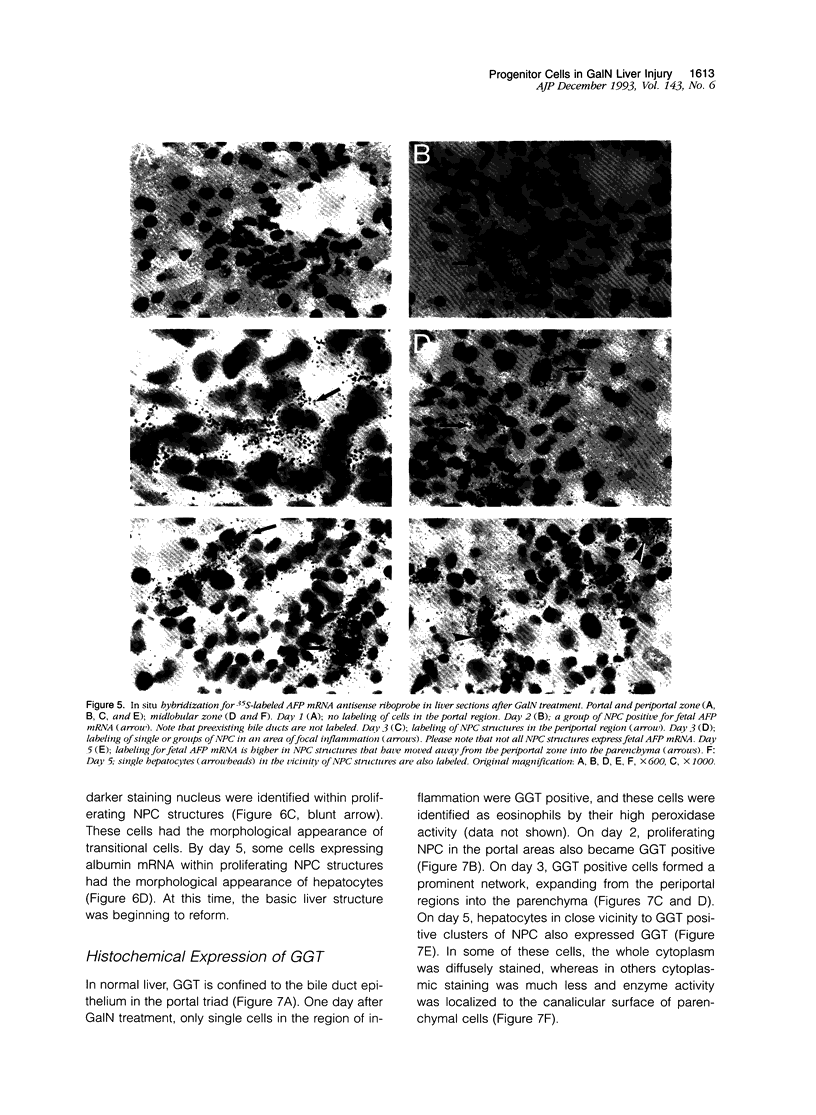
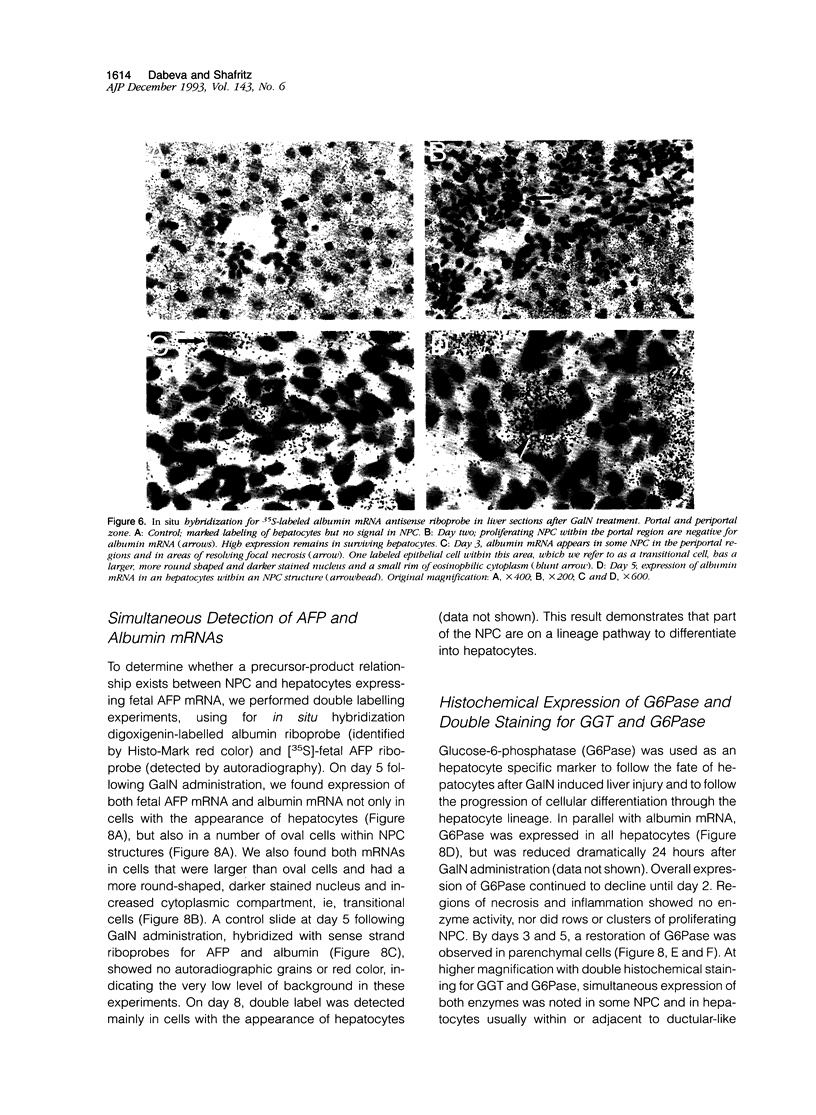
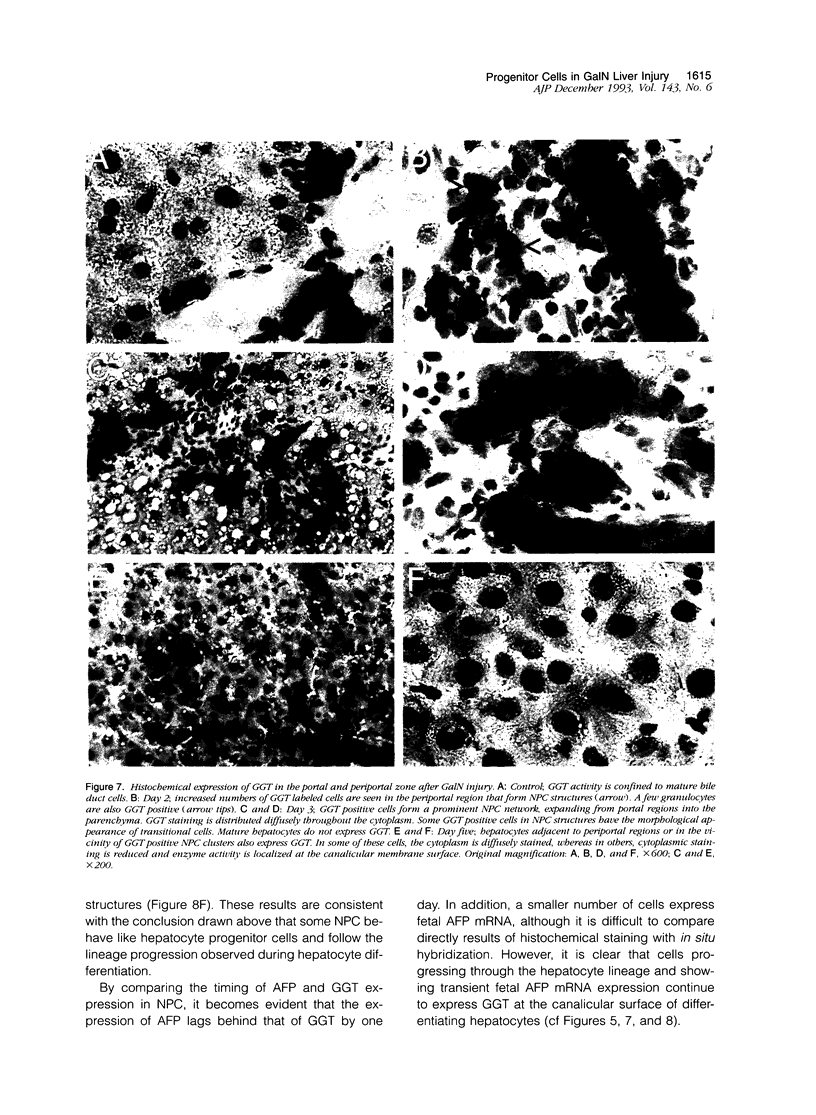
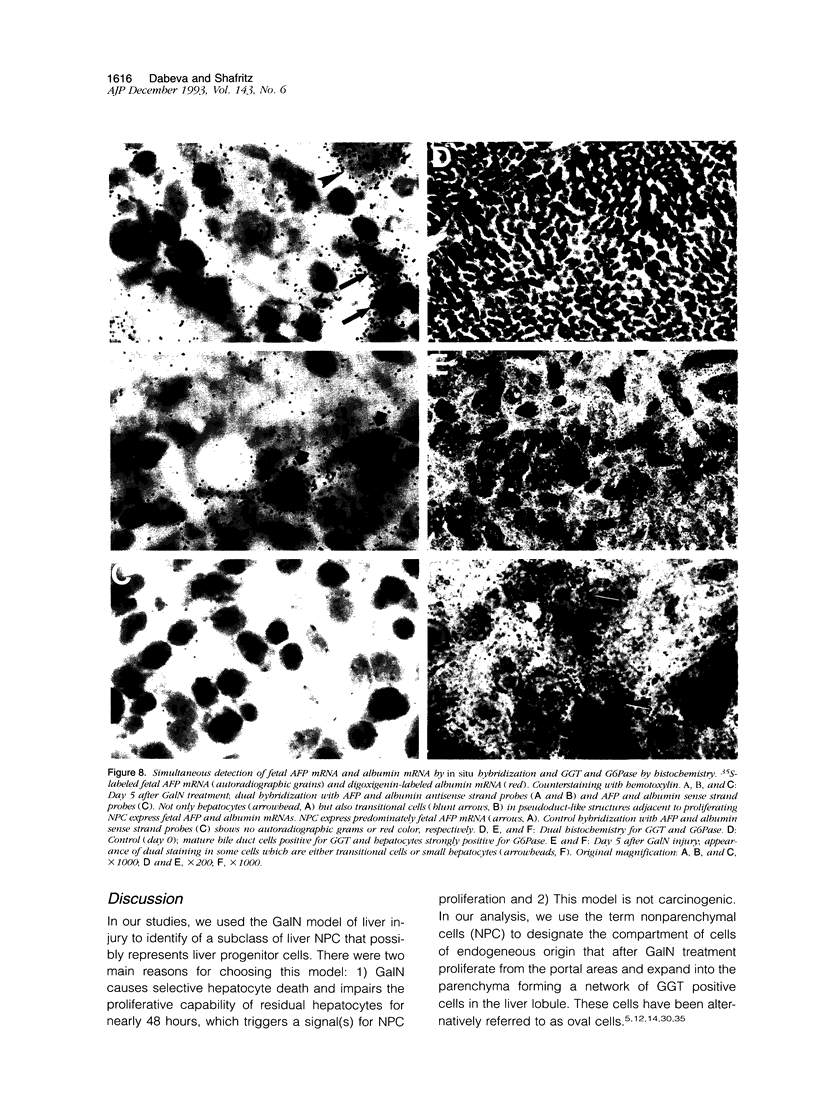




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpini G., Aragona E., Dabeva M., Salvi R., Shafritz D. A., Tavoloni N. Distribution of albumin and alpha-fetoprotein mRNAs in normal, hyperplastic, and preneoplastic rat liver. Am J Pathol. 1992 Sep;141(3):623–632. [PMC free article] [PubMed] [Google Scholar]
- Braun L., Goyette M., Yaswen P., Thompson N. L., Fausto N. Growth in culture and tumorigenicity after transfection with the ras oncogene of liver epithelial cells from carcinogen-treated rats. Cancer Res. 1987 Aug 1;47(15):4116–4124. [PubMed] [Google Scholar]
- Braun L., Mikumo R., Fausto N. Production of hepatocellular carcinoma by oval cells: cell cycle expression of c-myc and p53 at different stages of oval cell transformation. Cancer Res. 1989 Mar 15;49(6):1554–1561. [PubMed] [Google Scholar]
- Chiu J. F., Gabryelak T., Commers P., Massari R. The elevation of alpha-fetoprotein messenger RNA in regenerating rat liver. Biochem Biophys Res Commun. 1981 Jan 15;98(1):250–254. doi: 10.1016/0006-291x(81)91895-7. [DOI] [PubMed] [Google Scholar]
- Coleman W. B., Wennerberg A. E., Smith G. J., Grisham J. W. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993 May;142(5):1373–1382. [PMC free article] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- DuBois R. D. Early changes in gene expression during liver regeneration: what do they mean? Hepatology. 1990 Jun;11(6):1079–1082. doi: 10.1002/hep.1840110626. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Fausto N. Hepatocyte differentiation and liver progenitor cells. Curr Opin Cell Biol. 1990 Dec;2(6):1036–1042. doi: 10.1016/0955-0674(90)90153-6. [DOI] [PubMed] [Google Scholar]
- Fausto N., Mead J. E. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989 Jan;60(1):4–13. [PubMed] [Google Scholar]
- GRISHAM J. W., PORTA E. A. ORIGIN AND FATE OF PROLIFERATED HEPATIC DUCTAL CELLS IN THE RAT: ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDIES. Exp Mol Pathol. 1964 Jun;86:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- Germain L., Blouin M. J., Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988 Sep 1;48(17):4909–4918. [PubMed] [Google Scholar]
- Germain L., Goyette R., Marceau N. Differential cytokeratin and alpha-fetoprotein expression in morphologically distinct epithelial cells emerging at the early stage of rat hepatocarcinogenesis. Cancer Res. 1985 Feb;45(2):673–681. [PubMed] [Google Scholar]
- Goyette M., Faris R., Braun L., Hixson D., Fausto N. Expression of hepatocyte and oval cell antigens in hepatocellular carcinomas produced by oncogene-transfected liver epithelial cells. Cancer Res. 1990 Aug 1;50(15):4809–4817. [PubMed] [Google Scholar]
- Hixson D. C., Faris R. A., Thompson N. L. An antigenic portrait of the liver during carcinogenesis. Pathobiology. 1990;58(2):65–77. doi: 10.1159/000163565. [DOI] [PubMed] [Google Scholar]
- Ibsen K. H., Fishman W. H. Developmental gene expression in cancer. Biochim Biophys Acta. 1979 Aug 10;560(2):243–280. doi: 10.1016/0304-419x(79)90021-0. [DOI] [PubMed] [Google Scholar]
- Inaoka Y. Significance of the so-called oval cell proliferation during azo-dye hepatocarcinogenesis. Gan. 1967 Aug;58(4):355–366. [PubMed] [Google Scholar]
- Jonker A. M., Dijkhuis F. W., Kroese F. G., Hardonk M. J., Grond J. Immunopathology of acute galactosamine hepatitis in rats. Hepatology. 1990 Apr;11(4):622–627. doi: 10.1002/hep.1840110415. [DOI] [PubMed] [Google Scholar]
- Kuhlmann W. D., Wurster K. Correlation of histology and alpha 1-fetoprotein resurgence in rat liver regeneration after experimental injury by galactosamine. Virchows Arch A Pathol Anat Histol. 1980;387(1):47–57. doi: 10.1007/BF00428428. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Fausto N. Multiple alpha-fetoprotein RNAs in adult rat liver: cell type-specific expression and differential regulation. Cancer Res. 1991 Sep 1;51(17):4656–4664. [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Lesch R., Reutter W., Keppler D., Decker K. Liver restitution after acute galactosamine hepatitis: autoradiographic and biochemical studies in rats. Exp Mol Pathol. 1970 Feb;12(1):58–69. doi: 10.1016/0014-4800(70)90075-4. [DOI] [PubMed] [Google Scholar]
- MacDonald J. R., Beckstead J. H., Smuckler E. A. An ultrastructural and histochemical study of the prominent inflammatory response in D(+)-galactosamine hepatotoxicity. Br J Exp Pathol. 1987 Apr;68(2):189–199. [PMC free article] [PubMed] [Google Scholar]
- Marceau N. Cell lineages and differentiation programs in epidermal, urothelial and hepatic tissues and their neoplasms. Lab Invest. 1990 Jul;63(1):4–20. [PubMed] [Google Scholar]
- Medline A., Schaffner F., Popper H. Ultrastructural features in galactosamine-induced hepatitis. Exp Mol Pathol. 1970 Apr;12(2):201–211. doi: 10.1016/0014-4800(70)90050-x. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Novikoff P. M., Ikeda T., Hixson D. C., Yam A. Characterizations of and interactions between bile ductule cells and hepatocytes in early stages of rat hepatocarcinogenesis induced by ethionine. Am J Pathol. 1991 Dec;139(6):1351–1368. [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Minase T., Onhoe T. Demonstration of glucose 6-phosphatase activity in the oval cells of rat liver and the significance of the oval cells in azo dye carcinogenesis. Cancer Res. 1974 Dec;34(12):3379–3386. [PubMed] [Google Scholar]
- Panduro A., Shalaby F., Weiner F. R., Biempica L., Zern M. A., Shafritz D. A. Transcriptional switch from albumin to alpha-fetoprotein and changes in transcription of other genes during carbon tetrachloride induced liver regeneration. Biochemistry. 1986 Mar 25;25(6):1414–1420. doi: 10.1021/bi00354a034. [DOI] [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985 Nov;45(11 Pt 2):5762–5768. [PubMed] [Google Scholar]
- Petropoulos C., Andrews G., Tamaoki T., Fausto N. alpha-Fetoprotein and albumin mRNA levels in liver regeneration and carcinogenesis. J Biol Chem. 1983 Apr 25;258(8):4901–4906. [PubMed] [Google Scholar]
- RUBIN E. THE ORIGIN AND FATE OF PROLIFERATED BILE DUCTULAR CELLS. Exp Mol Pathol. 1964 Jun;86:279–286. doi: 10.1016/0014-4800(64)90059-0. [DOI] [PubMed] [Google Scholar]
- Reid L. M. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990 Feb;2(1):121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Scoazec J. Y., Moreau A., Feldmann G., Bernuau D. Cellular expression of alpha-fetoprotein gene and its relation to albumin gene expression during rat azo-dye hepatocarcinogenesis. Cancer Res. 1989 Apr 1;49(7):1790–1796. [PubMed] [Google Scholar]
- Sell S. Comparison of oval cells induced in rat liver by feeding N-2-fluorenylacetamide in a choline-devoid diet and bile duct cells induced by feeding 4,4'-diaminodiphenylmethane. Cancer Res. 1983 Apr;43(4):1761–1767. [PubMed] [Google Scholar]
- Sell S. Distribution of alpha-fetoprotein- and albumin-containing cells in the livers of Fischer rats fed four cycles of N-2-fluorenylacetamide. Cancer Res. 1978 Sep;38(9):3107–3113. [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Sell S., Salman J. Light- and electron-microscopic autoradiographic analysis of proliferating cells during the early stages of chemical hepatocarcinogenesis in the rat induced by feeding N-2-fluorenylacetamide in a choline-deficient diet. Am J Pathol. 1984 Feb;114(2):287–300. [PMC free article] [PubMed] [Google Scholar]
- Sells M. A., Katyal S. L., Shinozuka H., Estes L. W., Sell S., Lombardi B. Isolation of oval cells and transitional cells from the livers of rats fed the carcinogen DL-ethionine. J Natl Cancer Inst. 1981 Feb;66(2):355–362. [PubMed] [Google Scholar]
- Shalaby F., Shafritz D. A. Exon skipping during splicing of albumin mRNA precursors in Nagase analbuminemic rats. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2652–2656. doi: 10.1073/pnas.87.7.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirica A. E., Cihla H. P. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res. 1984 Aug;44(8):3454–3466. [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Tatematsu M., Ho R. H., Kaku T., Ekem J. K., Farber E. Studies on the proliferation and fate of oval cells in the liver of rats treated with 2-acetylaminofluorene and partial hepatectomy. Am J Pathol. 1984 Mar;114(3):418–430. [PMC free article] [PubMed] [Google Scholar]
- Teutsch H. F. Improved method for the histochemical demonstration of glucose-6-phosphatase activity. Histochemistry. 1978 Aug 25;57(2):107–117. doi: 10.1007/BF00496675. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson S. S. Hepatic stem cells. Am J Pathol. 1993 May;142(5):1331–1333. [PMC free article] [PubMed] [Google Scholar]
- Uriel J. Retrodifferentiation and the fetal patterns of gene expression in cancer. Adv Cancer Res. 1979;29:127–174. doi: 10.1016/s0065-230x(08)60847-7. [DOI] [PubMed] [Google Scholar]