Abstract
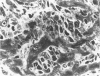
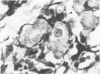
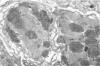
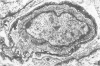
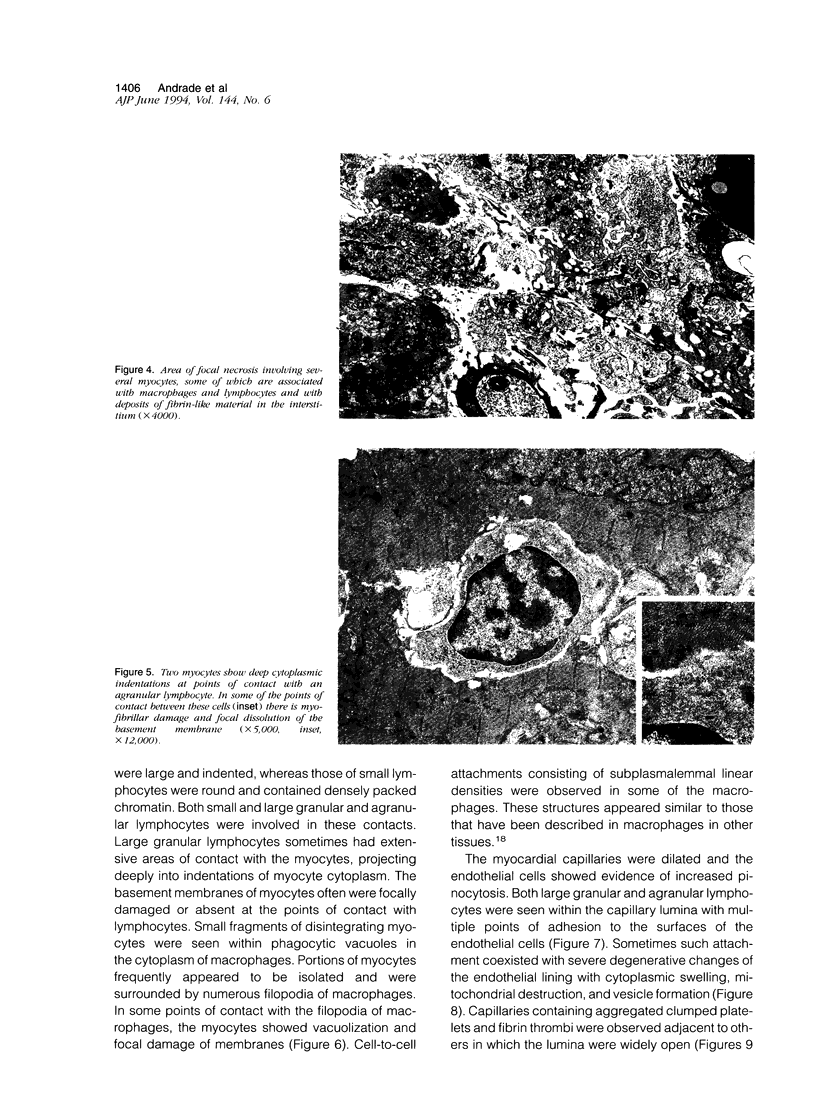
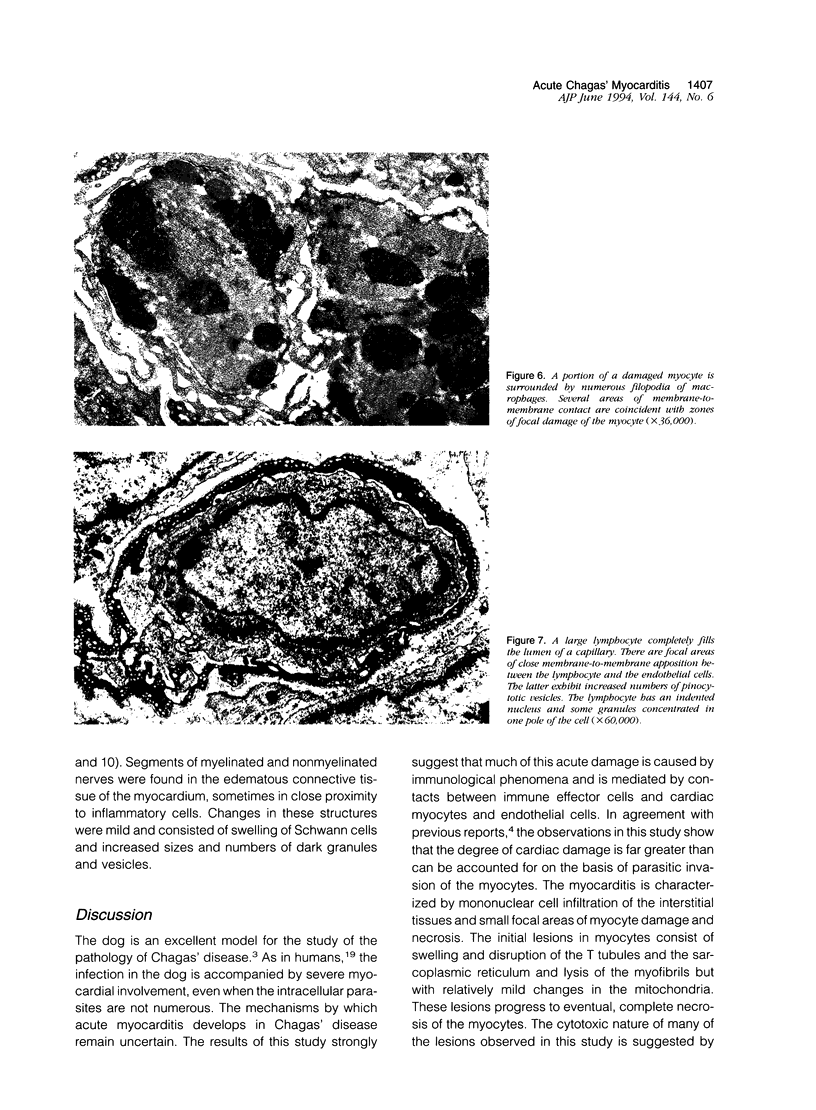
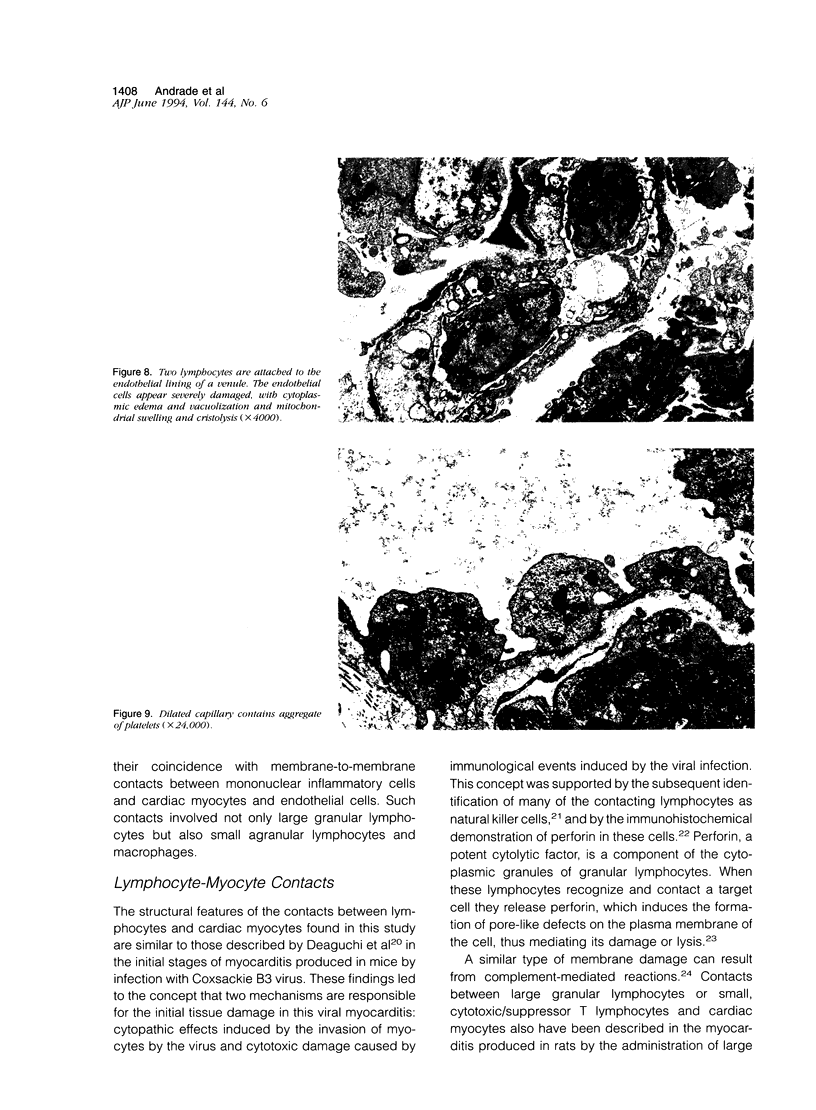
Histological and ultrastructural studies of the hearts of dogs sacrificed 18 to 26 days after intraperitoneal inoculation with 4 x 10(5) blood forms of the 12 SF strain of Trypanosoma cruzi/kg of body weight disclosed myocarditis characterized by parasitic invasion of some myocytes, damage and necrosis of nonparasitized myocytes, and interstitial infiltration by mononuclear cells. Nonparasitized myocytes showed alterations ranging from mild edema to severe myocytolysis. These changes often were accompanied by contacts of myocytes with lymphocytes (both granular and agranular) and macrophages. These contacts were characterized by focal loss of the myocyte basement membrane and close approximation of the plasma membranes of the two cells. Contacts between lymphocytes and capillary endothelial cells were also frequent. Platelet aggregates and fibrin microthrombi were observed in some capillaries. Our findings suggest that immune effector cells play a major role in the pathogenesis of the myocyte damage and the microangiopathy in acute Chagas' disease.
Full text
PDF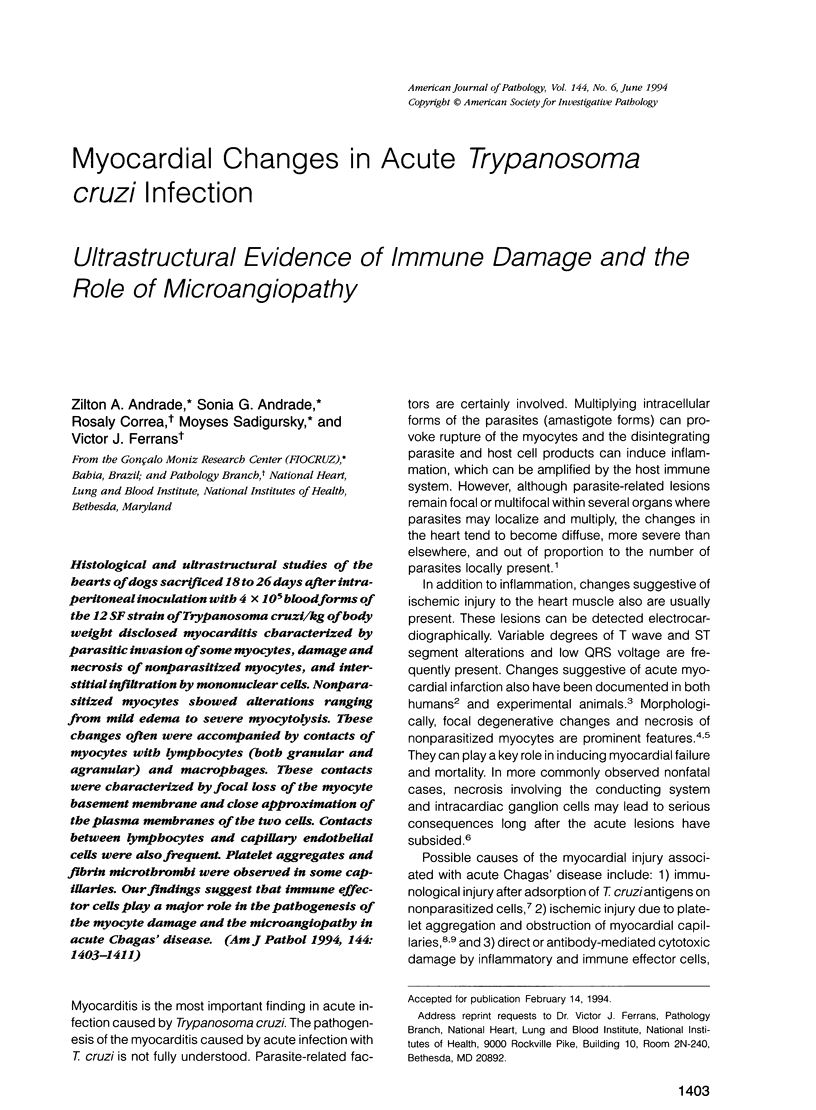





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade S. G., Grimaud J. A. Chronic murine myocarditis due to Trypanosoma cruzi--an ultrastructural study and immunochemical characterization of cardiac interstitial matrix. Mem Inst Oswaldo Cruz. 1986 Jan-Mar;81(1):29–41. doi: 10.1590/s0074-02761986000100004. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G., Oliveira G. B., Alonso D. R. Histopathology of the conducting tissue of the heart in Chagas' myocarditis. Am Heart J. 1978 Mar;95(3):316–324. doi: 10.1016/0002-8703(78)90362-9. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G., Sadigursky M. Damage and healing in the conducting tissue of the heart (an experimental study in dogs infected with Trypanosoma cruzi). J Pathol. 1984 Jun;143(2):93–101. doi: 10.1002/path.1711430204. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A. Pathogenesis of Chagas' disease. Res Immunol. 1991 Feb;142(2):126–129. doi: 10.1016/0923-2494(91)90021-a. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Martin W. B., Huber S. A. Role of CD4+ (helper) T cells in the pathogenesis of murine cytomegalovirus myocarditis. Lab Invest. 1992 Jun;66(6):755–761. [PubMed] [Google Scholar]
- Deguchi H., Kitaura Y., Hayashi T., Kotaka M., Kawamura K. Cell-mediated immune cardiocyte injury in viral myocarditis of mice and patients. Jpn Circ J. 1989 Jan;53(1):61–77. doi: 10.1253/jcj.53.61. [DOI] [PubMed] [Google Scholar]
- Deguchi H., Kitaura Y., Morita H., Kotaka M., Kawamura K. Cell-mediated immunity in Coxsackie B3 virus myocarditis in mice--in situ characterization by monoclonal antibody of mononuclear cell infiltrates. Heart Vessels Suppl. 1985;1:221–227. doi: 10.1007/BF02072397. [DOI] [PubMed] [Google Scholar]
- Jorgensen L., Rowsell H. C., Hovig T., Glynn M. F., Mustard J. F. Adenosine diphosphate-induced platelet aggregation and myocardial infarction in swine. Lab Invest. 1967 Dec;17(6):616–644. [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Crystal R. G. Subplasmalemmal linear densities in cells of the mononuclear phagocyte system in lung. Am J Pathol. 1980 Jul;100(1):131–150. [PMC free article] [PubMed] [Google Scholar]
- Lopes E. R., Tafuri W. L., Bogliolo L., Almeida H. O., Chapadeiro E., Raso P. Miocardite chagásica aguda humana (ganglionite subepicárdica; agressão à fibra cardíaca por linfócitos; relaço entre amastigotas e fibra muscular). Rev Inst Med Trop Sao Paulo. 1977 Sep-Oct;19(5):301–309. [PubMed] [Google Scholar]
- Marin-Neto J. A., Marzullo P., Marcassa C., Gallo Júnior L., Maciel B. C., Bellina C. R., L'Abbate A. Myocardial perfusion abnormalities in chronic Chagas' disease as detected by thallium-201 scintigraphy. Am J Cardiol. 1992 Mar 15;69(8):780–784. doi: 10.1016/0002-9149(92)90505-s. [DOI] [PubMed] [Google Scholar]
- Molina H. A., Kierszenbaum F. Eosinophil activation in acute and chronic chagasic myocardial lesions and deposition of toxic eosinophil granule proteins on heart myofibers. J Parasitol. 1989 Feb;75(1):129–133. [PubMed] [Google Scholar]
- Molina H. A., Kierszenbaum F. Interaction of human eosinophils or neutrophils with Trypanosoma cruzi in vitro causes bystander cardiac cell damage. Immunology. 1989 Feb;66(2):289–295. [PMC free article] [PubMed] [Google Scholar]
- Petry K., Eisen H. Chagas disease: a model for the study of autoimmune diseases. Parasitol Today. 1989 Apr;5(4):111–116. doi: 10.1016/0169-4758(89)90052-5. [DOI] [PubMed] [Google Scholar]
- Ridolfi R. L., Hutchins G. M., Bell W. R. The heart and cardiac conduction system in thrombotic thrombocytopenic purpura. A clinicopathologic study of 17 autopsied patients. Ann Intern Med. 1979 Sep;91(3):357–363. doi: 10.7326/0003-4819-91-3-357. [DOI] [PubMed] [Google Scholar]
- Rossi M. A., Carobrez S. G. Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice: histochemical evidence of hypoxic changes in the myocardium. Br J Exp Pathol. 1985 Apr;66(2):155–160. [PMC free article] [PubMed] [Google Scholar]
- Rossi M. A., Gonçalves S., Ribeiro-dos-Santos R. Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice. The potential role of intravascular platelet aggregation in its genesis. Am J Pathol. 1984 Feb;114(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Rossi M. A. Myocardial damage in Trypanosoma cruzi myocarditis: a role for macrophages. Can J Cardiol. 1990 Sep;6(7):293–298. [PubMed] [Google Scholar]
- Sadigursky M., Acosta A. M., Santos-Buch C. A. Muscle sarcoplasmic reticulum antigen shared by a Trypanosoma cruzi clone. Am J Trop Med Hyg. 1982 Sep;31(5):934–941. doi: 10.4269/ajtmh.1982.31.934. [DOI] [PubMed] [Google Scholar]
- Santos-Buch C. A. American trypanosomiasis: Chagas' disease. Int Rev Exp Pathol. 1979;19:63–100. [PubMed] [Google Scholar]
- Seko Y., Shinkai Y., Kawasaki A., Yagita H., Okumura K., Takaku F., Yazaki Y. Expression of perforin in infiltrating cells in murine hearts with acute myocarditis caused by coxsackievirus B3. Circulation. 1991 Aug;84(2):788–795. doi: 10.1161/01.cir.84.2.788. [DOI] [PubMed] [Google Scholar]
- Szarfman A., Terranova V. P., Rennard S. I., Foidart J. M., de Fatima Lima M., Scheinman J. I., Martin G. R. Antibodies to laminin in Chagas' disease. J Exp Med. 1982 Apr 1;155(4):1161–1171. doi: 10.1084/jem.155.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz H. B., Burns E. R., Sinha A. K., Kahn N. N., Morris S. A., Factor S. M., Hatcher V. B., Bilezikian J. P., Baum S. G., Wittner M. Enhanced platelet adherence and aggregation in Chagas' disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990 Sep;43(3):274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- Towbin H., Rosenfelder G., Wieslander J., Avila J. L., Rojas M., Szarfman A., Esser K., Nowack H., Timpl R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl(alpha 1-3)-galactose epitopes. J Exp Med. 1987 Aug 1;166(2):419–432. doi: 10.1084/jem.166.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekita L. F., Mota I. In-vitro lysis of sensitized Trypanosoma cruzi by platelets: role of C3b receptors. Parasite Immunol. 1989 Sep;11(5):561–566. doi: 10.1111/j.1365-3024.1989.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Yi E. S., Ulich T. R. Endotoxin, interleukin-1, and tumor necrosis factor cause neutrophil-dependent microvascular leakage in postcapillary venules. Am J Pathol. 1992 Mar;140(3):659–663. [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Liu C. C., Persechini P. M., Cohn Z. A. Perforin-dependent and -independent pathways of cytotoxicity mediated by lymphocytes. Immunol Rev. 1988 Mar;103:161–202. doi: 10.1111/j.1600-065x.1988.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yu Z. X., Hilbert S. L., Yamaguchi M., Chadwick D. P., Herman E. H., Ferrans V. J. Cardiotoxicity of human recombinant interleukin-2 in rats. A morphological study. Circulation. 1993 Apr;87(4):1340–1353. doi: 10.1161/01.cir.87.4.1340. [DOI] [PubMed] [Google Scholar]
- dos Santos R. R., Rossi M. A., Laus J. L., Silva J. S., Savino W., Mengel J. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med. 1992 Jan 1;175(1):29–39. doi: 10.1084/jem.175.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]