Abstract
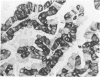
The human Class II major histocompatibility (MHC) antigens, or Ia antigens, which are thought to regulate immune cell interaction, can be detected in paraffin-embedded tissues by immunoperoxidase staining with a recently developed monoclonal antibody (LK8D3). HLA-DR antigens were observed in lymphoid tissues, Langerhans cells of the skin, some epithelial cells, and pulmonary alveolar macrophages. The expression of HLA-DR antigens was analyzed in formalin-paraffin sections by immunoperoxidase in 86 normal and abnormal thyroid epithelial tissues. All patients with Hashimoto's disease (8/8) and most patients with Graves' disease (6/8) expressed HLA/DR antigens in the thyroid epithelial cells and in adjacent inflammatory cells. Most papillary carcinomas (12/18), including 3 of 5 follicular variant of papillary thyroid carcinomas, had HLA-DR antigens detected in epithelial cells; whereas medullary thyroid carcinomas (0/5), follicular carcinomas (0/5), and multinodular goiters (0/4) did not have detectable HLA-DR immunoreactivity. A few other thyroid lesions had HLA-DR antigens detected in epithelial cells, including anaplastic carcinomas (2/5), Hurthle-cell tumors (1/16), and thyroid lymphomas (2/2). Monoclonal antibody LK8D3 and two other commercially available monoclonal antibodies against HLA-DR-stained tissues equally well in cryostat sections, but only antibody LK8D3 was effective in formalin-fixed paraffin-embedded tissue sections. These results indicate that epithelial cells from thyroids of patients with autoimmune diseases commonly express HLA-DR antigens. The presence of HLA-DR antigens in most papillary thyroid carcinomas may be helpful diagnostically in cases of follicular variants of papillary carcinomas. The role of HLA-DR expression in autoimmune thyroid disease and in papillary thyroid carcinoma remains to be determined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Canonica G. W., Bagnasco M., Moretta L., Cocco R., Ferrini O., Giordano G. Human T-lymphocyte subpopulations in Hashimoto's disease. J Clin Endocrinol Metab. 1981 Mar;52(3):553–556. doi: 10.1210/jcem-52-3-553. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Daynes R. A., Emam M., Krueger G. G., Roberts L. K. Expression of Ia antigen on epidermal keratinocytes after the grafting of normal skin to nude mice. J Immunol. 1983 Apr;130(4):1536–1539. [PubMed] [Google Scholar]
- Erlich H., Stetler D., Sheng-Dong R., Saiki R. Analysis by molecular cloning of the human class II genes. Fed Proc. 1984 Dec;43(15):3025–3030. [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Harach H. R., Williams E. D. Fibrous thyroiditis--an immunopathological study. Histopathology. 1983 Sep;7(5):739–751. doi: 10.1111/j.1365-2559.1983.tb02286.x. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Eisinger M., Albino A. P., Cairncross J. G., Old L. J. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982 Dec 1;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. A., Haynes B. F., Burch W. M., Shimizu K., Bowring M. A., Eisenbarth G. S. Ia+ T cells in new onset Graves' disease. J Clin Endocrinol Metab. 1984 Aug;59(2):187–190. doi: 10.1210/jcem-59-2-187. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Peterson P. A. Hormonal regulation of the expression of Ia antigens on mammary gland epithelium. Eur J Immunol. 1980 Dec;10(12):958–963. doi: 10.1002/eji.1830101212. [DOI] [PubMed] [Google Scholar]
- Moens H., Farid N. R., Sampson L., Noel E. P., Barnard J. M. Hashimoto's thyroiditis is associated with HLA-DRw3. N Engl J Med. 1978 Jul 20;299(3):133–134. doi: 10.1056/NEJM197807202990306. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Cordiali-Fei P., Cavaliere R., Di Filippo F., Quaranta V., Pellegrino M. A., Ferrone S. Ia-like antigens on freshly explanted human melanoma. Clin Immunol Immunopathol. 1981 May;19(2):250–259. doi: 10.1016/0090-1229(81)90067-2. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Hanafusa T., Chiovato L., Bottazzo G. F. Lectin-induced expression of DR antigen on human cultured follicular thyroid cells. Nature. 1983 Jul 7;304(5921):71–73. doi: 10.1038/304071a0. [DOI] [PubMed] [Google Scholar]
- Sridama V., Pacini F., DeGroot L. J. Decreased suppressor T-lymphocytes in autoimmune thyroid diseases detected by monoclonal antibodies. J Clin Endocrinol Metab. 1982 Feb;54(2):316–319. doi: 10.1210/jcem-54-2-316. [DOI] [PubMed] [Google Scholar]
- Strakosch C. R., Wenzel B. E., Row V. V., Volpé R. Immunology of autoimmune thyroid diseases. N Engl J Med. 1982 Dec 9;307(24):1499–1507. doi: 10.1056/NEJM198212093072407. [DOI] [PubMed] [Google Scholar]
- Thompson J. J., Herlyn M. F., Elder D. E., Clark W. H., Steplewski Z., Koprowski H. Expression of DR antigens in freshly frozen human tumors. Hybridoma. 1982;1(2):161–168. doi: 10.1089/hyb.1.1982.1.161. [DOI] [PubMed] [Google Scholar]
- Volpé R. The role of autoimmunity in hypoendocrine and hyperendocrine function: with special emphasis on autoimmune thyroid disease. Ann Intern Med. 1977 Jul;87(1):86–99. doi: 10.7326/0003-4819-87-1-86. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Herzig M. A., Lloyd R. V. Immunoperoxidase staining for Ia-like antigens in paraffin-embedded tissues from human melanoma and lung carcinoma. Am J Pathol. 1984 Apr;115(1):102–108. [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Indiveri F., Pellegrino M. A., Ferrone S. DR (Ia-like) antigens on human melanoma cells. Serological detection and immunochemical characterization. J Exp Med. 1979 Mar 1;149(3):658–668. doi: 10.1084/jem.149.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman K., Curman B., Forsum U., Klareskog L., Malmnäs-Tjernlund U., Rask L., Trägårdh L., Peterson P. A. Occurrence of Ia antigens on tissues on non-lymphoid origin. Nature. 1978 Dec 14;276(5689):711–713. doi: 10.1038/276711a0. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Kunkel H. G. The human Ia system. Adv Immunol. 1979;28:221–292. [PubMed] [Google Scholar]
- Winchester R. J., Wang C. Y., Gibofsky A., Kunkel H. G., Lloyd K. O., Old L. J. Expression of Ia-like antigens on cultured human malignant melanoma cell lines. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6235–6239. doi: 10.1073/pnas.75.12.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]