Abstract
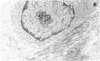
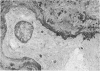
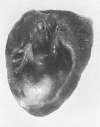
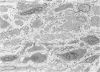
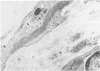
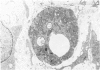
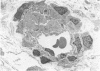
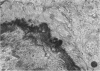
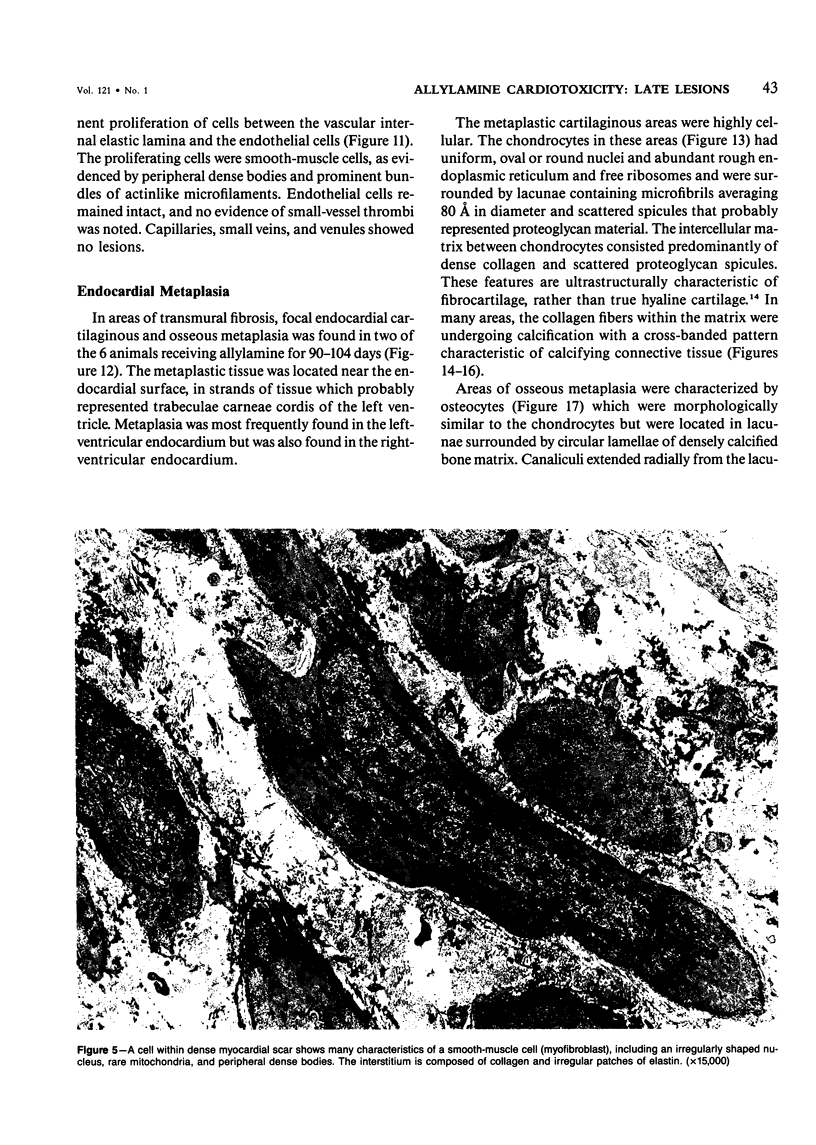
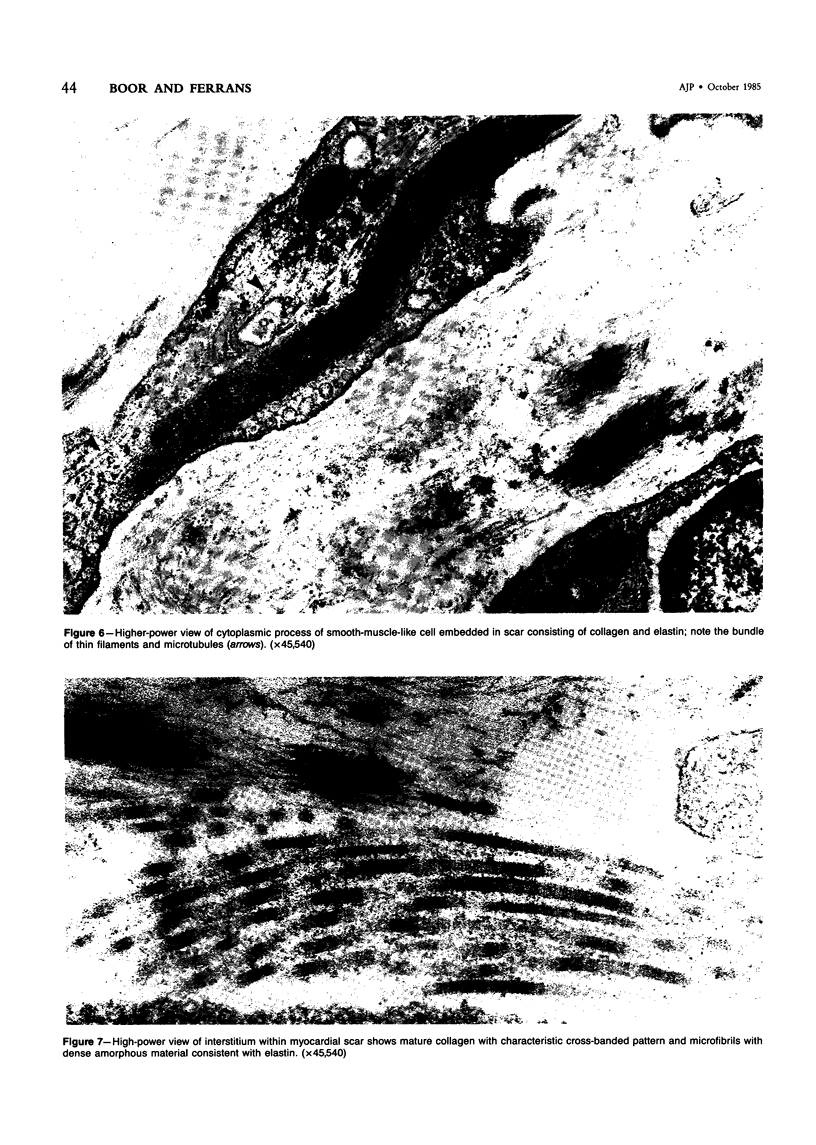
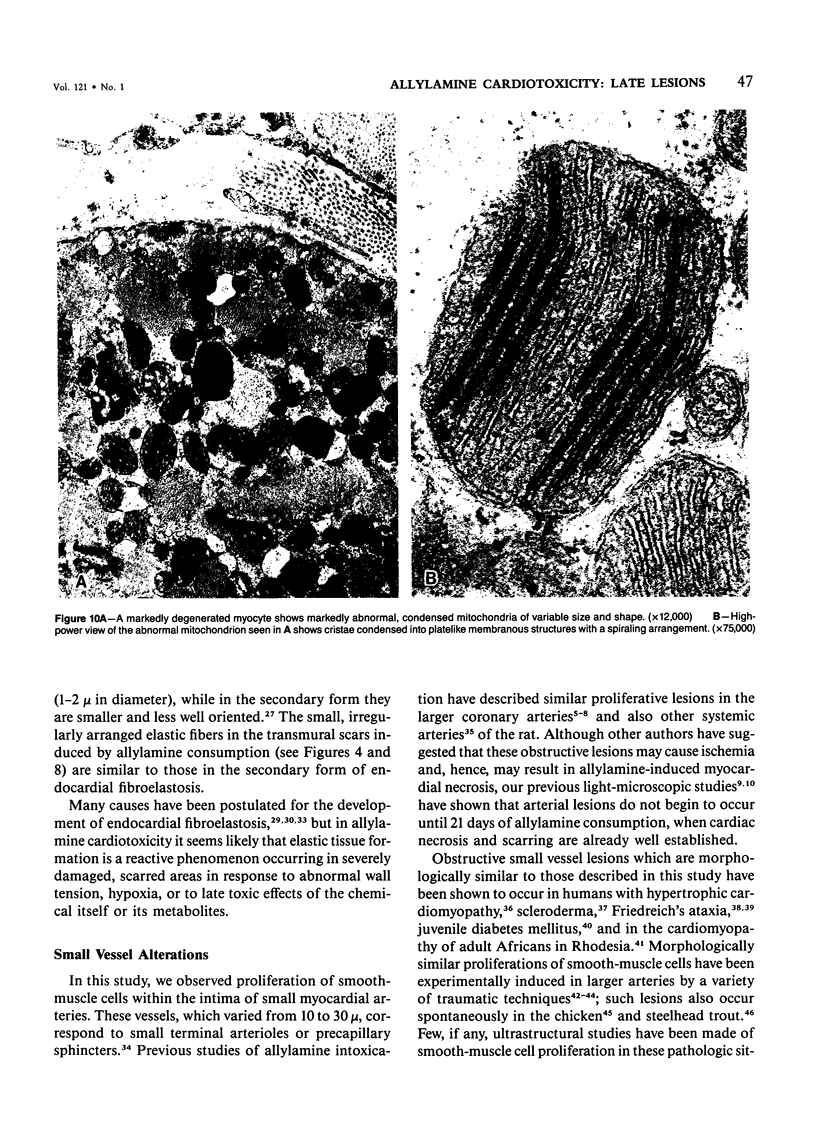
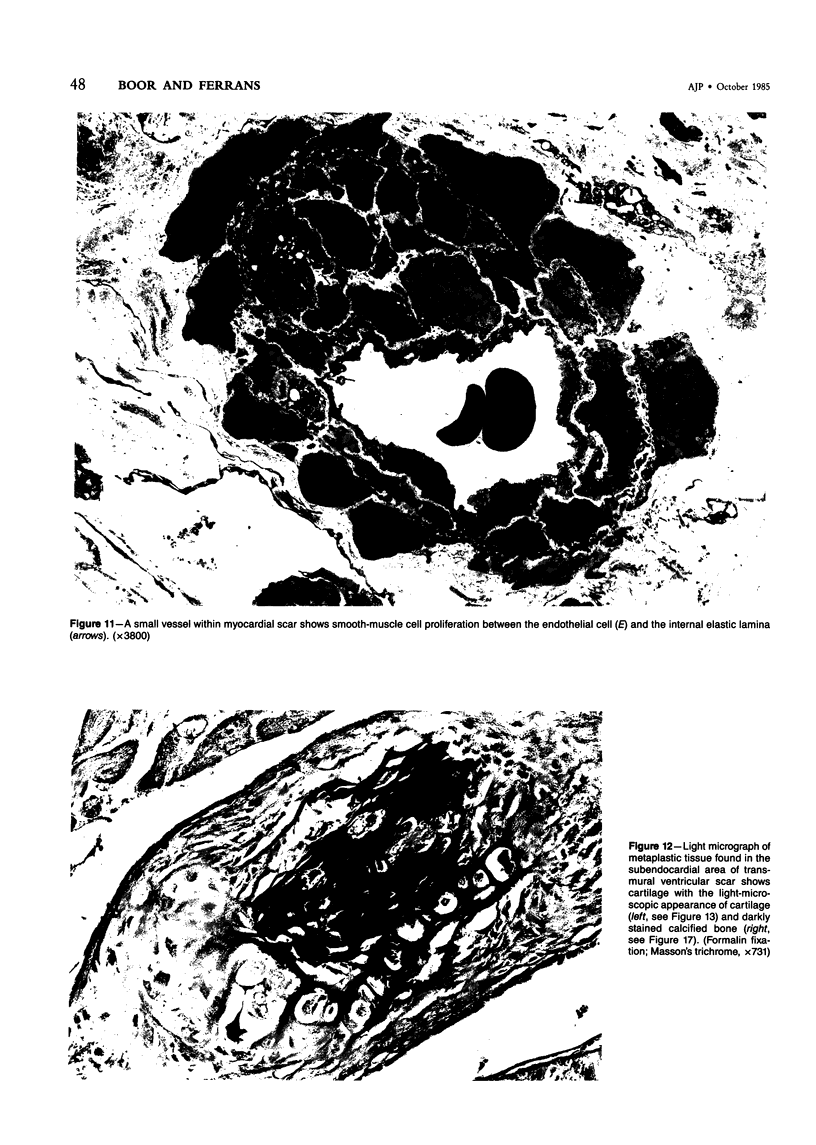
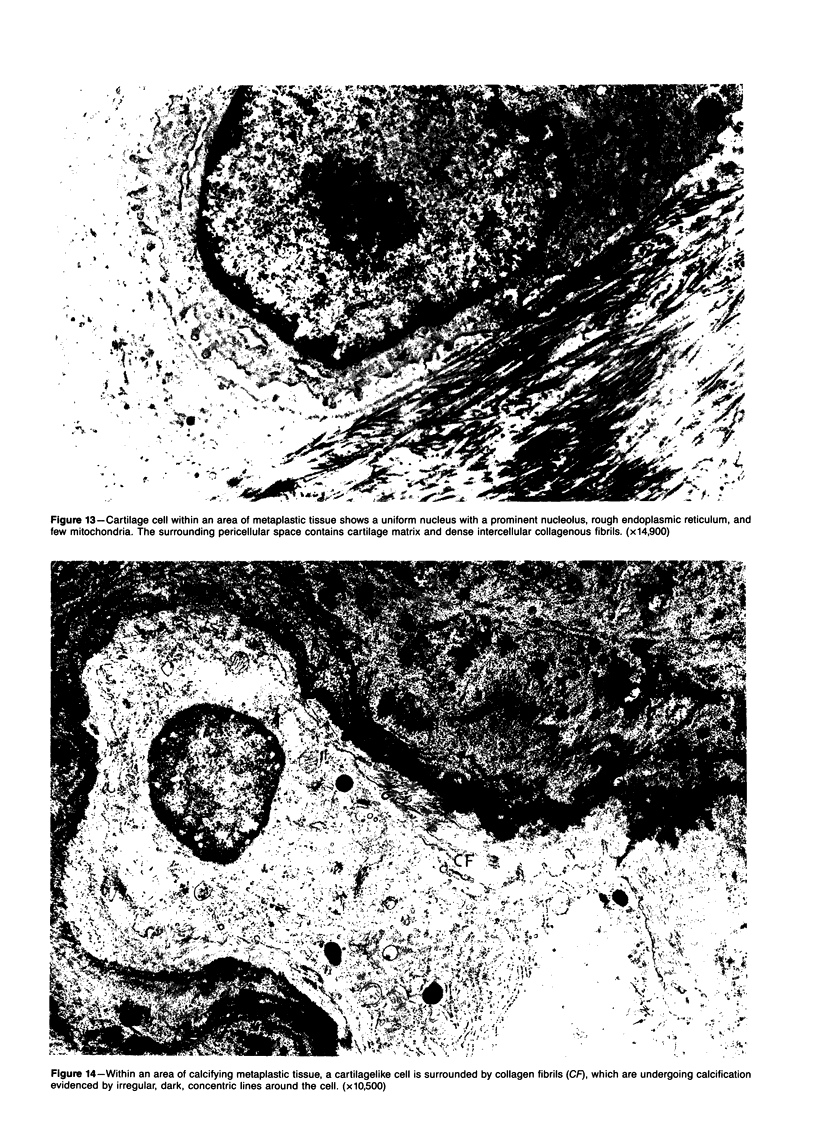
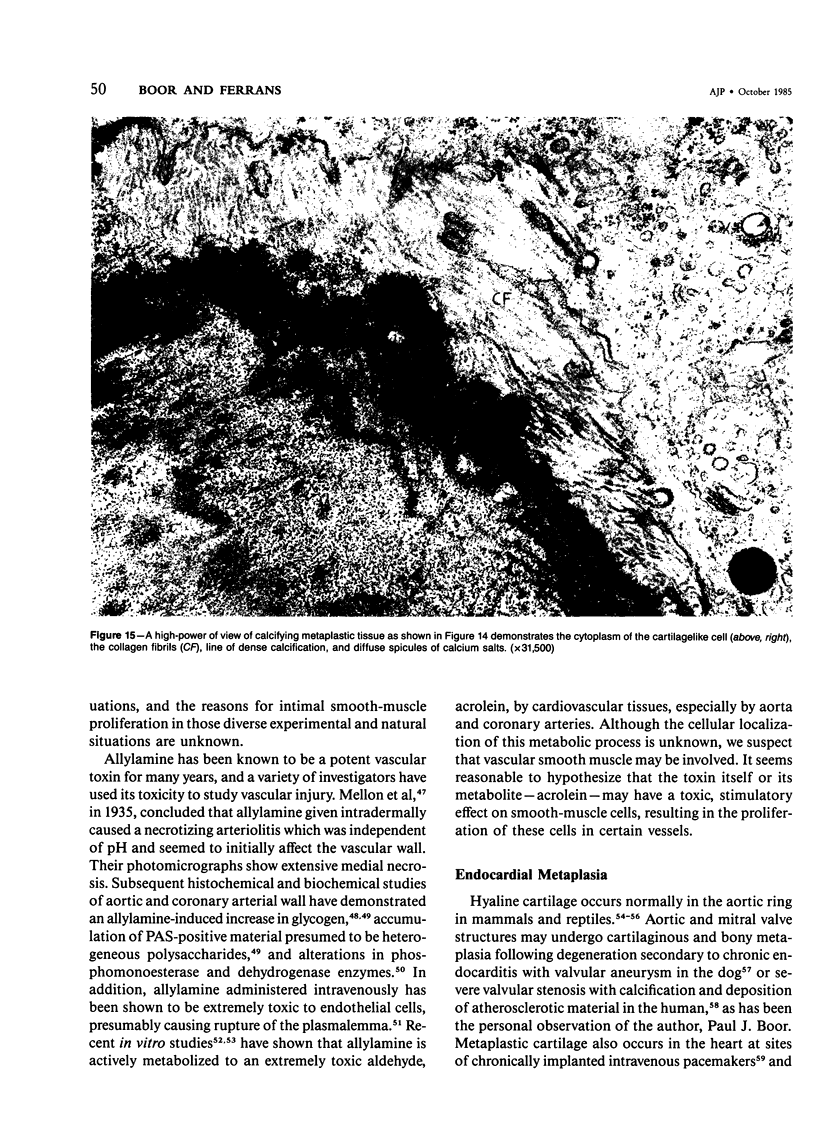
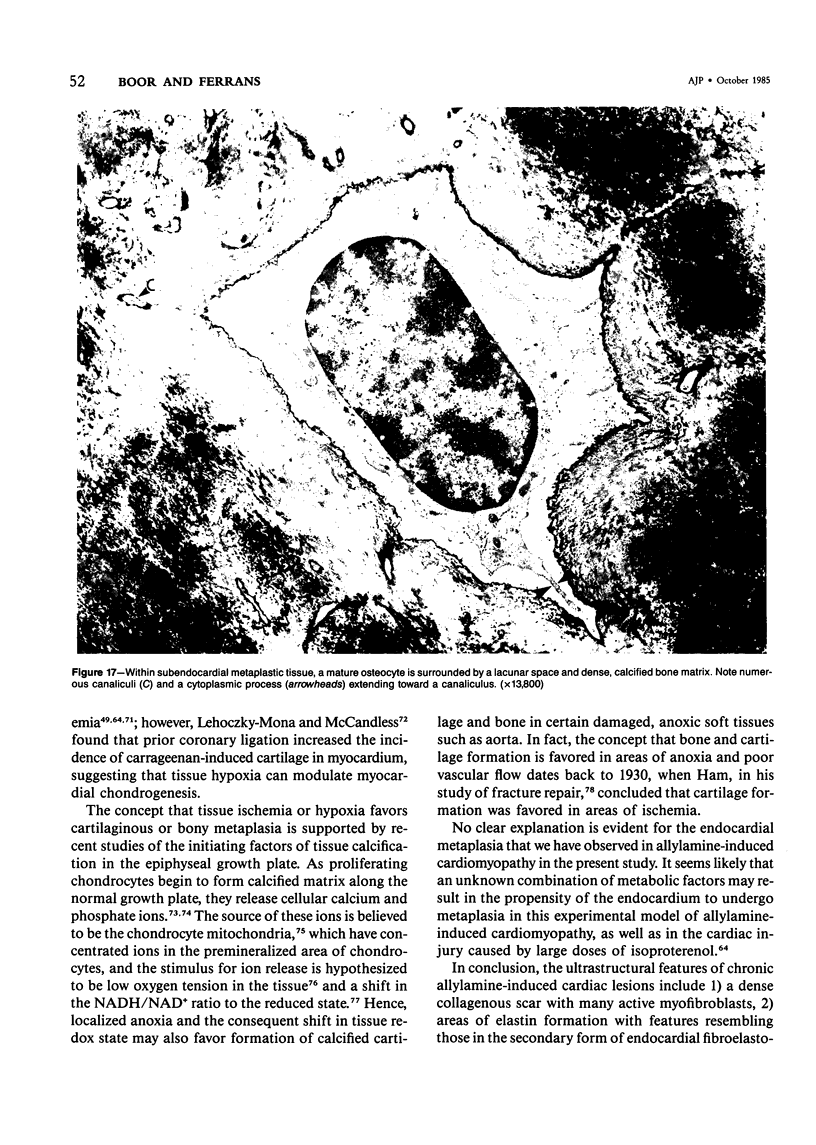
The late myocardial and vascular ultrastructural changes in rat hearts following consumption of the cardiovascular toxin allylamine were studied. Rats were given 0.1% allylamine HCl in drinking water for 10-104 days. From 10 to 21 days, there was organization of acute myocardial necrosis by macrophages and scattered polymorphonuclear leukocytes with prominent interstitial-cell proliferation. Alterations at 21-104 days included extensive scarring with formation of dense mature collagen with scattered fibroblasts present, grossly evident left-ventricular aneurysm, and gross and microscopic changes similar to those observed in the secondary form of endocardial fibroelastosis. Areas of scar contained highly cellular foci of smooth-muscle cells, myofibroblasts, and abundant extracellular elastin. Cardiac myocytes frequently showed markedly disorganized myofilaments, bizarrely distorted mitochondria with condensed cristae, and other severe degenerative changes. Small vessels within and adjacent to scar showed proliferation of intimal smooth-muscle cells. Endothelial lesions or recent or organized thrombi were not seen. Focal endocardial metaplasia, consisting of both chondroid and osseous tissue, was found in areas of transmural scarring, or ventricular aneurysm. Chondrocytes had the overall nuclear and cellular morphology, abundant rough endoplasmic reticulum, and surrounding lacunae typical of mature fibrocartilage. In some areas, the collagen matrix was undergoing calcification with the typical cross-banded pattern of calcifying connective tissue. Osteocytes were located in a densely calcified bone matrix and displayed characteristic cellular extensions into surrounding canaliculi. These findings indicate a severe myocardial, small-vessel, and endocardial injury during the course of chronic allylamine intoxication.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON K. J., DINGWALL J. A., SCHMIDT J., LECOCQ J. F., CLAWSON D. K. Induced connective tissue metaplasia. I. Heterogenous bone extract implants in the rat anterior eye chamber. A preliminary report. Plast Reconstr Surg Transplant Bull. 1960 Apr;25:399–405. [PubMed] [Google Scholar]
- BLOOR C. M., LOWMAN R. M. EXPERIMENTAL CORONARY ARTERIOGRAPHY. I. THE DISTRIBUTION AND EXTENT OF ALLYLAMINE-INDUCED VASCULAR LESIONS IN THE DOG. Radiology. 1963 Nov;81:770–777. doi: 10.1148/81.5.770. [DOI] [PubMed] [Google Scholar]
- BRIDGES J. B., PRITCHARD J. J. Bone and cartilage induction in the rabbit. J Anat. 1958 Jan;92(1):28–38. [PMC free article] [PubMed] [Google Scholar]
- Baur P. S., Larson D. L., Stacey T. R. The observation of myofibroblasts in hypertrophic scars. Surg Gynecol Obstet. 1975 Jul;141(1):22–26. [PubMed] [Google Scholar]
- Boor P. J., Ferrans V. J. Ultrastructural alterations in allylamine- induced cardiomyopathy: early lesions. Lab Invest. 1982 Jul;47(1):76–86. [PubMed] [Google Scholar]
- Boor P. J., Moslen M. T., Reynolds E. S. Allylamine cardiotoxicity: I. Sequence of pathologic events. Toxicol Appl Pharmacol. 1979 Sep 30;50(3):581–592. doi: 10.1016/0041-008x(79)90413-7. [DOI] [PubMed] [Google Scholar]
- Boor P. J., Nelson T. J. Biotransformation of the cardiovascular toxin, allylamine, by rat and human cardiovascular tissue. J Mol Cell Cardiol. 1982 Nov;14(11):679–682. doi: 10.1016/0022-2828(82)90165-1. [DOI] [PubMed] [Google Scholar]
- Boor P. J., Nelson T. J., Chieco P. Allylamine cardiotoxicity: II. Histopathology and histochemistry. Am J Pathol. 1980 Sep;100(3):739–764. [PMC free article] [PubMed] [Google Scholar]
- Boyde A., Shapiro I. M. Energy dispersive X-ray elemental analysis of isolated epiphyseal growth plate chondrocyte fragments. Histochemistry. 1980;69(1):85–94. doi: 10.1007/BF00508369. [DOI] [PubMed] [Google Scholar]
- Brighton C. T., Heppenstall R. B. Oxygen tension in zones of the epiphyseal plate, the metaphysis and diaphysis. An in vitro and in vivo study in rats and rabbits. J Bone Joint Surg Am. 1971 Jun;53(4):719–728. [PubMed] [Google Scholar]
- Brighton C. T., Hunt R. M. Mitochondrial calcium and its role in calcification. Histochemical localization of calcium in electron micrographs of the epiphyseal growth plate with K-pyroantimonate. Clin Orthop Relat Res. 1974 May;(100):406–416. [PubMed] [Google Scholar]
- CONRAD L. L., GONZALEZ I. E., JOEL W., FURMAN R. H. Histochemical evaluation of canine coronary artery and aortic lesions induced by intravenous allylamine. Circ Res. 1956 May;4(3):263–267. doi: 10.1161/01.res.4.3.263. [DOI] [PubMed] [Google Scholar]
- CROXATTO O. C. Producción experimental de cartilago en la aorta de perro; relación con la medionecrosis quística de aorta humana. Medicina (B Aires) 1953 Apr;13(2):98–108. [PubMed] [Google Scholar]
- Chamay A., Gabbiani G. Digital contracture deformity after implantation of a silicone prosthesis: light and electron microscopic study. J Hand Surg Am. 1978 May;3(3):266–270. doi: 10.1016/s0363-5023(78)80090-2. [DOI] [PubMed] [Google Scholar]
- Constantinides P., Robinson M. Ultrastructural injury of arterial endothelium. II. Effects of vasoactive amines. Arch Pathol. 1969 Aug;88(2):106–112. [PubMed] [Google Scholar]
- Ferrans V. J., Thiedemann K. U., Maron B. J., Jones M., Roberts W. C. Spherical microparticles in human myocardium: an ultrastructural study. Lab Invest. 1976 Oct;35(4):349–368. [PubMed] [Google Scholar]
- Fishbein M. C., Ferrans V. J., Roberts W. C. Histologic and ultrastructural features of primary and secondary endocardial fibroelastosis. Arch Pathol Lab Med. 1977 Jan;101(1):49–54. [PubMed] [Google Scholar]
- Fishbein M. C., Maclean D., Maroko P. R. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978 Jan;90(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- Fishbein M. C., Tan K. S., Beazell J. W., Schulman J. H., Hirose F. M., Criley J. M. Cardiac pathology of transvenous pacemakers in dogs. Am Heart J. 1977 Jan;93(1):73–81. doi: 10.1016/s0002-8703(77)80174-9. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Hüttner I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978 Mar;76(3):561–568. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Majno G. Dupuytren's contracture: fibroblast contraction? An ultrastructural study. Am J Pathol. 1972 Jan;66(1):131–146. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. Reparative processes in mammalian wound healing: the role of contractile phenomena. Int Rev Cytol. 1977;48:187–219. doi: 10.1016/s0074-7696(08)61745-3. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Karnovsky M. J. Smooth muscle cell proliferation in the occluded rat carotid artery: lack of requirement for luminal platelets. Am J Pathol. 1979 Mar;94(3):585–602. [PMC free article] [PubMed] [Google Scholar]
- HOMMES F. A., van LEEUWEN, ZILLIKEN F. Induction of cell differentiation. II. The isolation of a chondrogenic factor from embryonic chick spinal cords and notochords. Biochim Biophys Acta. 1962 Jan 29;56:320–325. doi: 10.1016/0006-3002(62)90569-3. [DOI] [PubMed] [Google Scholar]
- Hollander C. F. Cartilaginous focus at the base of the non-coronary semilunar valve of the aorta in rats of different ages. Exp Gerontol. 1968 Dec;3(4):303–307. doi: 10.1016/0531-5565(68)90041-7. [DOI] [PubMed] [Google Scholar]
- House E. W., Benditt E. P. The ultrastructure of spontaneous coronary arterial lesions in steelhead trout (Salmo gairdneri). Am J Pathol. 1981 Sep;104(3):250–257. [PMC free article] [PubMed] [Google Scholar]
- Hutchins G. M., Vie S. A. The progression of interstitial myocarditis to idiopathic endocardial fibroelastosis. Am J Pathol. 1972 Mar;66(3):483–496. [PMC free article] [PubMed] [Google Scholar]
- Imparato A. M., Baumann F. G., Pearson J., Kim G. E., Davidson T., Ibrahim I., Nathan I. Electron microscopic studies of experimentally produced fibromuscular arterial lesions. Surg Gynecol Obstet. 1974 Oct;139(4):497–504. [PubMed] [Google Scholar]
- JAMES T. N., FISCH C. OBSERVATIONS ON THE CARDIOVASCULAR INVOLVEMENT IN FRIEDREICH'S ATAXIA. Am Heart J. 1963 Aug;66:164–175. doi: 10.1016/0002-8703(63)90031-0. [DOI] [PubMed] [Google Scholar]
- JOHNS T. N. P., OLSON B. J. Experimental myocardial infarction. I. A method of coronary occlusion in small animals. Ann Surg. 1954 Nov;140(5):675–682. doi: 10.1097/00000658-195411000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T. N. De subitaneis mortibus. VIII. Coronary arteries and conduction system in scleroderma heart disease. Circulation. 1974 Oct;50(4):844–856. doi: 10.1161/01.cir.50.4.844. [DOI] [PubMed] [Google Scholar]
- James T. N., Marshall T. K. De subitaneis mortibus. XII. Asymmetrical hypertrophy of the heart. Circulation. 1975 Jun;51(6):1149–1166. doi: 10.1161/01.cir.51.6.1149. [DOI] [PubMed] [Google Scholar]
- James T. N. Small arteries of the heart. Circulation. 1977 Jul;56(1):2–14. doi: 10.1161/01.cir.56.1.2. [DOI] [PubMed] [Google Scholar]
- Kajikawa K., Yamaguchi T., Katsuda S., Miwa A. An improved electron stain for elastic fibers using tannic acid. J Electron Microsc (Tokyo) 1975;24(4):287–289. [PubMed] [Google Scholar]
- Kelsall M. A., Visci M. Aortic cartilage in the heart of Syrian hamsters. Anat Rec. 1970 Apr;166(4):627–633. doi: 10.1002/ar.1091660409. [DOI] [PubMed] [Google Scholar]
- LEHOCZKY-MONA J., MCCANDLESS E. L. ISCHEMIC INDUCTION OF CHONDROGENESIS IN MYOCARDIUM. Arch Pathol. 1964 Jul;78:37–42. [PubMed] [Google Scholar]
- Lalich J. J., Allen J. R., Paik W. C. Myocardial fibrosis and smooth muscle cell hyperplasia in coronary arteries of allylamine-fed rats. Am J Pathol. 1972 Feb;66(2):225–240. [PMC free article] [PubMed] [Google Scholar]
- Lalich J. J. Coronary artery hyalinosis in rats fed allylamine. Exp Mol Pathol. 1969 Feb;10(1):14–26. doi: 10.1016/0014-4800(69)90045-8. [DOI] [PubMed] [Google Scholar]
- Lalich J. J., Paik W. C. Influence of hydralazine consumption on allylamine induced myocardial fibrosis and hypertrophy in rats. Exp Mol Pathol. 1974 Aug;21(1):29–39. doi: 10.1016/0014-4800(74)90076-8. [DOI] [PubMed] [Google Scholar]
- MCCANDLESS E. L., LEHOCZKY J. M., RODBARD S. Aortic cartilage produced by intramural carrageenan. Arch Pathol. 1963 May;75:507–516. [PubMed] [Google Scholar]
- Mecham R. P. ELastin biosynthesis: a look at the current scene. Connect Tissue Res. 1981;8(3-4):155–160. doi: 10.3109/03008208109152366. [DOI] [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. II. The spontaneous plaque in the chicken. Lab Invest. 1970 Sep;23(3):231–245. [PubMed] [Google Scholar]
- NADAS A. S., ALIMURUNG M. M., SIERACKI L. A. Cardiac manifestations of Friedreich's ataxia. N Engl J Med. 1951 Feb 15;244(7):239–244. doi: 10.1056/NEJM195102152440701. [DOI] [PubMed] [Google Scholar]
- NORCIA L. N., GONZALEZ I. E., SHETLAR M. R., PETER J. A., FURMAN R. H. Alterations of protein, lipid and polysaccharide composition of canine aortas induced by allylamine, gonadal steroids and castration. Am J Physiol. 1958 Dec;195(3):759–768. doi: 10.1152/ajplegacy.1958.195.3.759. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Boor P. J. Allylamine cardiotoxicity--IV. Metabolism to acrolein by cardiovascular tissues. Biochem Pharmacol. 1982 Feb 15;31(4):509–514. doi: 10.1016/0006-2952(82)90152-6. [DOI] [PubMed] [Google Scholar]
- Neustein H. B., Lurie P. R., Fugita M. Endocardial fibroelastosis found on transvascular endomyocardial biospsy in children. Arch Pathol Lab Med. 1979 May;103(5):214–219. [PubMed] [Google Scholar]
- Paasch L. H., Zook B. C. The athogenesis of endocardial fibroelastosis in Burmese cats. Lab Invest. 1980 Feb;42(2):197–204. [PubMed] [Google Scholar]
- Paik W. C., Lalich J. Factors which contribute to aortic fibrous repair in rats fed beta-aminopropionitrile. Lab Invest. 1970 Jan;22(1):28–35. [PubMed] [Google Scholar]
- RHODIN J. A. Fine structure of vascular walls in mammals with special reference to smooth muscle component. Physiol Rev Suppl. 1962 Jul;5:48–87. [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Roberts W. C., Bulkley B. H., Morrow A. G. Pathologic anatomy of cardiac valve replacement: a study of 224 necropsy patients. Prog Cardiovasc Dis. 1973 May-Jun;15(6):539–587. doi: 10.1016/s0033-0620(73)80024-6. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Abraham J., Vecchione T., Guber S., Woodward M. Myofibroblasts and free silicon around breast implants. Plast Reconstr Surg. 1978 Aug;62(2):185–196. doi: 10.1097/00006534-197808000-00006. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Woodward M. Spatial orientation of microtubules in contractile fibroblasts in vivo. Anat Rec. 1978 Jun;191(2):169–181. doi: 10.1002/ar.1091910204. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Cliff W. J., Gabbiani G., Irlé C., Montandon D., Statkov P. R., Majno G. Myofibroblasts in human granulation tissue. Hum Pathol. 1974 Jan;5(1):55–67. doi: 10.1016/s0046-8177(74)80100-0. [DOI] [PubMed] [Google Scholar]
- Sandberg L. B., Leslie J. G., Oakes B. W. In vitro studies of elastin metabolism. Connect Tissue Res. 1981;8(3-4):219–225. doi: 10.3109/03008208109152379. [DOI] [PubMed] [Google Scholar]
- Schryer M. J., Karnauchow P. N. Endocardial fibroelastosis; etiologic and pathogenetic considerations in children. Am Heart J. 1974 Nov;88(5):557–565. doi: 10.1016/0002-8703(74)90238-5. [DOI] [PubMed] [Google Scholar]
- Shapiro I. M., Golub E. E., Kakuta S., Hazelgrove J., Havery J., Chance B., Frasca P. Initiation of endochondral calcification is related to changes in the redox state of hypertrophic chondrocytes. Science. 1982 Sep 3;217(4563):950–952. doi: 10.1126/science.7112108. [DOI] [PubMed] [Google Scholar]
- Simpson C. F. Coronary and thyroid arteriopathy induced by allylamine and beta-aminopropionitrile. Exp Mol Pathol. 1982 Dec;37(3):382–392. doi: 10.1016/0014-4800(82)90050-8. [DOI] [PubMed] [Google Scholar]
- Sottiurai V. S., Fry W. J., Stanley J. C. Ultrastructure of medial smooth muscle and myofibroblasts in human arterial dysplasia. Arch Surg. 1978 Nov;113(11):1280–1288. doi: 10.1001/archsurg.1978.01370230070008. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Kim D. N. Biology of disease. Atherosclerosis as a hyperplastic and/or neoplastic process. Lab Invest. 1983 Mar;48(3):245–255. [PubMed] [Google Scholar]
- Van Vleet J. F., Schollmeyer M. P., Engle W. R., Tacker W. A., Jr, Bourland J. D. Cardiovascular alterations induced by chronic transvenous implantation of an automatic defibrillator electrode catheter in dogs. J Electrocardiol. 1981;14(1):67–72. doi: 10.1016/s0022-0736(81)80031-3. [DOI] [PubMed] [Google Scholar]
- VanVleet J. F., Tacker W. A., Jr, Bourland J. D., Kallok M. J., Schollmeyer M. P. Cardiac damage in dogs with chronically implanted automatic defibrillator electrode catheters and given four episodes of multiple shocks. Am Heart J. 1983 Aug;106(2):300–307. doi: 10.1016/0002-8703(83)90196-5. [DOI] [PubMed] [Google Scholar]