Abstract
The diagnosis of limb girdle muscular dystrophy (LGMD) type 2A (due to mutations in the gene encoding for calpain-3) is currently based on protein analysis, but mutant patients with normal protein expression have also been identified. In this study we investigated 150 LGMD patients with normal calpain-3 protein expression, identified gene mutations by an allele-specific polymerase chain reaction test, and analyzed the mutant calpain-3 catalytic activity. Four different mutations were found in eight patients (5.5%): a frame-shifting deletion (550 A del) and three missense (R490Q, R489Q, R490W). Patients with normal calpain-3 protein expression on Western blot are a considerable proportion (20%) of our total LGMD2A population. While in control muscle the calpain-3 Ca++-dependent autocatalytic activity was evident within 5 minutes and was prevented by ethylene diaminetetraacetic acid, in all mutant patient samples the protein was not degraded, indicating that the normal autocatalytic function had been lost. By this new functional test, we show that conventional protein diagnosis fails to detect some mutant proteins, and prove the pathogenetic role of R490Q, R489Q, R490W missense mutations. We suggest that these mutations impair protein activity by affecting interdomain protein interaction, or reduce autocatalytic activity by lowering the Ca++ sensitivity.
Autosomal recessive limb girdle muscular dystrophies (LGMD type 2) are a clinically and genetically heterogeneous group of disorders, characterized by progressive involvement of proximal limb girdle muscles. LGMD2A (MIM number 253600), whose locus has been mapped to chromosome 15q15.1, 1 is considered to be the most frequent form of recessive LGMD. LGMD2A is caused by single or small nucleotide changes widespread along a 40-kb gene, named CAPN-3. 2 More than 110 different CAPN-3 gene mutations have been described 3-10 ; about 45% are null mutations and half are missense.
LGMD2A is caused by defects in a protein with an enzymatic rather than structural function. CAPN-3 gene encodes for calpain-3 (originally named p94), the muscle-specific member of a family of Ca++-activated neutral proteases, which also includes the ubiquitous m- and μ-calpains. Calpain-3 is a multidomain protein, characterized by 3 exclusive sequence inserts (NS, IS1, IS2) 11 ; domain I has regulatory role, domain II is the proteolytic module, domain III has a C2-domain like Ca++-binding function (probably involved in Ca++-dependent translocation of calpain to the membrane), and domain IV binds Ca++ ions.
The pathophysiological role of calpain-3 is still under investigation. Most of the biochemical information on calpain-3 has been inferred from comparison with the ubiquitous calpains, 12 as protein purification is not feasible due to its high in vitro instability. 13 Some gene mutations are expected to impair the main biochemical activities of calpain-3 11,14 : autocatalytic activity, α-fodrin proteolysis, and binding to titin.
The search for specific substrates of calpain-3 has been so far unsuccessful. Due to its nuclear localization signal sequence, calpain-3 might act in the nucleus in particular conditions and might be involved in the regulation of transcription factors controlling survival genes and apoptosis. 15,16 Several molecules with regulatory functions have been reported to be substrates for calpains in cell culture, but various lines of research have suggested that calpains may be involved in cytoskeletal or myofibrillar protein degradation. 5,17,18 While it is unlikely that calpain-3 is implicated in myoblast fusion, being fusion-effective in muscle of LGMD2A patients, it might act in the disassembly of myofibrils during early stages of turnover 17 or cause a postfusion defect in muscle maturation. 19
The pathological consequences of CAPN-3 gene mutations have been investigated on both protein and clinical phenotype: patients homozygous for null mutations usually have severe phenotype and absent protein, whereas patients homozygous for missense mutations have variable protein amount and disease severity. 3,4,9 Moreover, the same mutation can produce a wide variation at both the clinical 3,9,20 and protein level (from normal to absent). 10
Recently, the diagnosis of LGMD2A has been based on protein testing, but only patients who show a deficiency are selected for subsequent mutation screening. This approach is useful for diagnosing previously undetermined LGMD patients, 8,21,22 but it is not completely sensitive; some patients with normal protein expressions do have CAPN-3 gene mutations. 8,23 The functional characterization of CAPN-3 gene mutations could be helpful in explaining why some missense mutations lead to the synthesis of a protein that loses its function but preserves normal expression. The only functional studies were conducted by site-directed mutagenesis on animal cultured cells 13,14,24 or transgenic mice 19,25 and demonstrated that some mutants lost autocatalytic activity, while others lost fodrinolytic capacity.
In this study we investigated a group of LGMD2A patients who showed normal calpain-3 protein expression to identify CAPN-3 mutant alleles and, further, characterized the function of the resulting abnormal protein by biochemical assays.
Materials and Methods
Patients
Our muscle biopsy bank contains more than 5300 specimens which have been collected since 1980 for diagnostic purposes. Reviewing the diagnosis of all samples, about 20% of total (1100) were classified as “muscular dystrophy” on the basis of clinical, histopathological, and molecular findings. For the purpose of this study, we excluded 644 muscular dystrophy biopsies because our multiple-protein screening (for dystrophin, α-sarcoglycan, dysferlin, calpain-3, caveolin-3, merosin, and emerin) identified one deficient protein and/or a molecular diagnosis was assessed (199 DMD, 164 BMD, 50 D/BMD carrier, 34 LGMD2A, 33 LGMD2B, 19 LGMD2D, 7 LGMD2C, 6 LGMD2E, 1 LGMD2F, 9 LGMD1C, 3 EDMD1, 22 merosin-deficient congenital dystrophy, 71 myotonic dystrophy, 26 facio-scapulo-humeral dystrophy). An additional 306 patients were excluded because their phenotype was considered not compatible with LGMD2A (dominant LGMD, distal myopathy, scapulo-peroneal dystrophy, oculo-pharyngeal dystrophy, and merosin-positive congenital dystrophy).
A total of 150 patients were selected for CAPN-3 mutation screening since they matched the following criteria: normal expression of above mentioned muscle proteins, muscle biopsy histopathology consistent with a dystrophic or myopathic process, and increased creatine kinase (CK) levels (> 500 U/L). Of these 150 patients, 129 were diagnosed as affected with LGMD, 18 with proximal myopathy, and three were presymptomatic with high CK.
The clinical severity of muscle disease was graded as 1) asymptomatic: elevated CK level, calf hypertrophy, cramps after effort; 2) mild: muscle weakness in lower and/or upper girdle; 3) moderate: waddling gait, Gowers’ sign, difficulty climbing stairs; and 4) severe: significant loss of muscle strength or loss of ambulation.
Muscle Biopsy Histopathology and Electron Microscopy
Microscopy inspection of routinely stained sections was used to determine the degree of the dystrophic/myopathic process, on the following graded scale: 1, active dystrophic process (marked increase of fiber size variability, active degeneration and regeneration, marked increase of connective tissue); 2, moderate dystrophic process (marked increase of fiber size variability, increased central nuclei, few degenerating and regenerating fibers, slight increase of connective tissue); and 3, mild myopathic picture (moderate increase of fiber size variability, increased central nuclei) (see examples of each category in Figure 1 ▶ ).
Figure 1.

Muscle biopsy sections stained with trichrome (a) and hematoxylin and eosin routine stains (b to d). In the “active stage dystrophic process” (a and b) increased fiber size variability, fibro-fatty replacement, and degenerating and regenerating fibers are observed. “Moderate dystrophic process” (c) is characterized by increased fiber size variability, central nuclei, regenerating, and ring fibers. “Mild myopathic process” (d) shows slight fiber size variability and central nuclei. Magnification, ×400.
For electron microscopy, muscle tissue was fixed for 3 hours at 4°C in 5% glutaraldehyde buffered with 0.1 mol/L cacodylate buffer (pH 7.2), washed overnight at 4°C with cacodylate buffer with 0.1 mol/L sucrose, postfixed in 1% osmium tetroxid (Sigma, St. Louis, MO), dehydrated, infiltrated, and embedded in Epon 812 epoxy resin (Electron Microscopy Sciences, Fort Washington, PA). Longitudinal ultrathin sections were stained with uranyl acetate and lead nitrate and examined with a Jeol transmission electron microscope JEM-1200 EXII.
Immunoblot Studies
Western blot analysis was performed as described previously, 21 with minor modifications. Briefly, muscle biopsy sections were dissolved in loading buffer (0.05 mol/L dithiothreitol, 0.1 mol/L ethylenediaminetetraacetic acid (EDTA), 0.125 mol/L Tris, 4% sodium dodecyl sulfate (SDS), 0.005% bromophenol blue), boiled, and centrifuged. Proteins were resolved by SDS-PAGE and electroblotted to nitrocellulose membrane (Hoefer Pharmacia, San Francisco, CA). Posttransfer gels were stained with Coomassie blue, whereas the blots were blocked with bovine serum albumin (BSA) and then incubated with antibodies against calpain-3 (Calp12A2, diluted 1:800; Novocastra, Newcastle-upon-Tyne, UK), nebulin (1:400; Sigma), α-actinin (1:400; Novocastra), dystrophin (C-terminus, 1:1000; Novocastra), desmin (1:500; Novocastra), and β-dystroglycan (1:400; Novocastra). Immunoreactive bands were visualized using avidin-biotin peroxidase and chemiluminescent method (ECL; Amersham, Rainham, UK).
Biochemical Studies
Calpain-3 Autocatalytic Assay
Several sets of control muscle biopsy sections were dissolved in 50 μl of sterile saline solution and incubated at room temperature for different lengths of time (0, 1, 5, 10, 20, and 30 minutes) before further adding 50 μl of loading buffer. To conserve muscle tissue, the same experiment was conducted on patient samples for only 5 and 20 minutes of incubation, on the basis of the results obtained in the controls. To test the role of Ca++ ions in calpain-3 autocatalytic activity, an additional set of sections was incubated in saline solution with 10 mmol/L EDTA. All samples were then processed as described for immunoblot analysis, using antibodies against calpain-3.
Calpain-3 Proteolytic Assay
The role of time-dependent calpain-3 proteolytic activity on several muscle proteins (dystrophin, β-dystroglycan, α-actinin, desmin, and nebulin) has been tested by immunoblot on muscle samples in the experimental condition described for calpain-3 autocatalytic assay; multiple loading of each sample was used, followed by labeling with different antibodies.
Molecular Studies
DNA Extraction
Genomic DNA was extracted from blood leukocytes or muscle biopsy, using GenElute Mammalian Genomic DNA kit and the procedure recommended by the manufacturer (Sigma). DNA concentration and integrity were tested by optical density measurement and by 0.8% agarose gel electrophoresis, respectively. A working dilution of 100 ng/μl DNA sample was prepared.
PCR Amplification with Allele-Specific Primers
Fifteen different CAPN-3 gene mutations were selected for our allele-specific screening on the basis of their frequency or because they were expected to result in normal protein expression. 8,12,14,23,26 Selected mutations have been previously reported in other patients, and the possibility that they were polymorphic variants was excluded. 4 Seven mutations (550 del. A, G222R, R448H, R489W, R572Q, R572W, R748Q) were studied using classic ARMS-PCR test (Amplification Refractory Mutation System), and eight mutations (D77N, T184 mol/L, R448C, R489Q, R490W, R490Q, G496R, S606L) using tetra-primer ARMS-PCR. 27 In the latter method, one pair of outer control primers and one pair of inner primers (specific for mutant and wild-type alleles) are used in the same reaction. By positioning the outer primers at different distances from mutation point, two different small allele-specific and one large control PCR products are produced. To enhance the allelic specificity, a second deliberate mismatch at position −2 from the 3′ terminus was incorporated in the inner primers, according to the rules described by Little. 28
We choose this method because of its high specificity and rapidity, and because it provides an internal control reaction that reduces false negative results. The primer sequences used for ARMS were those reported elsewhere, 4 except one for R448H. The primers for this mutation by ARMS and for tetra-primer ARMS (sequence available on request) were designed using Primer software (http://www.genome.wi.mit.edu/genome software/other/primer3.html), with the human calpain-3 sequence as reference (GenBank accession number AF209502). PCR reactions were prepared in a 12.5-μl mixture containing 100 ng of genomic DNA, 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 200 μmol/L of each dNTP (Applied Biosystems, Foster City, CA), 1.25 U AmpliTaq Gold polymerase (Applied Biosystems), 0.2 μmol/L of each primer (Sigma Genosys, Woodlands, TX) for ARMS or 5 pmol of each inner primer and 1.25 pmol of each outer primer for tetra-primer ARMS (the higher concentration of inner primers was used to enhance the amplification of the two allele-specific products). PCR amplifications were performed (9700 thermal cycler; Applied Biosystems) using the following conditions: denaturation at 95°C for 10 minutes, followed by 35 cycles of denaturation at 95°C for 40 seconds, annealing for 30 seconds, extension at 72°C for 30 seconds, and final extension at 72°C for 10 minutes. To reduce specific products and optimize the allele-specific reaction, a touchdown program was used: in the first cycle the temperature was set 5°C higher than the annealing temperature, it was decreased by half a degree per cycle for 10 cycles, and then held constant for a further 25 cycles. PCR products were electrophoresed on a 2% horizontal agarose gel stained with ethidium bromide. Each experiment was designed to run in the same gel PCR products from patients, one normal control and one mutation-positive control, when available. Each mutant allele identified was confirmed by direct sequence analysis.
PCR Amplification of Genomic DNA Sequence and DNA Sequencing
For DNA sequence analysis, the genomic sequence of exons 4 and 11 (the only exons where mutations were found) were amplified as described above. For exon 4, we used the primer sequence reported elsewhere, 4 whereas for exon 11 the primer sequences used were: forward 5′-TGTAGGGAATAGAAATAAATGG-3′; reverse 5′-CCAGGAGTCCTGTGGGTCA-3′. PCR products were purified using the Microcon Amicon PCR kit (Millipore, Bedford, MA), quantified on agarose gel, and directly sequenced in both directions using the Big Dye dideoxy-terminator cycle sequencing kit and the ABI-PRISM model 377 automated sequencer (Applied Biosystems), at the CRIBI Biotechnology Center (http://bmr.cribi.unipd.it), University of Padova. Sequence analysis was obtained using Chromas 1.45 software (http://www.technelysium.com.au/chromas.html).
Results
Patients
Our ARMS screening detected mutations in the CAPN-3 gene in 8 patients with normal calpain-3 protein expression (Table 1) ▶ . All patients presented an LGMD phenotype, and they had a variable age of onset (from 10 to 37 years) and different degree of clinical severity at biopsy. Their clinical follow-up showed that the disease progression was widely variable, even between the 4 patients homozygous for the same missense R490Q mutation (Table 1) ▶ .
Table 1.
Clinical and Molecular Data
| Patient, sex | Family history | Age at onset (years) | Age at biopsy (years) | CK at biopsy (U/L) | Muscle pathology severity | Clinical grade at biopsy | Clinical grade at last exam | Nucleotide change | Amino acid change | Exon | Zygosity | Mutation type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1, M | − | 17 | 29 | 1603 | Active | Severe | Severe (46) | G1469A | R490Q | 11 | Homoz. | Missense |
| 2, F | + | 28 | 30 | 783 | Active | Moderate | Severe (36) | G1469A | R490Q | 11 | Homoz. | Missense |
| 3, M* | + | 37 | 43 | 4348 | Mild | Mild | Mild (49) | G1469A | R490Q | 11 | Homoz. | Missense |
| 4, M* | + | 10 | 10 | 5960 | Mild | Asymptom | Moderate (26) | G1469A | R490Q | 11 | Homoz. | Missense |
| 5, M | − | 11 | 16 | 3200 | Mild | Asymptom. | − | G1469A | R490Q | 11 | Heteroz. | Missense |
| G1466A | R489Q | 11 | Heteroz. | Missense | ||||||||
| 6, M | − | 20 | 20 | 5200 | Moderate | Mild | Mild (26) | 550 del. A | − | 4 | Heteroz. | Frame-shift |
| G1469A | R490Q | 11 | Heteroz. | Missense | ||||||||
| 7, M | − | 11 | 18 | 2500 | Moderate | Moderate | Moderate/severe (25) | 550 del. A | − | 4 | Heteroz. | Frame-shift |
| C1468T | R490W | 11 | Heteroz. | Missense | ||||||||
| 8, M | − | 16 | 21 | 8600 | Moderate | Mild | Moderate (30) | C1468T# | R490W | 11 | Heteroz. | Missense |
*, second-degree cousins;
Age of patient at last clinical follow-up is indicated in parentheses,
#, only one mutant allele was found.
asymptom., asymptomatic, homoz., homozygous; heteroz., heterozygous
Morphological Features
We observed by histopathological analysis that patients 1 and 2 presented an active dystrophic process (Figure 1, a and b ▶ , respectively) with clusters of regenerating and degenerating fibers, while patients 6 to 8 had a moderate dystrophic process (Figure 1c ▶ , patient 6), and patients 3 to 5 revealed a mild myopathic picture (Figure 1d ▶ , patient 3). There was a correlation between the severity of muscle histopathology and the clinical phenotype: patients with an active dystrophic process had severe form of muscular dystrophy, whereas patients with mild pathological changes showed a slowly progressive myopathy (Table 1) ▶ . The normal expression of several muscle-specific proteins indicates that the primary calpain-3 defect does not affect the integrity of either the plasma or the nuclear membrane. Ultrastructural muscle examination showed aspecific changes, consisting of focal myofibrillar disorganization leading to the disruption of the sarcomeric structure, fragmented nuclei with focally detached chromatin, and increased subsarcolemmal glycogen deposits.
CAPN-3 Gene Mutation Identification
Screening gene mutations by allele-specific primers allowed us to detect four different CAPN-3 gene mutations in eight of 150 patients (5.5%) (Table 1 ▶ ; Figure 2 ▶ ): an A deletion at position 550 (550 del. A) in 2 heterozygous patients; a C to T substitution at position 1468, causing an arginine to tryptophan amino acid change at position 490 (R490W) in two heterozygous patients; a G to A substitution at position 1466 causing an arginine to glutamine amino acid change at position 489 (R489Q) in one heterozygous patient; a G to A substitution at position 1469 causing an arginine to glutamine amino acid change at position 490 (R490Q) in four homozygous and two heterozygous patients (10 of 16 mutant alleles). All of the mutations found by ARMS were confirmed by sequence analysis. In one patient (8) this method allowed to detect only one mutant allele (Table 1) ▶ . Two affected relatives (3 and 4) and a third unrelated patient (2) shared the same family name and a homozygous R490Q mutation (Table 1) ▶ ; though they were not aware of any parental consanguinity, a founder effect was suspected, because these patients came from the same town in the Venetian lagoon, in a region that in the past was genetically isolated.
Figure 2.
CAPN-3 gene mutation analysis by ARMS-PCR (A) and tetra-primer ARMS-PCR (B), and corresponding DNA sequence analysis. A: Left-hand figure shows the agarose gel electrophoresis of the 185-bp PCR products for the 550 del. A mutation. Normal control (C), homozygous control (ho), heterozygous control (he), and patients 6 and 7 are tested for both the wild-type (w) and the mutant (m) alleles. Patients 6 and 7 are heterozygote. The right-hand figure shows direct DNA sequence analysis in patient 6: the arrow points to the mutation site (550 del. A) with the consequent frame-shifting. B: The left-hand figure shows the agarose gel electrophoresis for the R490Q mutation: normal control (C) and patients 1 to 6 are tested for the wild-type (192 bp) and mutant alleles (168 bp) and the outer primer amplicon (304 bp). Patients 5 and 6 are heterozygous, and patients 1 to 4 are homozygous. The middle figure shows the results for the R489Q mutation: the amplification pattern of the wild-type allele (213 bp), the mutant allele (188 bp), and the outer primer amplicon (347 bp) indicates that patient 5 is heterozygous. The right-hand figure shows the results for the R490W mutation: the amplification pattern of the wild-type allele (176 bp), the mutant allele (194 bp) and the outer primer amplicon (315 bp) indicates that patient 7 is heterozygous. Direct DNA sequence analysis in patients 5 (compound heterozygote for both R489Q and R490Q) and 7 (heterozygote for R490W) is shown in the bottom figure; arrows indicate the mutation sites and the nucleotide changes.
Functional Characterization of Calpain-3 Protein
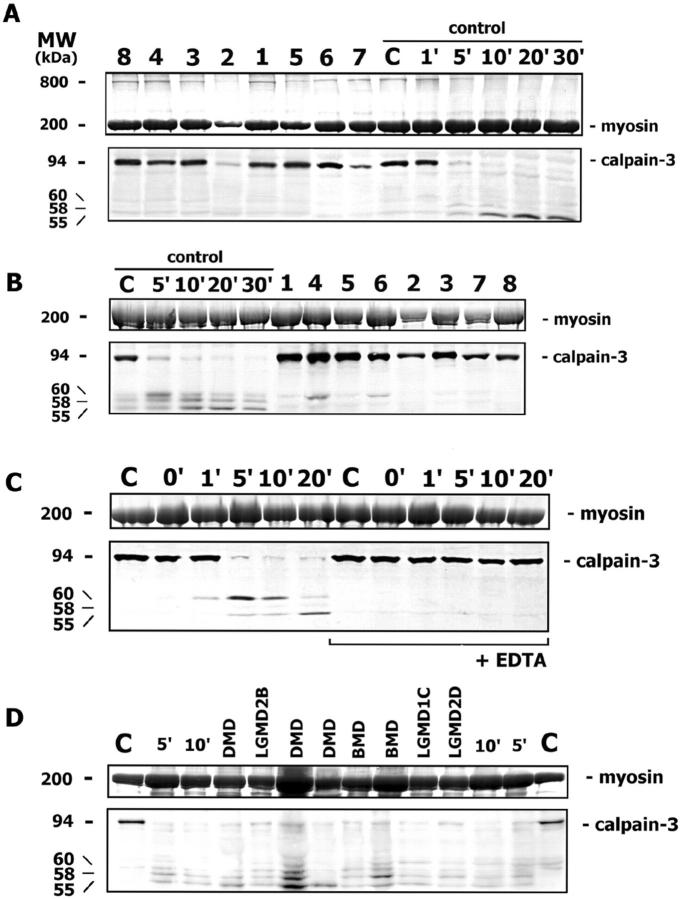
Immunoblot analysis for calpain-3 showed a normal protein expression in all mutant patients (Figure 3A) ▶ . The study of autocatalytic calpain-3 activity has shown that in control muscle the full-length protein (94 kd) is degraded after 5 minutes, and the three typical degradation bands 8,14,24,29 of lower molecular weight (60, 58, 55 kd) appear progressively (Figure 3, A to C) ▶ . On the other hand, under the same experimental condition, the full-length protein was still present at normal level in muscle of all patients, indicating that the normal autocatalytic activity of calpain-3 was lost (Figure 3B) ▶ . To check if autocatalysis could occur in patients’ muscle with a longer time, we incubated patient samples also for 20 minutes, and observed calpain-3 degradation as in control muscle (data not shown).
Figure 3.
A: Calpain-3 Western blotting in muscle biopsy sections incubated in loading buffer shows that all mutant patients (1 to 8) have calpain-3 of an amount comparable with the normal control (C), as determined by myosin in the posttransfer Coomassie blue-stained gel. The calpain-3 autocatalytic activity is tested in control muscle after different incubation times (from 1′ to 30′) in saline solution; the full-length calpain-3 band (94 kd) almost disappears after 5 minutes, and additional degradation bands at lower molecular weight (60, 58, and 55 kd) appear progressively. In the posttransfer Coomassie blue-stained gel, a high molecular-weight protein of about 800 kd is present in patient and control samples, where calpain-3 autocatalysis does not occur, and it disappears progressively during autolysis. B: The assay of calpain-3 autocatalytic activity is conducted in control muscle after various incubation times (from 5′ to 30′) and in patient samples (1 to 8) after 5 minutes. While in the control muscle the full-length calpain-3 almost disappears after 5 minutes and degradation bands appear progressively, in all patient samples the full-length calpain-3 is still present at an amount comparable with the non-autocatalyzed normal control. C: While an almost complete calpain-3 autocatalysis occurs in control muscle after 5 minutes of incubation, and degradation bands appear progressively, the addition of 10 mmol/L EDTA to the saline solution blocks the autocatalytic calpain-3 activity at all times. D: The calpain-3 autocatalytic activity is tested in muscle disease controls (DMD, BMD, LGMD2B, LGMD1C, LGMD2D) after 5 minutes of incubation: the full-length calpain-3 almost disappears in all patients and in control muscle after different incubation times (5′ and 10′), and degradation bands are present.
Calpain-3 autocatalytic activity was tested also in eight different disease control muscle biopsies (3 DMD, 2 BMD, 1 LGMD2B, 1 LGMD1C, 1 LGMD2D): all patients showed the marked reduction of 94 kd calpain-3 protein band and the appearance of degradation bands (Figure 3D) ▶ .
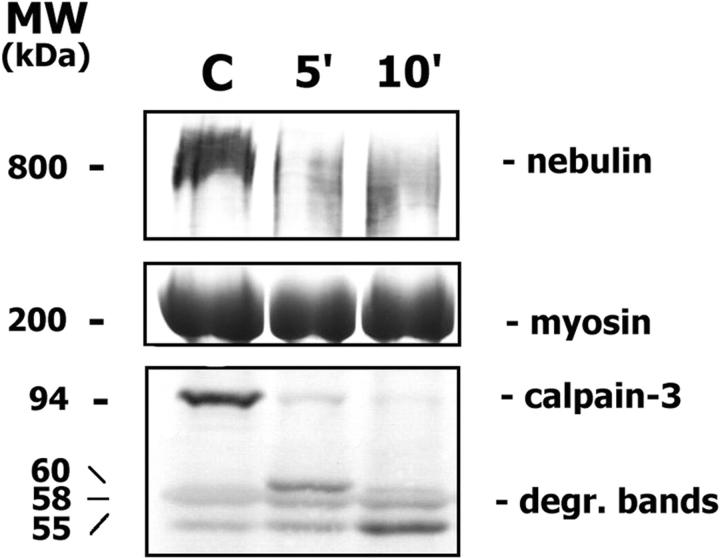
Calpain-3 autocatalysis did not occur in control muscle when 10 mmol/L EDTA was added (Figure 3C) ▶ , indicating that Ca++ ions (contained in the saline solution) are required for its normal function. A high molecular-weight band (of about 800 kd) was present in the posttransfer Coomassie blue-stained gels of control and patient samples where calpain-3 autocatalysis was avoided, and it progressively disappeared during autolysis (Figure 3A) ▶ . This band likely corresponds to a muscle-specific protein, possibly nebulin. Through immunoblot analysis, nebulin was normally expressed in both control and patient samples when calpain-3 autocatalysis was avoided; in the control muscle where calpain-3 autocatalysis occurred nebulin amount was highly reduced (Figure 4) ▶ . Other muscle proteins, such as desmin, α-actinin, β-dystroglycan, and dystrophin were normally expressed during time-dependent calpain-3 autocatalytic activity (data not shown).
Figure 4.
Nebulin is normally expressed in control muscle (C) where calpain-3 autocatalysis does not occur. Nebulin amount is highly reduced during calpain-3 autocatalysis (after 5′ and 10′ incubation time) and calpain-3 degradation bands progressively appear.
Discussion
Since calpain-3 antibodies have been available for protein testing the diagnosis of calpainopathy has shifted from molecular genetics toward biochemistry. Mutation detection on multiple genes is not used as a first-line diagnostic approach for unclassified LGMD patients, because of the high cost and effort required. The validity of protein testing has been fully recognized, 6,8,21,22 as most patients with protein defect do indeed have CAPN-3 gene mutations. However, CAPN-3 mutant patients who show normal protein expression have occasionally been reported, 8,23 and they represent a potential pitfall of using only protein testing. In this study, we systematically screened a large series of undiagnosed LGMD patients with normal calpain-3 protein expression using an allele-specific mutation test, and we have demonstrated that this approach is effective in the detection of CAPN-3 gene mutations.
One important conclusion of this study is that conventional calpain-3 protein screening fails to detect mutant proteins in a considerable number of cases. We calculated that mutant cases expressing normal protein are 5.5% of unclassified LGMD cases in our study, but this number would probably be higher if a complete gene sequence analysis had been done. The importance of the approach by allele-specific screening for diagnostic testing is documented by the detection of an additional 20% of primary LGMD2A patients in our population (8 of 42), as 80% of cases have been identified by calpain-3 deficiency on immunoblot. Thus, LGMD2A is more frequent than would appear when calculated only from protein testing, and in our population it accounts for about 19% of all LGMD patients diagnosed so far. As linkage analysis and genetic epidemiology from 85 LGMD brazilian families estimated that 30% of all LGMD cases are LGMD type 2A, 30 it seems likely that the same proportion would result also in european countries if a complete CAPN-3 gene analysis is done in all unclassified LGMD patients.
Following the molecular characterization of LGMD2A patients by allele-specific test, we analyzed the mutant calpain-3 activity. Another new result of our study is the demonstration that the mutant protein was fully expressed in muscle tissue from a series of molecularly proven LGMD2A patients, but it had lost its normal autocatalytic function.
Unlike muscular dystrophies where structural proteins are involved, calpainopathy is the result of an enzyme defect, where, as in enzymatic disorders, the mutant protein may be inactive even if it is present. Diagnosing LGMD2A, clinicians should be careful if they draw conclusions based on calpain-3 protein testing alone, unless a functional protein test (such as autocatalysis) has been used as well. The availability of a simple method to test calpain-3 autocatalytic capacity in patients’ muscle offers a novel diagnostic improvement. This test should be associated with conventional calpain-3 protein studies to select LGMD patients where Western blot analysis does not reveal an etiology.
Earlier studies of site-directed mutagenesis suggested that there might be a pathogenetic effect in some CAPN-3 gene mutations in COS-7 cells, mouse muscle cells 13,14,24 or transgenic mice, 19,25 but such studies have not yet been done on human biopsies. In our study the functional test was useful to demonstrate the pathogenetic role of R490W, R490Q, and R489Q missense mutations in LGMD2A, which usually have unpredictable consequences at protein level. By comparison with ubiquitous calpains, these missense mutations could either be affecting intramolecular domain interaction, or be causing an alteration in the charge of side chains involved in internal salt links. 12
The Ca++-dependence of calpain-3 has been controversial, with some authors arguing that calpain-3 is Ca++-independent in vitro, while others have argued that it requires very low Ca++ level concentration. In this study we offer indirect evidence that calpain-3 has Ca++ dependence: when Ca++ ions were subtracted through the addition of EDTA to the incubation medium, normal autocatalytic activity is blocked.
Recent investigations in COS cells 14 further suggested that some mutations (including the R490W) possibly affect interdomain protein interaction, resulting in a reduction of the autocatalytic activity by lowering Ca++ sensitivity. The salt bridge region in domain III would exert a conformational constraint on the movement of domain IIb 31 ; if mutations in domain III produce an altered Ca++ requirement, the R490W, R490Q, and R489Q mutations described in this study (located in domain III) could have a similar effect on protein activity as well.
The characterization of CAPN-3 gene mutations and their corresponding effect on protein function makes it possible to draw up a kind of functional genetic map. To date, only few missense mutations have been reported to produce a normal expression of calpain-3 protein in LGMD2A patients: T184M and G222R (both in domain IIa) 8 might disrupt domain IIa-domain IIb interaction 12 ; G496R and S606L found in one compound heterozygote patient 23 (both in domain III) possibly act in a similar way, causing disruption of salt links and impairing domain IIa-domain III interaction. 12 Our study adds three additional missense mutations (R490W, R490Q, R489Q) which are associated with normal calpain-3 expression, and suggests that domain III is of crucial importance for protein function.
To better understand the pathogenetic role of calpain-3, we also studied muscle-specific proteins in a proteolytic assay. Desmin, α-actinin, β-dystroglycan, and dystrophin did not show any time-dependent loss. On the contrary, nebulin degradation was found only in control muscle where there was normal calpain-3 autocatalytic activity and was not found in any of our eight mutant patients. This could agree with previous studies suggesting an involvement of calpains in cytoskeletal or myofibrillar protein degradation 5,17,18 ; however, we could not experimentally verify if nebulin degradation is directly related to calpain-3 activity or if it is simply a secondary phenomenon.
In conclusion, our study suggests that LGMD2A patients with normal calpain-3 protein expression are relatively frequent (20%) and that a functional test of calpain-3 protein activity is useful to prove the pathogenetic effect of some missense mutations in the domain III of calpain-3.
Footnotes
Address reprint requests to M. Fanin, Department of Neurosciences, University of Padova, via Giustiniani 5, 35128 Padova, Italy. E-mail: marina.fanin@unipd.it.
Supported by grants from Telethon-Italy (GTF02009 and 9UP030516) and the Italian Ministry of University and Scientific Research MURST (2001068328).
References
- 1.Beckmann JS, Richard I, Hillaire D, Broux O, Antignac C, Bois E, Cann H: A gene for limb girdle muscular dystrophy maps to chromosome 15 by linkage. CR Acad Sci III 1991, 312:141-148 [PubMed] [Google Scholar]
- 2.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C, Hillaire D, Passos-Bueno MR, Zatz M, Tischfield JA, Fardeau M, Jackson CE, Cohen D, Beckmann JS: Mutations in the proteolytic calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995, 81:27-40 [DOI] [PubMed] [Google Scholar]
- 3.Richard I, Brenguier L, Dincer P, Roudaut C, Bady B, Burgunder JM, Chemaly R, Garcia CA, Halaby G, Jackson CE, Kurnit DM, Lefranc G, Legum C, Loiselet J, Merlini L, Nivelon-Chevallier A, Ollagnon-Roman E, Restagno G, Topaloglu H, Beckmann JS: Multiple independent molecular etiology for limb-girdle muscular dystrophy type 2A patients from various geographical origins. Am J Hum Genet 1997, 60:1128-1138 [PMC free article] [PubMed] [Google Scholar]
- 4.Richard I, Roudaut C, Saenz A, Pogue R, Grimbergen JEMA, Anderson LVB, Beley C, Cobo AM, de Diego C, Eymard B, Gallano P, Ginjaar HB, Lasa A, Pollitt C, Topaloglu H, Urtizberea JA, de Visser M, van der Kooi A, Bushby K, Bakker E, Lopez de Munain A, Fardeau M, Beckmann JS: Calpainopathy: a survey of mutations and polymorphisms. Am J Hum Genet 1999, 64:1524-1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae J, Minami N, Jin Y, Nakagawa M, Murayama K, Igarashi F, Nonaka II: Calpain-3 gene mutations: genetic and clinico-pathologic findings in limb girdle muscular dystrophy. Neuromuscul Disord 2001, 11:547-555 [DOI] [PubMed] [Google Scholar]
- 6.Pollitt C, Anderson LVB, Pogue R, Davison K, Pyle A, Bushby KMD: The phenotype of calpainopathy: diagnosis based on a multidisciplinary approach. Neuromuscul Disord 2001, 11:287-296 [DOI] [PubMed] [Google Scholar]
- 7.Chou FL, Angelini C, Daentl D, Garcia C, Greco C, Hausmanowa-Petrusewicz I, Fidzianska A, Wessel H, Hoffman EP: Calpain-III mutation analysis of a heterogeneous limb-girdle muscular dystrophy population. Neurology 1999, 52:1015-1020 [DOI] [PubMed] [Google Scholar]
- 8.Anderson LVB, Davison K, Moss JA, Richard I, Fardeau M, Tomè FMS, Hubner C, Lasa A, Colomer J, Beckmann JS: Characterisation of monoclonal antibodies to calpain-3 and protein expression in muscle from patients with limb girdle muscular dystrophy type 2A. Am J Pathol 1998, 153:1169-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fardeau M, Hillaire D, Mignard C, Feingold N, Feingold J, Mignard D, de Ubeda B, Collin H, Tomè FMS, Richard I, Beckmann JS: Juvenile limb-girdle muscular dystrophy: clinical, histopathological and genetic data from a small community living in the Reunion Island. Brain 1996, 199:295-308 [DOI] [PubMed] [Google Scholar]
- 10.Minami N, Nishino I, Kobayashi O, Ikezoe K, Goto Y, Nonaka I: Mutations of calpain-3 gene in patients with sporadic limb-girdle muscular dystrophy in Japan. J Neurol Sci 1999, 171:31-37 [DOI] [PubMed] [Google Scholar]
- 11.Sorimachi H, Imajoh-Ohmi S, Emori Y, Kawasaki H, Ohno S, Minami Y, Suzuki K: Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and μ-types: specific expression of the mRNA in skeletal muscle. J Biol Chem 1989, 264:20106-2011 [PubMed] [Google Scholar]
- 12.Jia Z, Petrounevitch V, Wong A, Moldoveanu T, Davies PL, Elce JS, Beckmann JS: Mutations in calpain 3 associated with limb girdle muscular dystrophy analysis by molecular modeling and by mutation in m-calpain. Biophys J 2001, 80:2590-2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorimachi H, Toyama-Sorimachi N, Saido TC, Kawasaki H, Sugita H, Miyasaka M, Arahata K, Ishiura S, Suzuki K: Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J Biol Chem 1993, 268:10593-10605 [PubMed] [Google Scholar]
- 14.Ono Y, Shimada H, Sorimachi H, Richard I, Saido TC, Beckmann JS, Ishiura S, Suzuki K: Functional defects of a muscle-specific calpain, p94, caused by mutations associated with limb-girdle muscular dystrophy type 2A. J Biol Chem 1998, 273:17073-17078 [DOI] [PubMed] [Google Scholar]
- 15.Baghdiguian S, Martin M, Richard I, Pons F, Astier C, Bourg N, Hay RT, Chemaly R, Halaby G, Loiselet J, Anderson LVB, Lopez de Munain A, Fardeau M, Mangeat P, Beckmann JS, Lefranc G: Calpain-3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkBα/NF-kB pathway in limb girdle muscular dystrophy type 2A. Nat Med 1999, 5:503-511 [DOI] [PubMed] [Google Scholar]
- 16.Richard I, Roudaut C, Marchand S, Baghdiguian S, Herasse M, Stockholm D, Ono Y, Suel L, Bourg N, Sorimachi H, Lefranc G, Fardeau M, Sebille A, Beckmann JS: Loss of calpain 3 proteolytic activity leads to muscular dystrophy and to apoptosis-associated IκB-α/nuclear factor κB pathway perturbation in mice. J Cell Biol 2000, 151:1583-1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Fosberg NE: Role of calpain in skeletal-muscle protein degradation. Proc Natl Acad Sci USA 1998, 95:12100-12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poussard S, Duvert M, Balcerzak D, Ramassamy S, Brustis JJ, Cottin P, Ducastaing A: Evidence for implication of muscle-specific calpain (p94) in myofibrillar integrity. Cell Growth Diff 1996, 7:1461-1469 [PubMed] [Google Scholar]
- 19.Spencer MJ, Guyon JR, Sorimachi H, Potts A, Richard I, Herasse M, Chamberlain J, Dalkilic I, Kunkel LM, Beckmann JS: Stable expression of calpain 3 from a muscle transgene in vivo: immature muscle in transgenic mice suggests a role for calpain 3 in muscle maturation. Proc Natl Acad Sci USA 2002, 99:8874-8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penisson-Besnier I, Richard I, Beckmann JS, Fardeau M: Phenotypic variations of calpain deficiency in two siblings. Muscle Nerve 1998, 21:1078-1080 [DOI] [PubMed] [Google Scholar]
- 21.Fanin M, Pegoraro E, Matsuda-Asada C, Brown RH, Angelini C: Calpain-3 and dysferlin protein screening in patients with limb-girdle dystrophy and myopathy. Neurology 2001, 56:660-665 [DOI] [PubMed] [Google Scholar]
- 22.Pogue R, Anderson LVB, Pyle A, Sewry C, Pollitt C, Johnson MA, Davison K, Moss JA, Marcuri E, Muntoni F, Bushby KMD: Strategy for mutation analysis in the autosomal recessive limb-girdle muscular dystrophies. Neuromuscul Disord 2001, 11:80-87 [DOI] [PubMed] [Google Scholar]
- 23.Talim B, Ognibene A, Mattioli E, Richard I, Anderson LVB, Merlini L: Normal calpain expression in genetically confirmed limb-girdle muscular dystrophy type 2A. Neurology 2001, 56:692-693 [DOI] [PubMed] [Google Scholar]
- 24.Herasse M, Ono Y, Fougerousse F, Kimura E, Stockholm D, Beley C, Montarras D, Pinset C, Sirimachi H, Suzuki K, Beckmann JS, Richard I: Expression and functional characteristics of calpain 3 isoforms generated through tissue-specific transcriptional and post-transcriptional events. Mol Cell Biol 1999, 19:4047-4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagawa K, Taya C, Hayashi Y, Nakagawa M, Ono Y, Fukuda R, Karasuyama H, Toyama-Sorimachi N, Katsui Y, Hata S, Ishiura S, Nonaka I, Seyama Y, Arahata K, Yonekawa H, Sorimachi H, Suzuki K: Myopathy phenotype of transgenic mice expressing active site-mutated inactive p94 skeletal muscle-specific calpain, the gene product responsible for limb-girdle muscular dystrophy type 2A. Hum Mol Genet 2000, 9:1393-1402 [DOI] [PubMed] [Google Scholar]
- 26.Ono Y, Sorimachi H, Suzuki K: New aspect of the research on limb-girdle muscular dystrophy 2A: a molecular biologic and biochemical approach to pathology. Trends Cardiovasc Med 1999, 9:114-118 [DOI] [PubMed] [Google Scholar]
- 27.Ye S, Dhillon S, Ke X, Collins AR, Day NM: An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 2001, 29:88-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little S: ARMS analysis of point mutations. Taylor GR eds. Laboratory Methods for the Detection of Mutations and Polymorphisms in DNA. 1997:pp 45-51 CRC Press, Boca Raton, FL
- 29.Kinbara K, Ishiura S, Tomioka S, Sorimachi H, Jeong S, Amano S, Kawasaki H, Kolmerer B, Kimura S, Labeit S, Suzuki K: Purification of native p94, a muscle-specific calpain, and characterization of its autolysis. Biochem J 1998, 335:589-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zatz M, Vainzof M, Passos-Bueno MR: Limb-girdle muscular dystrophy: one gene with different phenotypes, one phenotype with different genes. Curr Opin Neurol 2000, 13:511-517 [DOI] [PubMed] [Google Scholar]
- 31.Hosfield CM, Moldoveanu T, Davies PL, Elce JS, Jia Z: Calpain mutants with increased Ca++ sensitivity and implications for the role of the C2-like domain. J Biol Chem 2001, 276:7404-7407 [DOI] [PubMed] [Google Scholar]