Abstract
Placebo-induced expectancies have been shown to decrease pain in a manner reversible by opioid antagonists, but little is known about the central brain mechanisms of opioid release during placebo treatment. This study examined placebo effects in pain by using positron-emission tomography with [11C]carfentanil, which measures regional μ-opioid receptor availability in vivo. Noxious thermal stimulation was applied at the same temperature for placebo and control conditions. Placebo treatment affected endogenous opioid activity in a number of predicted μ-opioid receptor-rich regions that play central roles in pain and affect, including periaqueductal gray and nearby dorsal raphe and nucleus cuneiformis, amygdala, orbitofrontal cortex, insula, rostral anterior cingulate, and lateral prefrontal cortex. These regions appeared to be subdivided into two sets, one showing placebo-induced opioid activation specific to noxious heat and the other showing placebo-induced opioid reduction during warm stimulation in anticipation of pain. These findings suggest that a mechanism of placebo analgesia is the potentiation of endogenous opioid responses to noxious stimuli. Opioid activity in many of these regions was correlated with placebo effects in reported pain. Connectivity analyses on individual differences in endogenous opioid system activity revealed that placebo treatment increased functional connectivity between the periaqueductal gray and rostral anterior cingulate, as hypothesized a priori, and also increased connectivity among a number of limbic and prefrontal regions, suggesting increased functional integration of opioid responses. Overall, the results suggest that endogenous opioid release in core affective brain regions is an integral part of the mechanism whereby expectancies regulate affective and nociceptive circuits.
Keywords: neuroimaging, periaqueductal gray, expectancy, affective neuroscience
Placebo effects are treatment effects caused not by the physical properties of a treatment but by the meaning ascribed to it. They are particularly strong in experimental and clinical studies of pain. However, much remains to be learned about the neural and cognitive mechanisms by which placebo treatments have their effects. Placebo analgesic treatments elicit expectations of pain relief, which are thought to change the affective and motivational context in which nociceptive signals are interpreted (1–6). Although ample evidence exists that placebo expectancies reduce reported pain, the neurobiology of how expectancies interact with nociceptive brain processes is relatively unexplored.
Early pharmacological studies showed that placebo analgesia is reduced by the opioid antagonist naloxone (7), suggesting that endogenous opioids play a crucial role. Follow-up work pointed to both opioid and nonopioid mechanisms of placebo (8). An emerging idea is that expectancy-based placebo effects are opioid-mediated, but conditioned placebo effects may depend on other mechanisms (9–13). Although these studies support a strong role for opioids, little is known about the brain mechanisms that link expectancy with opioid release and pain relief in humans. Opioid-mediated placebo effects are thought to involve the periaqueductal gray (PAG), which contains many of the brain's opioid-containing neurons and has been linked in a large animal literature with pain relief (14–21). Opioid activity in PAG mediates the analgesic effects of frontal stimulation in rats, and PAG is a target for human therapeutic treatments for chronic pain (22–25).
Previously, we found that placebo induced increases in functional MRI (fMRI) activity in lateral prefrontal cortex (PFC) and orbitofrontal cortex (OFC). The magnitude of these activations was correlated with increases in the midbrain around the PAG, supporting the hypothesis that PAG and opioids play a role in expectancy-based placebo (26). However, the only previous direct study of regional placebo-induced opioid activity found effects in regions related to motivation and reward value, including the nucleus accumbens (NAC) and anterior insula (aINS), but not the PAG (27). Thus, involvement of PAG opioid activity in placebo analgesia has not yet been directly demonstrated, and the relationships between endogenous opioid activity in PAG and other brain regions, such as PFC and rostral anterior cingulate (rACC), are unknown.
In this study, we aimed to address these gaps in knowledge by testing placebo effects on μ-opioid activity in PAG and other opioid-rich regions of interest (ROIs). We delivered identical thermal stimuli to patches of skin treated with identical, pharmacologically inert placebo and control creams. The conditions differed only in the instructions: Participants were told that the placebo treatment was a “highly effective pain reliever,” and that the control treatment would “have no effect on pain.” We examined placebo-induced changes in μ-opioid receptor binding potential (BP) as measured with 11C-radiolabeled carfentanil (a μ-opioid receptor-selective agonist) and positron-emission tomography (PET). With this method, endogenous opioids interfere with the binding of [11C]carfentanil to the receptors, resulting in reductions in BP (28). Thus, the reduction in BP for placebo vs. control treatment is a measure of placebo-induced opioid system activation (27).
With this design, we can address another important unresolved issue. In Zubieta et al. (27), placebo and control conditions were designed to produce equal subjective pain by using an adaptive procedure. Because noxious input differed for placebo and control conditions, it is possible that (placebo − control) opioid effects could be caused by differences in input or even minimized because of the psychological equivalence of the conditions. Here, we examine whether there are placebo effects on opioid activity when noxious input is equivalent.
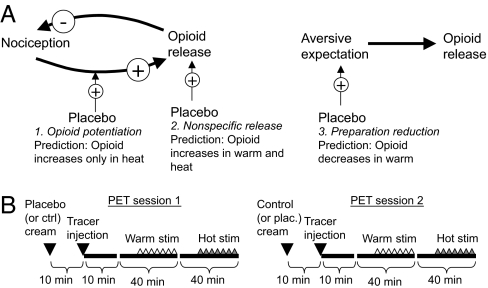
Placebo expectancy may affect opioid activity in several ways, three of which are shown in Fig. 1A. Placebo may enhance endogenous opioid release caused by noxious stimulation (mechanism no. 1), in which case placebo-induced μ-opioid activity should be higher during hot than warm stimulation; thus, a temperature (hot vs. warm) × placebo (placebo vs. control) interaction is predicted. This effect is consistent with studies showing larger placebo effects with more intense pain (29). Alternatively, application of the placebo cream may itself trigger endogenous opioid activity (mechanism no. 2), which should result in evidence for placebo-induced opioid increases with both hot and warm stimulation. Finally, a function of opioid analgesia may be to prepare an organism to deal with an imminent threat, in which case pain anticipation may result in opioid release (mechanism no. 3; Fig. 1A Right). In this case, placebo may reduce threat (30, 31) and thus reduce μ-opioid release during the anticipatory warm stimulation period. Different brain regions may independently show evidence for one or more of these predicted patterns. Our hypothesis was that placebo treatment would potentiate pain-related opioid release (no. 2) in opioid-rich regions such as PAG, OFC, and aINS.
Fig. 1.
Study hypotheses and procedures. (A) Mechanisms by which placebo may affect opioid binding. See text for details. +, positive effect; −, inhibitory effect. (B) Study procedures.
In addition to regional opioid activity, we sought to test for placebo effects on the functional integration of opioid responses in frontal, limbic, and brainstem regions, which relate to different components of the endogenous analgesic response (28). We expected frontal regions, which may maintain expectancies for pain relief, to be more correlated with limbic regions and PAG under placebo conditions. Connectivity between frontal cortex and brainstem appears to play an important role in the modulation of pain by attention (32, 33) and placebo (26), as predicted from animal studies (14, 34). Recent evidence suggests that correlations between rACC and PAG activity are stronger with placebo (35) and hypnotic analgesia (36). In the present study, multivariate analyses, which combined nonmetric multidimensional scaling (NMDS) and permutation tests with hierarchical clustering, allowed us to identify several functional opioid subsystems and to test for the modulation of connectivity within and between subsystems by placebo.
Results
Pain-Specific Placebo Effects.
The four conditions in the study were as follows: placebo with painful heat (PH), control with painful heat (CH), placebo with nonpainful warmth (PW), and control with nonpainful warmth (CW). Placebo treatment led to significant reported analgesia during noxious heat [(PH − CH) in pain reports, 5.58 for CH vs. 5.07 for PH, t (13) = 1.87, P = 0.042, one-tailed test], shown in supporting information (SI) Fig. 6C. In the brain, we first tested for opioid activation differences in the temperature × placebo interaction contrast, (CH − PH) − (CW − PW), in which positive values indicate larger placebo-induced opioid activation in heat relative to warm stimulation (mechanism no. 1 in Fig. 1A).
Of the 11 key ROIs, 8 showed significant positive values, indicating placebo-induced μ-opioid system activation [(P < 0.05, small volume corrected (SVC)], and none showed decreases. Across all 27 ROIs, 14 regions showed significant placebo-induced increases and none showed decreases. At the set level, activation of three key ROIs or five ROIs in the complete set were required for the number of significant ROIs to reach the P < 0.05 corrected level. Thus, findings of 8 of 11 key ROIs and 14 of 27 in the full set of ROIs were highly significant (P < 0.001 for both tests), demonstrating that many more regions showed placebo increases in opioid activity than would be expected under chance.
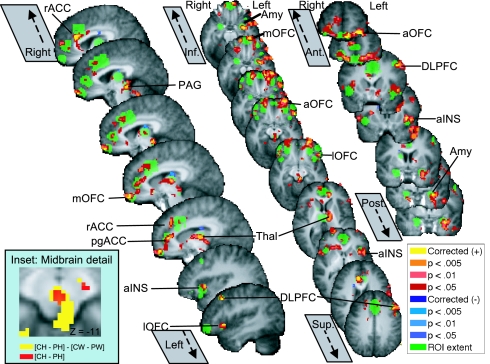
ROIs are shown in green in Fig. 2 and SI Fig. 6B, and voxels that reached corrected significance within ROIs (P < 0.05, SVC) are shown in yellow. Significant regions include PAG, rACC, pregenual anterior cingulate (pgACC), multiple loci within OFC, aINS, thalamus, dorsolateral PFC (DLPFC), and amygdala. The activation extent is shown in Fig. 2 and subsequent figures at P < 0.005, 0.01, and 0.05 (two-tailed) in hot colors. Activation volumes and center-of-mass coordinates in Montreal Neurological Institute (MNI) space are presented in SI Table 1. Placebo effect magnitudes ranged from 5.0% of baseline BP (left DLPFC) to 16.4% (left amygdala), large enough to be of practical significance and comparable to previous work (27).
Fig. 2.
ROIs showing significant pain-specific opioid activation, [(CH − PH) − (CW − PW)]. ROI extent is shown in green, and significant voxels in or contiguous with ROIs are shown in red/yellow (positive effects) or lavender/blue (negative effects). Amy, amygdala; aOFC, anterior OFC; lOFC, lateral OFC/inferior frontal border; mOFC, medial OFC; thal, thalamus.
Pain-Specific Placebo Effects Predict Reported Placebo Analgesia.
We expected correlations between temperature × placebo interactions in significant ROIs and reported placebo analgesia, demonstrating BP differences between relative placebo “responders” and “nonresponders.” We first tested for correlations in the exact voxels that showed the significant group effects above. Opioid activity effect magnitudes were significantly negatively correlated with reported placebo analgesia in four of these regions: PAG, right lateral OFC (lOFC), pgACC, and rACC (partial r's = −0.47 to −0.77; SI Table 1), indicating, contrary to our initial hypotheses, greater placebo opioid activation in low responders. We return to a discussion of these effects below. Two other regions, left anterior OFC and lOFC, showed negative trends (r's = −0.57 to −0.60). (For more information on robust partial correlations, see SI Methods.) Only one region showed a positive correlation, left DLPFC (r = 0.79). Correlation scatter plots are shown in SI Fig. 7.
Tests within key ROIs revealed three additional negatively correlated regions: PAG, right lOFC, and right NAC (P < 0.05, SVC, for each; set-level P = 0.04). Two regions in the extended ROI set showed positive correlations, both within left DLPFC (set-level P = 0.12). Overall, opioid temperature × placebo interactions were both significant in the group and predicted the magnitude of reported analgesia, supporting mechanism no. 1 (Fig. 1).
Placebo Effects in Heat Vs. Anticipation.
If placebo potentiates opioid release during pain (mechanism no. 1), then the effects reported above should be caused by placebo-related increases in opioid system activity during heat (CH > PH). Alternatively, the observed temperature × placebo interactions could be caused by placebo-induced opioid decreases during pain anticipation (PW > CW; mechanism no. 3). Therefore, we assessed simple effects of placebo in heat (CH − PH) and warmth (CW − PW) separately.
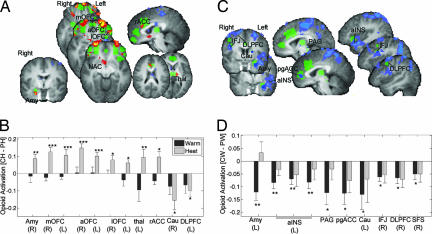
Results revealed separate sets of regions that showed effects in heat and anticipation (P < 0.05, SVC). Significant results for (CH − PH) are shown in Fig. 3A and SI Table 2. During heat, opioid activity increases were found primarily in OFC areas [anterior OFC, medial OFC (mOFC), and lOFC bilaterally] and right amygdala, and at lower thresholds, thalamus (P < 0.005), rACC (P < 0.005), and NAC (P < 0.01, all two-tailed). In these regions, there was virtually no effect of placebo during warm stimulation (Fig. 3B). Of these regions, significant negative correlations between average (CH − PH) and reported placebo analgesia were found in right amygdala (r = −0.61), mOFC (r = −0.78), and lOFC (r = −0.74). Voxel-wise searches within ROIs yielded the same set of regions (P < 0.05, SVC). Scatter plots and coordinates are shown in SI Fig. 8. Subsequent analyses suggested that negative correlations could be caused by greater nonspecific release in control conditions in placebo responders (SI Fig. 9 and SI Text), which was observed in areas including medial PFC, aINS, mOFC, and lOFC.
Fig. 3.
Placebo effects in heat vs. anticipation. (A) Regions showing placebo-induced opioid increases during heat (CH > PH). Color coding is as in Fig. 1. (B) Bar graphs showing placebo-induced opioid activity in warm (black bars) and heat (gray bars) averaged across regions (x axis). R, right; L, left. Other labels are as in Fig. 2. (C) Regions showing placebo-induced anticipatory opioid decreases (PW > CW). (D) Bar graphs for effects in C. Amy, amygdala; aOFC, anterior OFC; Cau, caudate; IFJ, inferior frontal junction; lOFC, lateral OFC/inferior frontal border; mOFC, medial OFC; thal, thalamus
A different set of regions showed evidence of placebo-induced anticipatory decreases (PW > CW; mechanism no. 3) (Fig. 3C). These regions include left amygdala, left ventral aIns, pgACC, dorsal PAG, caudate, and right PFC [DLPFC, superior frontal sulcus (SFS), and inferior frontal junction, (IFJ)]. Notable is the dissociation between rACC, reported in Wager et al. (26), and pgACC, reported in Zubieta et al. (27): rACC responded specifically during pain, whereas pgACC responded specifically during anticipatory warm stimulation. Right vs. left amygdala showed a similar distinction. Among these regions, negative correlations between (CH − PH) and reported analgesia were found in right DLPFC (r = −0.65; P < 0.05, SVC) and positive correlations in left ventral aINS (r = 0.67; SI Fig. 8).
Detailed Analysis of Midbrain.
Results of exploratory analysis (P < 0.05, two-tailed) in midbrain nuclei are shown in SI Fig. 10. Anticipatory placebo decreases in opioid activity (PW > CW) were found in the dorsal PAG and overlying superior colliculus. Placebo increases during heat (CH > PH) were found in midventral regions of the PAG and most strongly in the nucleus cuneiformis (NCF) and dorsal raphe nucleus (DRN) (inferior to the red nucleus), extending into the ventral tegmental area (VTA). The temperature × placebo interaction (Fig. 2 Inset and SI Fig. 10) was significant and positive in both dorsal and midventral PAG, extending into DRN as well. These results are consistent with mechanisms nos. 1 and 3.
Multivariate Connectivity Analysis.
Portions of ROIs that showed placebo effects in one or more contrasts, 32 contiguous regions in all were selected for network analysis. We analyzed average opioid BP across conditions for each participant, computing Spearman's ρ values between pairs of regions. These interregion correlations can reveal whether individual differences in opioid binding are consistent across the brain or whether there are functional subsystems in opioid responses that show different patterns of individual differences.
To test for functional subsystems, the matrix of ρ's for all pairs was decomposed with nonmetric multidimensional scaling (NMDS) (eight-dimensional solution; SI Fig. 11 A and B and SI Text), and hierarchical cluster analysis with average linkage was used on component scores to group regions into sets (“subsystems”). Nonparametric tests were used to determine the number of subsystems and to establish significance. We found that the best solution was a seven-subsystem solution, and this was reliably better than the null hypothesis of no functional subsystems (P < 0.0001, Z = 4.0) (for details, see SI Fig. 11 C and D and SI Text).
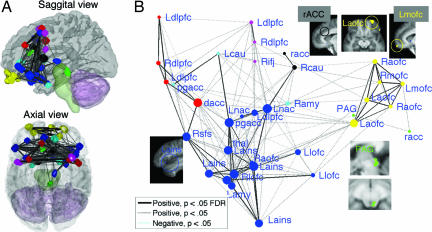
Fig. 4A shows locations of the 32 regions in the brain, and Fig. 4B shows the relationships between regions (marked with circles) in the space of the first two components. Each circle represents a unique region, although some regions share the same name (e.g., DLPFC). Regions closer together on the graph were on average more highly correlated, and the marker color indicates subsystem membership. Lines show pairs of regions with significant bivariate ρ's [gray, P < 0.05; black, false discovery rate (FDR) (37) corrected P < 0.05]. The size of each region indicates its centrality in the overall network; regions with larger circles are more centrally connected hubs.
Fig. 4.
Connectivity analysis of opioid binding potential. (A) 3D rendering of connectivity among regions that show placebo opioid responses. (B) Nonmetric multidimensional scaling (NMDS) connectivity graph of the regions in A. See text for explanation. L, left; R, right; amy, amygdala; aofc, anterior OFC; cau, caudate; ifj, inferior frontal junction; lofc, lateral OFC/inferior frontal border; mofc, medial OFC; thal, thalamus.
Fig. 4 shows that OFC regions (yellow) have high connectivity within-subsystem and low connectivity with other subsystems, as do two separable prefrontal subsystems (red and magenta). PAG–rACC (green) and amydgala–pgACC subsystems (light blue) also show patterns of binding distinguishable from the other subsystems. A large, interconnected subsystem of limbic regions (dark blue) includes NAC, insula, lateral OFC, thalamus, and some frontal and cingulate regions.
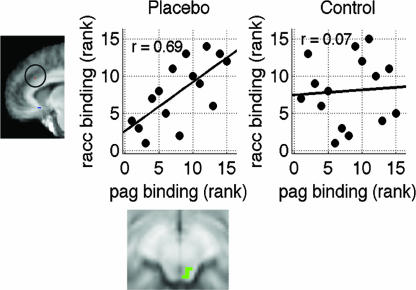
Averaging μ-opioid BP within subsystems, we tested whether connectivity between networks was stronger with painful than with warm stimulation and stronger with placebo than with control during heat (ref. 38; see SI Fig. 12 and SI Text). This analysis can test whether individual differences are more coherent across brain subsystems with heat (analysis 1) and with placebo (analysis 2). Increased connectivity with placebo would indicate that placebo induces coherent opioid activity across multiple regions that varies on an individual basis and would support the idea that there are true individual differences in placebo responses. We found that heat increased integration of OFC with DLPFC and the limbic subsystem (statistics in SI Table 3). Correlations between the PAG–rACC and the rACC–caudate networks were stronger with placebo than with control treatment (ρplacebo = 0.67 vs. ρcontrol = 0.09, Z = 2.25, P = 0.02, two-tailed). Placebo treatment also increased connectivity between the PAG and the rACC themselves, (ρplacebo = 0.71 vs. ρcontrol = 0.06, Z = 2.31, P = 0.02). Scatter plots are shown in Fig. 5.
Fig. 5.
Placebo-modulated connectivity between PAG and rACC.
Finally, we tested for placebo-induced increases in functional integration among regions as a whole. We compared interregion correlations under PH with those under CH, and counted the number of increased vs. decreased correlations (SI Fig. 12). We found 36 increased pairwise correlations under placebo compared with 5 decreased (difference = 26, P < 0.0001 by using a permutation test). All significant placebo effects on pairwise correlations are listed in SI Table 4. Notable is the increased connectivity among limbic regions (scatter plots for left NAC and left aINS are in SI Fig. 12 Inset 2) and between limbic regions and DLPFC (scatter plots for left DLPFC–amygdala are in SI Fig. 12 Inset 3).
Discussion
Understanding expectancy effects on endogenous opioid neurotransmission is an important step in explaining how cognitive processes modulate perception and affect, with implications for healthy endogenous regulation and clinical pain syndromes (39). Consistent with behavioral studies (7, 11, 40), the results indicate that placebo treatment induces increases in endogenous opioid activity in μ-opioid-rich limbic and paralimbic regions, including PAG, NCF, DRN, OFC, amygdala, two dissociable regions of the anterior cingulate (rACC and pgACC), ventral aINS, and thalamus. All of these regions have shown a response to placebo or expectancy effects in pain or pharmacological administration of opioids. NCF and DRN are and have been reported in recent fMRI studies of central pain modulation (41–43).
The results suggest that placebo may affect opioid responses in multiple ways. Placebo treatment worked in a subset of regions [OFC, rACC, NAC, NCF, DRN, ventral tegmental area (VTA), right amygdala, and thalamus] by potentiating the opioid release caused by noxious stimulation (mechanism no. 1 in Fig. 1A). In another set of regions (lateral prefrontal cortex, aIns, pgACC, and left amygdala), placebo treatment appears to reduce anticipatory opioid activity, perhaps by reducing anticipatory threat responses (mechanism no. 3 in Fig. 1A). These regions may be involved in preparing for upcoming pain. Connectivity analyses supported this distinction, as the regions showing each pattern of responses belonged to different, separable subsystems. Although these results suggest a prominent role for endogenous opioids in placebo analgesia, placebo analgesia does not appear to be opioid-mediated in all conditions; for example, after conditioning with a nonopioid analgesic (11) and in a recent study of irritable bowel syndrome (44).
Placebo Effects in the Midbrain.
The finding of PAG opioid increases in placebo is important because it is perhaps the key region linking placebo expectancies with analgesia at both the spinal and supraspinal levels. In addition to placebo modulation of μ-opioid activity, we found that correlations between the PAG and rACC during noxious stimulation were strengthened with placebo, corroborating findings of Bingel et al. (35) on placebo-dependent connectivity between rACC and PAG in fMRI. Interestingly, however, the strongest midbrain placebo effects during noxious heat were found in and around the NCF and DRN (SI Fig. 10), two key nuclei in descending antinociceptive pathways (16, 45) that have been reported in recent imaging studies to show expectancy-related effects (41–43).
Functional Integration.
We assessed interregional connectivity across participants (rather than on dynamic measurements over time), as has much of the previous work on connectivity in pain (32, 33, 35, 36). Such analyses are informative about the pattern of individual differences in brain activity. Connectivity analyses suggested that there are multiple subsystems in the human μ-opioid system, as evidenced by the findings that opioid BP levels were not correlated across ROIs. Rather, opioid BP levels were correlated within subsets of anatomically distributed but functionally coherent regions (identified by cluster analysis). For example, our findings suggest that one subsystem is composed of left and right lateral and midlateral OFC. Opioid BP levels among these regions was correlated (although the regions are distinct and each is at least 2 cm from each other one) and correlations with other regions are lower. Other subsystems included dissociable superior and mid-dorsolateral subsystems, a limbic subsystem, a midline (rACC and PAG) subsystem, and perilimbic subsystems (comprised of pgACC, amygdala, caudate, and one prefrontal region). These are shown in Fig. 4. These findings argue against the notion of a unitary “opioid system.”
In addition to increasing the PAG–rACC connectivity hypothesized a priori, placebo treatment increased functional connectivity among the distinct subsystems (SI Fig. 12), particularly among subcortical regions, including NAC, thalamus, amygdala, and insula, and between these regions and DLPFC and OFC regions in other subsystems. The most straightforward interpretation of increased opioid–BP connectivity with placebo is that individuals differ in the magnitude of their opioid response to placebo and that an individual's response magnitude is consistent across “connected” regions. That is, the “placebo responders” in one region are the same as those in other regions, implying a central common mechanism for placebo-induced opioid release that varies across individuals. These results suggest that it may be meaningful to characterize individuals on the basis of brain activity, i.e., as “opioid placebo responders,” in future studies.
Correlations Between Reported Placebo and Opioid Activation.
Opioid activity increases in a substantial proportion of ROIs was correlated with reported analgesia, in many cases in the same voxels that showed group effects. However, the correlations were negative in most regions, suggesting that greater reported reduction in pain is associated with more modest opioid increases. One reason may be that placebo responders show nonspecific opioid activity across both placebo and control conditions and thus smaller (CH − PH) differences. There was evidence for this effect in a number of regions; a representative example in the right lateral OFC is shown in SI Fig. 9 A–C. Strong placebo responders showed evidence of higher opioid activation (lower BP values) averaged across task conditions (r = 0.56, P = 0.03; SI Fig. 9B) as well as evidence of higher opioid activation (lower BP) in the CH condition (r = 0.72, P = 0.003). SI Fig. 9D shows whole-brain maps of correlations between reported placebo and BP in the CH and CW conditions. The widespread negative correlations reinforce the notion that placebo responders show relatively lower BP in many opioid-rich regions. This could occur for two reasons: Placebo responders may release more endogenous opioids in response to the experimental context or to the manipulation phase preceding PET, or placebo responders may have higher receptor-binding affinity, which would lead to lower overall BP levels for these participants in the context of an existing opioid tone. In the former case, inclusion of the expectancy manipulation may be an important experimental difference from previous work (27), because it may elicit opioid release by itself. In the later case, affinity differences may help explain observed correlations between opiate- and placebo-induced analgesic responses (11). More detail and discussion of additional alternatives is provided in SI Text.
Conclusions
Placebo-induced expectancies of pain relief have been shown to decrease pain in a manner reversible by opioid antagonists, but little is known about the central brain mechanisms of opioid release during placebo treatment. This study examined placebo effects on endogenous opioid neurotransmission with PET and the μ-opioid receptor-selective radiotracer [11C]carfentanil in relation to several alternative mechanisms: Placebo treatment may potentiate either anticipatory or pain-related endogenous opioid release or elicit opioid release itself. Noxious stimulation under placebo and control conditions differed only in contextual instructions (temperatures were equivalent). Increases in endogenous opioid neurotransmission (decreases in μ-opioid receptor availability in vivo) were found in a number of key opioid-rich regions identified in previous studies of placebo and cognitive regulation, including the PAG, amygdala, OFC, insula, rACC, and lateral PFC. Two distinct patterns were found: First, placebo-induced increases in opioid activity specific to heat in OFC, right amygdala, and rACC suggests that placebo potentiates pain-related opioid release; opioid activity in many of these regions was correlated with reported placebo analgesia. Second, placebo-induced decreases in opioid activity specific to anticipation in pregenual cingulate, insula, and PFC suggests that anticipatory anxiety may result in opioid release, and placebo may block this release (although this is speculative); these anticipatory opioid responses were not correlated with reported placebo analgesia. Finally, an application of nonmetric multidimensional scaling and cluster analysis revealed distinct clusters of regions with similar opioid activity across participants, suggesting that coherent subsystems within the endogenous opioid system can be identified. Painful heat (vs. warm stimulation) increased the functional integration of opioid activity in OFC with those in other areas. Placebo treatment increased connectivity between the PAG and rACC and increased functional integration among limbic regions and between limbic regions and PFC. Overall, placebo treatment had widespread effects on endogenous opioid activity in cortical and subcortical regions critical for the determination of affective value and context-based control of pain.
Materials and Methods
Experimental Design.
Fifteen healthy volunteers (details in SI Text) underwent two 90-min scans with PET and [11C]carfentanil (Fig. 1B). Each scan consisted of 30 warm trials (one per minute) and 30 noxious heat trials (one per minute), in that order. We stimulated the placebo-treated forearm region during one scan and the control-treated region in the other, with order randomized, counterbalanced, and controlled for by regression in all analyses. Carfentanil was delivered at subpharmacological doses, 0.026 ± 0.01 μg/kg per scan. These doses occupy <0.5% of the available μ-opioid receptors and are devoid of physiological effects.
During each trial, a 1-s warning tone was followed by 4 s of anticipation. Next, 24.5 s of thermal stimulation (3 s ramp up, 17 s at target, 4.5 s ramp down) was applied using a Medoc TSA 2001 (Medoc Ltd., Chapel Hill, NC). Next, a warning tone cued participants to rate the stimulus intensity on a 0–10 visual analogue scale with the following verbal anchors: 0 was “no sensation”; 1 was “detectable sensation”; 2 was “nonpainful warmth”; 3 was “just painful”; and 10 was “unbearable.” The interval between stimulation offset and the next warning cue was 30.5 s to allow physiological recovery and prevent habituation (see SI Text and SI Fig. 6). Before scanning, warm and noxious temperatures were chosen on an individualized basis for each subject (3, 26, 46). Warm temperatures (44–46.5°C) were chosen at calibration level 2. Noxious temperatures (48–49.5°C) were chosen at level 8. After calibration, placebo and control treatments were applied to two regions of the volar forearm of each volunteer. As in previous work, before scanning, subjects also underwent an expectancy manipulation phase in which belief in the placebo was strengthened (3, 26, 46). The manipulation involves surreptitiously presenting reduced temperatures during stimulation on placebo-treated skin (using different skin areas from those used in scanning). Stimulation during PET scanning followed a minimum of 20 min after the manipulation phase. See SI Text for more details.
Before each scan, the control and placebo creams were reapplied to the forearm. During scanning, each block of warm or noxious trials was preceded by a 10-min rest period, which facilitates the use of Logan plots (47) used for BP quantification (see SI Text). The study was designed to maximize the sensitivity of the placebo vs. control comparison with noxious heat, which began 40 min after injection in each condition. We were also interested in the placebo vs. control comparison during warm stimulation (providing information relevant for mechanisms no. 2 and no. 3) and the pain (hot–warm) × placebo interaction (relevant for mechanism no. 1). Importantly, however, the design is not suited for a direct comparison between hot vs. warm stimulation, because these conditions were not randomized, and BP calculation bias may differ between early and late scanning periods. The comparison between control and pain states has been the subject of previous work (28, 48) from which we draw inferences about the effects of noxious stimulation on opioid binding.
Image Acquisition and Analysis.
[11C]carfentanil PET distribution volume ratio (DVR) [equal to BP + 1 or (Bmax/Kd) + 1] images for each condition were acquired, reconstructed, and corrected for attenuation by using a modified Logan plot analysis (27). High-resolution anatomical MRI images were warped into Montreal Neurologic Institute (MNI) standard space, and warping parameters were applied to DVR images (details in SI Text). DVR images were spatially smoothed with an 8-mm isotropic Gaussian filter. All opioid effects were localized with reference to the group mean warped anatomical MRI image, which was used as the anatomical underlay in all figures.
Consistent with previous studies (27), we define relative μ-opioid “activation” as the relative decrease μ-opioid receptor BP between conditions, i.e., a positive value for (CH − PH) in DVR indicates that placebo increases in endogenous opioid activity during heat. Similarly, positive (CW − PW) values indicate that placebo increases during anticipatory warm stimulation, and positive interaction values, [(CH − PH) − (CW − PW)] indicate that placebo-induced opioid increases greater during heat. Contrast values were entered into a random effects, multiple regression model at the second level with mean-centered reported placebo during heat (reported pain in CH − PH) and administration order (contrast coded) entered as covariates. Robust model estimation was performed using iteratively reweighted least squares (IRLS) in Matlab software (Mathworks, Natick, MA), which produces valid P values while minimizing the influence of outliers and violations of normality (49). See SI Text for details.
ROIs.
ROIs were identified on the basis of previous imaging studies. Each ROI met all of the following criteria: (i) a priori interest on the basis of at least one activation in placebo studies of fMRI or μ-opioid receptor BP; (ii) high BP in the current sample (> 1.1, reflecting specific binding; see SI Fig. 6); and (iii) activation in at least two previous studies of either placebo, opiate administration, or emotion regulation, from ref. 50 (SI Fig. 6). In some cases, ROIs were enlarged slightly to encompass high-BP regions in our sample. Eleven ROIs of primary interest included PAG, rACC, aINS, pgACC, medial orbital sulcus (MOS), lateral OFC/inferior frontal gyrus (LOFC), amygdala (Amy), and nucleus accumbens (NAC). Each of these regions has been shown to be important for placebo effects in fMRI and/or opioid-binding studies. Additional ROIs in the thalamus (6), DLPFC, medial OFC, and dorsal caudate were also identified as described above, making a complete set of 27 regions. Correction for multiple comparisons across regions was performed with a permutation test that compared the number of significant SVC ROIs with the number expected under the null hypothesis (see SI Text). For ROI sets that show a significant number of activated regions, we report SVC-corrected regions (yellow in all figures).
Because of the importance of brainstem pain modulation pathways, additional exploratory ROIs included the NCF (41, 43), lateral to PAG in the midbrain, the midline DRN (e.g., ref. 42; SI Fig. 10), and dopaminergic ventral tegmental area (VTA) due to opioid-dopamine interactions in this region (51, 52). NCF and DRN are heavily interconnected with PAG and the rostral ventral medulla and play key roles in spinal afferent inhibition and sensitization (45). Because too few imaging studies have examined these regions to define formal ROIs as above, we compared the locations of midbrain placebo effects with approximate locations of these nuclei as defined in refs. 41–43 and in the Duvernoy atlas (53).
Supplementary Material
Acknowledgments
We thank Drs. Ed Smith and Ken Casey for helpful comments and discussion; Raza Zaidi and Alex Sokolik for help with data collection; Matthew Davidson and Brent Hughes for technical assistance; the PET technologist at the University of Michigan PET Center for assistance; and the authors of Statistical Parametric Mapping and the Montreal Neurological Institute. Support was provided by the Mind, Brain, Body, and Health Initiative (T.D.W.), the John D. and Catherine T. MacArthur Foundation (T.D.W.), and National Science Foundation Grants 0631637 (to T.D.W.) and R01 AT 001415 (to J.K.Z.).
Abbreviations
- aINS
anterior insula
- BP
binding potential
- CH
control with painful heat
- CW
control with nonpainful warmth
- DLPFC
dorsolateral PFC
- DRN
dorsal raphe nucleus
- DVR
distribution volume ratio
- fMRI
functional MRI
- lOFC
lateral OFC
- mOFC
medial OFC
- NAC
nucleus accumbens
- NCF
nucleus cuneiformis
- OFC
orbitofrontal cortex
- PAG
periaqueductal gray
- PET
positron-emission tomography
- PFC
prefrontal cortex
- PH
placebo with painful heat
- pgACC
pregenual anterior cingulate
- PW
placebo with nonpainful warmth
- rACC
rostral anterior cingulate
- ROI
region of interest
- SVC
small volume corrected.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702413104/DC1.
References
- 1.De Pascalis V, Chiaradia C, Carotenuto E. Pain. 2002;96:393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- 2.Clark WC. J Abnorm Psychol. 1969;74:363–371. doi: 10.1037/h0027509. [DOI] [PubMed] [Google Scholar]
- 3.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 4.Vase L, Robinson ME, Verne GN, Price DD. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 5.Voudouris NJ, Peck CL, Coleman G. Pain. 1989;38:109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 6.Marchand S, Kupers RC, Bushnell MC, Duncan GH. Pain. 2003;105:481–488. doi: 10.1016/S0304-3959(03)00265-3. [DOI] [PubMed] [Google Scholar]
- 7.Fields HL, Levine JD. Am J Med. 1981;70:745–746. doi: 10.1016/0002-9343(81)90525-8. [DOI] [PubMed] [Google Scholar]
- 8.Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Nature. 1983;306:264–265. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- 9.Benedetti F, Arduino C, Amanzio M. J Neurosci. 1999;19:3639–3648. doi: 10.1523/JNEUROSCI.19-09-03639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetti F, Amanzio M, Baldi S, Casadio C, Maggi G. Eur J Neurosci. 1999;11:625–631. doi: 10.1046/j.1460-9568.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- 11.Amanzio M, Benedetti F. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollo A, Vighetti S, Rainero I, Benedetti F. Pain. 2003;102:125–133. doi: 10.1016/s0304-3959(02)00345-7. [DOI] [PubMed] [Google Scholar]
- 13.Levine JD, Gordon NC, Fields HL. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YQ, Tang JS, Yuan B, Jia H. Pain. 1997;72:127–135. doi: 10.1016/s0304-3959(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Tang JS, Yuan B, Jia H. Neurosci Lett. 1997;224:142–146. doi: 10.1016/s0304-3940(97)13478-4. [DOI] [PubMed] [Google Scholar]
- 16.Willis WD, Westlund KN. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen A, Zhang YX, Lund I, Lundeberg T, Yu LC. Brain Res. 2004;1001:87–94. doi: 10.1016/j.brainres.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 18.Nichols DS, Thorn BE. Pain. 1990;41:347–352. doi: 10.1016/0304-3959(90)90011-2. [DOI] [PubMed] [Google Scholar]
- 19.Morgan MM, Gold MS, Liebeskind JC, Stein C. Brain Res. 1991;545:17–23. doi: 10.1016/0006-8993(91)91264-2. [DOI] [PubMed] [Google Scholar]
- 20.Behbehani MM. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 21.Prado WA, Pelegrini-da-Silva A, Martins AR. Brain Res. 2003;972:207–215. doi: 10.1016/s0006-8993(03)02541-1. [DOI] [PubMed] [Google Scholar]
- 22.Barbaro NM. Prog Brain Res. 1988;77:165–173. doi: 10.1016/s0079-6123(08)62783-1. [DOI] [PubMed] [Google Scholar]
- 23.Hosobuchi Y. Appl Neurophysiol. 1983;46:112–115. doi: 10.1159/000101249. [DOI] [PubMed] [Google Scholar]
- 24.Hosobuchi Y, Rossier J, Bloom FE, Guillemin R. Science. 1979;203:279–281. doi: 10.1126/science.83674. [DOI] [PubMed] [Google Scholar]
- 25.Young RF, Chambi VI. J Neurosurg. 1987;66:364–371. doi: 10.3171/jns.1987.66.3.0364. [DOI] [PubMed] [Google Scholar]
- 26.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 27.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 29.Levine JD, Gordon NC, Bornstein JC, Fields HL. Proc Natl Acad Sci USA. 1979;76:3528–3531. doi: 10.1073/pnas.76.7.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGlashan TH, Evans FJ, Orne MT. Psychosom Med. 1969;31:227–246. doi: 10.1097/00006842-196905000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Evans FJ. In: Placebo: Theory, Research and Mechanisms. White L, Tursky B, Schwartz G, editors. New York: Guilford; 1985. pp. 215–228. [Google Scholar]
- 32.Lorenz J, Minoshima S, Casey KL. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 33.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Tang JS, Yuan B, Jia H. Brain Res. 1998;813:359–366. doi: 10.1016/s0006-8993(98)01050-6. [DOI] [PubMed] [Google Scholar]
- 35.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 36.Faymonville ME, Roediger L, Del Fiore G, Delgueldre C, Phillips C, Lamy M, Luxen A, Maquet P, Laureys S. Brain Res Cogn Brain Res. 2003;17:255–262. doi: 10.1016/s0926-6410(03)00113-7. [DOI] [PubMed] [Google Scholar]
- 37.Genovese CR, Lazar NA, Nichols T. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 38.Steiger JH. Psychol Bull. 1980;87:245–251. [Google Scholar]
- 39.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Matre D, Casey KL, Knardahl S. J Neurosci. 2006;26:559–563. doi: 10.1523/JNEUROSCI.4218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. J Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- 43.Zambreanu L, Wise RG, Brooks JCW, Iannetti GD, Tracey I. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Vase L, Robinson ME, Verne GN, Price DD. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Fields H. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 46.Wager TD, Matre D, Casey KL. Brain Behav Immun. 2006;20:219–230. doi: 10.1016/j.bbi.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 49.Wager TD, Keller MC, Lacey SC, Jonides J. NeuroImage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devine DP, Leone P, Pocock D, Wise RA. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- 52.Spanagel R, Herz A, Shippenberg TS. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Anatomy. New York: Springer; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.