Abstract
Respiratory infections constitute the most widespread human infectious disease, and a substantial proportion of them are caused by unknown etiological agents. Reoviruses (respiratory enteric orphan viruses) were first isolated from humans in the early 1950s and so named because they were not associated with any known disease. Here, we report a previously unknown reovirus (named “Melaka virus”) isolated from a 39-year-old male patient in Melaka, Malaysia, who was suffering from high fever and acute respiratory disease at the time of virus isolation. Two of his family members developed similar symptoms ≈1 week later and had serological evidence of infection with the same virus. Epidemiological tracing revealed that the family was exposed to a bat in the house ≈1 week before the onset of the father's clinical symptoms. Genome sequence analysis indicated a close genetic relationship between Melaka virus and Pulau virus, a reovirus isolated in 1999 from fruit bats in Tioman Island, Malaysia. Screening of sera collected from human volunteers on the island revealed that 14 of 109 (13%) were positive for both Pulau and Melaka viruses. This is the first report of an orthoreovirus in association with acute human respiratory diseases. Melaka virus is serologically not related to the different types of mammalian reoviruses that were known to infect humans asymptomatically. These data indicate that bat-borne reoviruses can be transmitted to and cause clinical diseases in humans.
Keywords: respiratory infection, zoonosis, human-to-human transmission, orthoreovirus, Pulau virus
Respiratory tract illness (RTI) accounts for a large portion of public health spending worldwide. Although several new etiological agents of RTI have been identified in the past few decades, a substantial proportion of these illnesses remain undiagnosed. Recently, three previously unknown coronaviruses have been identified as the causative agents for mild to very severe RTI, including the novel coronavirus responsible for the global outbreak of the severe acute respiratory syndrome (SARS) (1–3).
Reoviruses (respiratory enteric orphan viruses) are a large and diverse group of nonenveloped viruses with segmented dsRNA genomes that are taxonomically classified into 10 genera in the family Reoviridae (4, 5). Members of the genus Orthoreovirus contain 10 genome segments and have been isolated from a broad range of mammalian, avian, and reptilian hosts. Orthoreoviruses are divided into two subgroups, fusogenic and nonfusogenic, based on the ability of the virus to induce cell–cell fusion and syncytium formation (6, 7). The mammalian orthoreoviruses (MRV) are nonfusogenic, whereas the remaining members of the genus are fusogenic, including avian orthoreoviruses, baboon orthoreoviruses, reptilian orthoreoviruses, and Nelson Bay orthoreovirus (NBV). Since the first isolation of MRV from humans in 1951, it has been shown that MRV infection is quite common in the human population (8). However, although many diseases in animals have been attributed to orthoreovirus infection, from neurological symptoms in baboons and snakes to pneumonia and death in chickens, infections in humans are generally benign with very rare cases of mild upper respiratory tract illness or enteritis in infants and children (7).
Bats, probably the most abundant, diverse, and geographically dispersed vertebrates on earth, have recently been shown to be the reservoir hosts of a variety of zoonotic viruses responsible for severe human disease outbreaks, some with very high mortality (9). In the period from 1994 to 1999, four new viruses in the family Paramyxoviridae were discovered, and all appeared to have bats as a reservoir host. Hendra virus emerged in Queensland, Australia, in 1994, killing one human and 14 horses (10), and was responsible for at least four other sporadic outbreaks involving horse and human cases between 1994 and 2006 (11). The closely related Nipah virus (NiV) emerged in 1998–1999 in Peninsular Malaysia, resulting in the death of >100 people and the culling of >1 million pigs (12). Since then, several NiV outbreaks have been recorded in Bangladesh and India (11). Fruit bats in the genus Pteropus (flying foxes) are the natural reservoir of both Hendra virus and NiV. NiV is present in fruit bat populations in Indonesia, Thailand, Malaysia, and Cambodia (9). In 1997, another new paramyxovirus, Menangle virus (MenPV), emerged as the cause of a disease outbreak in pigs causing stillbirth and abortion in a commercial piggery near Sydney, Australia (13). Two workers who were exposed to infected pigs developed a flu-like illness with rash and high titers of antibodies to MenPV (14). Seropositive flying foxes were found in a colony near the piggery, although MenPV was not isolated. Two years later, the fourth new paramyxovirus from bats, Tioman virus, was isolated from pteropid bat urine samples from Tioman Island off the east coast of Peninsular Malaysia (15). Tioman virus is related to MenPV, but its disease-causing status in animals and humans remains unknown. During the same period (1994–1999), Australian bat lyssavirus (ABLV) spilled over from bats to humans, resulting in two fatal infections (9, 16). Recently, we and another group independently identified horseshoe bats (genus Rhinolophus) as the reservoir host for a group of genetically diverse SARS-like coronaviruses (17, 18), suggesting that the etiological agent responsible for SARS had a bat origin. Although the exact origin of Ebola virus is still unknown, recent serological and molecular studies suggested a potential link between Ebola virus and bats (19).
The first reovirus of bat origin, NBV, was isolated in 1968 from the heart blood of a flying fox (Pteropus poliocephalus) in New South Wales, Australia (20). NBV was the first mammalian orthoreovirus to display the fusogenic properties characteristic of avian orthoreoviruses (21). In 1999, during a search for NiV in pteropid bats on Tioman Island, a novel orthoreovirus, Pulau virus (PulV), was isolated from Pteropus hypomelanus (22, 23). Serological and sequence characterization revealed that PulV was closely related to NBV. It is not known whether these bat orthoreoviruses are capable of infecting and causing disease in animals or humans.
Here, we report the discovery and characterization of Melaka virus (MelV), the third virus in the NBV species group, and its isolation from a human patient with fever and acute respiratory illness. Our data indicate that not only was the virus capable of human-to-human transmission, but also that its origin was probably bats. Furthermore, serological survey data suggest that the related bat-derived PulV is also capable of infecting humans, raising the possibility that PulV might have caused respiratory illness that was undiagnosed or misdiagnosed in the past.
Results
Virus Isolation from a Patient with Acute Respiratory Disease.
On March 20, 2006, a 39-year-old male patient (MRA) with a 1-day history of high fever, cough, and sore throat was treated in a government outpatient clinic in the suburb of Melaka, the capital city of the state of Melaka, in Malaysia. His high-grade fever was associated with headache, myalgia, malaise, loss of appetite, generalized body weakness, and severe prostration. There was no associated giddiness, blurring of vision, photophobia, skin eruption, conjunctivitis, or arthritis or related episode of abdominal pain, nausea, vomiting, or diarrhea. The high fever and associated symptoms persisted for 4 days. The patient was treated by different doctors on three occasions during his illness. His cough was initially mild and associated with coryza. Over the subsequent week, his cough worsened progressively with production of yellowish mucoid sputum. However, there was no associated dyspnea, tachypnea, or hemoptysis, although there was one episode of epistaxis. His sore throat, described as severe in nature, persisted for 4 days and was associated with a sensation of tightness in the pharynx and neck, which led to some degree of difficulty in swallowing both liquid and solid food. He continued to feel weak and lethargic for nearly a fortnight after defervescence.
Physical findings at first examination showed MRA to be a Malay man of medium build who appeared unwell and tired. His oral temperature was 39°C and vital signs were stable, with a recorded blood pressure of 120/80 mmHg (1 mmHg = 133 Pa) and pulse rate of 80 beats per min. He was not cyanosed, jaundiced, or in respiratory distress. There was no cutaneous rash or petechiae. His throat displayed injection and hyperemia with slight enlargement of the tonsils but was free of white exudates. Air-entry into his lungs was equal and breath sound was bronchovesicular without other adventitious sounds. The abdominal examination was normal and hepatosplenomegaly was not noted. A mobile, slightly tender cervical lymph node (≈1 × 1.5 cm) was palpable in each anterior triangle of his neck. Other systemic examinations were essentially normal, and a provisional clinical diagnosis of influenza-like illness was made at first examination. Neither chest x-ray nor venous blood was taken, but a throat swab was collected and transported in viral transport medium to a laboratory for virus isolation.
A virus causing a syncytial cytopathic effect (CPE) in Madin–Darby canine kidney (MDCK) cells (Fig. 1) was isolated after 48 h incubation at 37°C. The virus was named “Melaka virus” (MelV) after the location of the index case. After two passages in MDCK cells, MelV replicated and caused syncytial CPE in all types of mammalian cell lines available in the laboratory and also in mosquito-derived C6/36 cells [see supporting information (SI) Table 2].
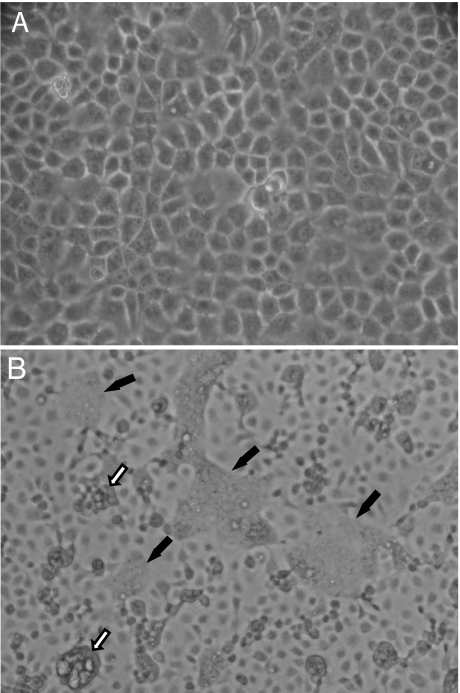
Fig. 1.
Syncytia formation in MelV-infected MDCK cells. (A) Mock-infected. (B) MelV-infected. Two types of syncytial cells were observed: cells still attached to culture flask surface (filled arrows) and cells detaching from the surface (open arrows).
MelV-infected cells failed to react to antiserum against most known respiratory viruses, including adenovirus, influenza A and B, parainfluenza 1, 2, and 3, and respiratory syncytial virus. PCR analyses of the original throat swab by using primers against these viruses were also negative (data not shown).
Morphological and Serological Characterization.
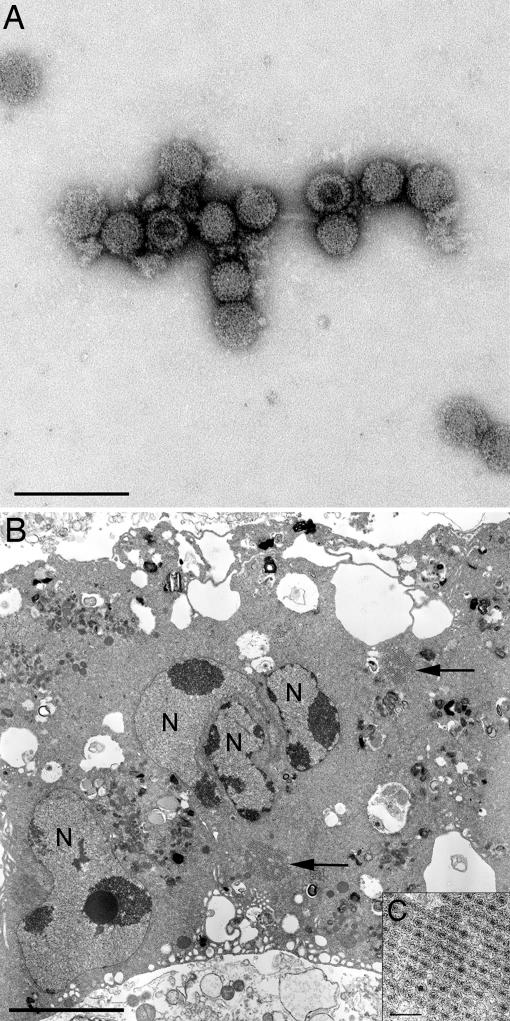
Transmission electron microscopical examination of ultrathin sections from MDCK and Vero cells exhibiting a syncytial CPE and associated negative-stained particles from the supernatant revealed icosahedral viruses resembling those of the family Reoviridae, genus Orthoreovirus. The particles possessed a mean diameter of 74.20 ± 5.02 nm (n = 43) in negative-stained preparations and 78.86 ± 5.31 nm (n = 37) for individual viruses in ultrathin sections. The viruses were naked and possessed a double capsid with conspicuous “spikes” or “turrets” situated on the inner core. Within ultrathin sections, viruses were observed in the cytoplasm as individual particles in paracrystalline arrays and associated with semi-electron-dense inclusion bodies (Fig. 2).
Fig. 2.
Electron micrographs of MelV. (A) Negative-stained MelV. (Scale bar: 200 nm.) (B) Image of an ultrathin section of a MelV-infected multinucleated Vero cell. N, nucleus; arrows, paracrystalline viral arrays (inclusion bodies not shown). (Scale bar: 5 μm.) (C) Higher magnification view of a paracrystalline array. (Scale bar: 200 nm.)
The growth characteristics of MelV and PulV were similar in a number of cell lines, although subtle differences existed in the degree of syncytial CPE that each virus induced (SI Table 2).
Results obtained from cross-neutralization studies (SI Table 3) indicated that serum from the patient (MRA) neutralized both MelV and PulV with equal efficacy. Similarly, rabbit anti-PulV serum cross-neutralized MelV but not any of the three MRV types. Vice versa, none of the three MRV-type sera used in this study was able to neutralize either MelV or PulV. All members of the family appeared to have preexisting anti-MRV2 antibodies that showed no correlation with their anti-MelV antibody status.
Molecular and Phylogenetic Characterization.
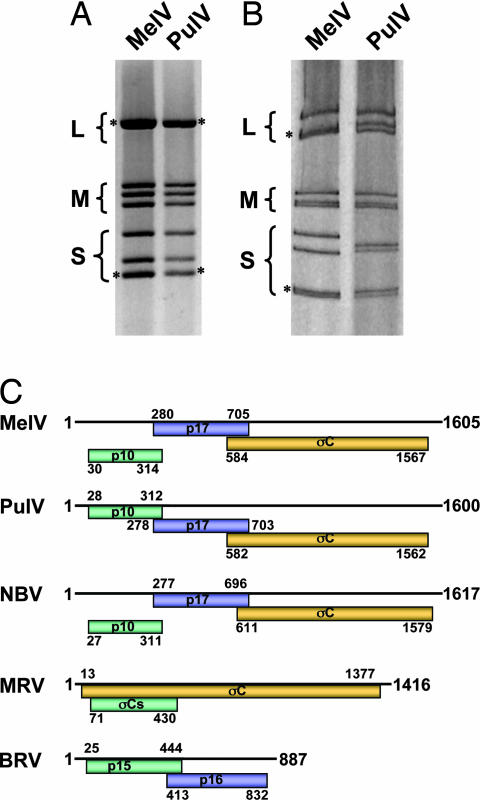
Comparison of genome segments by gel electrophoresis revealed almost identical electropherotypes for MelV and PulV (Fig. 3 A and B). Sequence analysis of the four small (S) segments indicated a very close genetic relationship between the two viruses. One S class genome segment of orthoreoviruses may be polycistronic, and its coding arrangement is characteristic of a particular species group (24). As shown in Fig. 3C, the S1 segment of MelV has an almost identical coding arrangement to that of PulV and NBV. Another genetic marker often used for species differentiation of orthoreoviruses is the highly conserved genome terminal sequences at the 5′ end of the positive-sense RNA (7). For MelV, the consensus sequence is 5′-GCUUwA (w = U or A), which is identical to that of PulV and NBV (SI Fig. 5). The deduced protein products encoded by the four S segments of the two viruses are very similar in size and share high levels of sequence identity (SI Figs. 6–9).
Fig. 3.
Comparison of genome segments and coding strategy of the polycistronic S-class genome segments. (A) Genome segments of MelV and PulV separated on a 1% agarose gel. (B) Genome segments of MelV and PulV separated on a 10% SDS-polyacrylamide gel. The classes of genome segments (L, large; M, medium; S, small) are labeled on the left, and the asterisks indicate comigrating bands where more than one segment is present. (C) Coding arrangement of the polycistronic S segments of three bat orthoreoviruses in comparison with two other mammalian orthoreovirus species. The line on top represents the RNA genome and the shaded boxes underneath depicture protein-coding regions in reading frames 1–3 (top to bottom). Numbers refer to size, in base pairs, of the genome segments and to the first and last nucleotides of the individual ORFs (excluding the stop codons). The names of the encoded proteins are indicated within the shaded boxes.
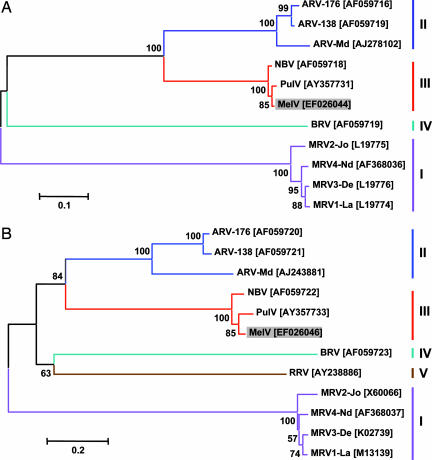
To establish the evolutionary relationship among MelV, PulV, and other known orthoreoviruses, phylogenetic trees were constructed based on deduced amino acid sequences of the major outer capsid protein (Fig. 4A) and the major inner capsid protein (Fig. 4B), respectively. In both phylogenetic trees, MelV was most closely related to PulV and was placed in the species group III together with NBV. Similar trees were obtained based on other proteins encoded by the S genome segments (data not shown).
Fig. 4.
Phylogenetic trees of orthoreoviruses based on deduced amino acid sequence of the major inner (A) and outer (B) capsid proteins. The GenBank accession number for each sequence is given in bracket next to the abbreviated virus name. ARV, avian reovirus; BRV, baboon reovirus; RRV, reptilian reovirus. Numbers at nodes indicate levels of bootstrap support calculated from 1,000 trees.
Epidemiological Investigation.
MRA presently holds the rank of sergeant in the Malaysian Army and works as an accounts clerk in a military base in Melaka. He has not been involved in jungle training or overseas field trips for the last 2 years. However, an unusual event occurred ≈1 week before the onset of his fever. On that day, a bat flew through the main door into his living room at ≈8 p.m. while he was watching television. The bat flew “frantically” for ≈2–3 min in the living room without making any “squeaky” noise or alighting on the ceiling and subsequently managed to fly out through the same door through which it entered.
MRA is married with five children aged 12, 11, 8, 6, and 2, and his wife was 8 months pregnant at the time of his illness. Approximately 6 days after the onset of his fever, his second child (his 11-year-old daughter) developed a high fever; his fourth child (his 6-year-old son) developed a high fever the next day. Both children were lethargic for 1 day but did not have a cough, sore throat, or skin rashes, although the fourth child had one episode of vomiting. His wife did not experience any illness or subjective feeling of being unwell. She gave birth to a normal, healthy baby girl on April 16, 2006.
MRA and his family live in a single-story linked house in a residential area of 30 houses situated in the suburb of Melaka. The family did not keep any pets or rear any poultry in the house compound, although a neighbor who lived five houses away kept a few parrots.
On follow-up investigation, venous blood samples were taken from MRA on three separate occasions. Venous blood samples were also taken from his family members on the third occasion. The sera from the patient and his family members were tested for the presence of antibodies against MelV, and the results are summarized in Table 1. Similar results were also obtained when the sera of the different family members were examined by immunogold electron microscopy. Whereas the sera of MRA, his wife, his 11-year-old daughter, and 6-year-old son were able to specifically react to MelV, the sera from his other sons were negative (data not shown).
Table 1.
Serological responses of the patient and his family members to MelV
| Person (age) | First bleed (April 3) | Second bleed (May 12) | Third bleed (June 9) |
|---|---|---|---|
| Immunofluorescence antibody tests titer IgM/IgG | |||
| MRA (39) | 1:80/1:640 | 1:40/1:2,560 | 1:10/1:2,560 |
| Wife (36) | NA | NA | 1:20/1:2,560 |
| Son (12) | NA | NA | −ve/−ve |
| Daughter (11) | NA | NA | 1:10/1:320 |
| Son (8) | NA | NA | −ve/−ve |
| Son (6) | NA | NA | 1:10/1:80 |
| Son (2) | NA | NA | −ve/−ve |
| Virus neutralization test titer | |||
| MRA (39) | 1:80 | 1:320 | 1:160 |
| Wife (36) | NA | NA | 1:40 |
| Son (12) | NA | NA | −ve |
| Daughter (11) | NA | NA | 1:160 |
| Son (8) | NA | NA | −ve |
| Son (6) | NA | NA | 1:40 |
| Son (2) | NA | NA | −ve |
NA, sera not available for testing; −ve, negative.
Seroprevalence.
To test the prevalence of MelV or other closely related reoviruses in the human population, we used a panel of human sera collected in 2001–2002 from volunteers on Tioman Island during serological surveillance for NiV (25). This panel of sera was chosen because a large number of fruit bats were present on the island and several isolates of PulV were made from bat urine specimens collected on the island at the time. The sera were tested against both PulV and MelV. The data obtained indicate that ≈13% of the 109 sera tested neutralized both viruses. Some sera neutralized both viruses equally well, but several showed a slightly higher neutralizing activity against PulV than MelV (see SI Table 4).
Discussion
The last decade has experienced a surge in the discovery of emerging viruses of bat origin, several of which have had significant impact on human and animal health, tourism, and trade (26–29). Isolation of MelV from a human patient and demonstration of human infection by the closely related PulV serve as the latest examples of this emerging trend. Although we cannot discount the role of modern detection technology in facilitating identification of new viruses, it is believed that ecological changes, which result in greater interaction of human and wildlife animals, are mainly responsible for the increased spillover events observed in recent years (26–29).
A number of viruses recently emerged from bats display a “promiscuous” host range. Such viruses may have coevolved with bats, which are considered to be among the most ancient mammalian species, and use cell-surface receptors that are conserved across a range of animal species. This is certainly the case for henipaviruses, which naturally infect >10 different species in six mammalian taxonomic orders, an unusual observation for paramyxoviruses whose host ranges are usually limited (11). Ephrin B2 has been identified as the main receptor for henipaviruses (30, 31), and its wide distribution and status as a member of a conserved family of cell-surface glycoprotein ligands (32) may help to account for the wide host range of henipaviruses in vivo and in vitro. Similarly, the receptor for the SARS coronavirus, angiotensin-converting enzyme 2 (33), is also conserved among different animals (34), an observation consistent with the finding that SARS coronavirus seems to be able to infect most mammalian species tested under experimental conditions (35). In this regard, it is worth noting that MelV and PulV are also capable of replicating in a wide range of cell lines, including one derived from mosquitoes (see SI Table 2).
The risk of virus spillover from bats as a result of increasing encroachment by animals and humans into bat habitats is enhanced by the genetic diversity observed among newly emergent bat-borne viruses. For henipaviruses, four to five genetically distinct virus strains have been detected with different virological and biological properties. For example, the NiV Bangladesh strains, but not the NiV Malaysian strains, are capable of human-to-human transmission (11). This is also true for the SARS-like coronaviruses in bats. A limited search among horseshoe bats in China already uncovered at least five different genetic variants (17, 18), and the number of variants is expected to increase dramatically when a systematic survey is conducted across different countries and geographic areas. The same applies to the NBV species group, which has three different members so far. Considering the wide distribution of pteropid bats in India, Southeast Asia, Australia, and the Pacific, one can expect that more bat-borne reoviruses in this group will be discovered in the future. It should be noted that although the epidemiological tracing data and the close genetic relationship of MelV to PulV and NBV strongly suggest that MelV originated from bats, at this stage we do not have experimental evidence to prove the bat origin of MelV and the direct bat-to-human transmission. Field surveillance of bat populations in Melaka will be necessary to resolve this issue.
MelV is a unique orthoreovirus capable of infecting and causing disease in humans. The infection of multiple members in the same family and the delayed onset (by 1 week) of clinical symptoms of the two children strongly suggest human-to-human transmission. It is not clear to us how and when the wife became infected and why she displayed no clinical symptoms. Fusogenicity is a known important virulence marker for many of the well characterized respiratory viruses, such as respiratory syncytial virus, henipaviruses, influenza, and parainfluenza viruses. Although widely present among human populations, none of the MRVs are fusogenic, an observation highlighting the potential importance of cell–cell fusion for the pathogenesis of orthoreoviruses in humans.
In a recent Foresight (www.foresight.gov.uk) report entitled “Infectious Diseases: Preparing for the Future,” eight categories of infectious diseases were identified for which improved detection systems would make a difference over the next 10–25 years (36). Of the eight categories, MelV represents categories one, three, and six: new diseases, zoonoses, and acute respiratory infections. With the advent of multiplexed diagnostic technologies both at the nucleic acid and protein levels, classic exclusion diagnosis will eventually be replaced with differential diagnosis. When a severe RTI patient presents at a hospital, it is important not only to exclude SARS or highly pathogenic avian influenza, but also to accurately determine the causative agent so that a targeted treatment regimen can be implemented. In this regard, identification of novel RTI agents will play a key role in shaping the future of RTI diagnosis, and the discovery of MelV, its relationship with PulV and NBV, and the potential involvement of fusogenic reoviruses as causes of RTI will undoubtedly make a significant contribution to the improvement of RTI diagnosis and treatment.
Materials and Methods
Virus Isolation and Purification.
The throat swab sample in viral transport medium was treated with antibiotics (100,000 units/ml penicillin and 100 μg/ml streptomycin). After 1 h of treatment, the sample was inoculated in duplicates (100 μl and 200 μl, respectively) into freshly confluent monolayers of MDCK (ATCC, CCL-34), Vero (ATCC, CCL-81), and Hep-2 (ATCC, CCL-23) cells cultured in a 24-well cell culture plate. The plate was incubated at 37°C in 5% CO2 and examined daily for the presence CPE in cultured cells. After two passages in MDCK, the virus (MelV) was passaged once in Vero cells in the presence of ciprofloxacin (10 μg/ml) to control mycoplasma contamination. The virus was purified by three consecutive limiting dilution passages in Vero cells in the presence of ciprofloxacin. After purification, the virus was passed twice at low multiplicity of infection in Vero cells and harvested 72 h postinfection to generate a working stock for analysis. Stock preparations of MelV had average titers of 4 × 106 TCID50 (tissue culture 50% infective dose)/ml. PulV was previously plaque-purified (23) and grown in Vero cells to a titer of 1 × 106 TCID50/ml. Virus preparations were purified for further analysis according to standard protocols by using Dounce homogenization and ultracentrifugation.
Electron Microscopy.
Vero cells displaying syncytial CPE were processed through to resin blocks and ultrathin sections examined at 120 kV in a Philips CM120 transmission electron microscope (Philips, Eindhoven, The Netherlands). For negative staining, paraformaldehyde-fixed (2%) purified MelV and stocks of PulV were adsorbed onto carbon-coated parlodion-filmed nickel grids (5 min), stained with 2% (wt/vol) phosphotungstic acid (pH 6.8) (1 min), and viewed as described above.
Antibody Tests.
Immunofluorescence antibody tests (IFAT) were conducted as follows. A freshly confluent monolayer of MDCK was inoculated with MelV at high multiplicity of infection and incubated at 37°C in 5% CO2. At full CPE, the infected cells were harvested, washed four times, and suspended in sterile PBS at a cell concentration of 3,000 cells per ml. Ten microliters of the infected cell suspension was carefully spotted onto each well of Teflon-coated slides, followed by air-drying over a warm plate and subsequent fixation in cold acetone for 10 min. Five microliters of each serum was subjected to serial 2-fold dilution with sterile PBS from 1:10 to 1:2,560. For assay of IgM, IgG was removed by absorption with protein A before serum dilution. Twenty microliters of diluted serum was transferred onto each respective well of the antigen-coated slide and incubated in a moist chamber for 30 min at 37°C. Subsequently, the slides were rinsed with PBS before being soaked for 10 min in PBS solution that was kept in gentle motion by a magnetic stirrer. The slides were allowed to air-dry over a warm plate and probed with 20 μl of 1:40 diluted fluorescein-conjugated respective rabbit anti-human IgM or IgG (Dako, Glostrup, Denmark). The slides were then incubated for another 30 min at 37°C in a moist chamber. The same process of washing and drying was carried before the slides were mounted with a commercially supplied mounting fluid, and the specific reactivity/labeling was read under a UV fluorescence microscope (BX50; Olympus, Tokyo, Japan) at ×400 magnification.
For the virus neutralization test of PulV or MelV, serial 2-fold dilutions of control and test sera were prepared in duplicate starting at 1:10. An equal volume of either MelV or PulV working stock containing 150 TCID50 was added to the diluted sera and incubated for 30 min. The preincubated virus/serum mix was added to confluent Vero cell monolayers and incubated for 1 h. The inoculum was removed, monolayers were washed three times with PBS, and cell media were replaced. All incubations were performed at 37°C in a humidified 5% CO2 incubator. Vero cell monolayers were observed for CPE 3 days later. The ability of sera to neutralize virus was determined by scoring the extent of CPE observed in duplicate wells.
For the virus neutralization test of MRV, MA104 cell monolayers were prepared in 96-well tissue culture plates seeded with 20,000 cells per well and incubated overnight at 37°C in a humidified 5% CO2 incubator. MRV types 1, 2, and 3 were diluted in cell media to give 4 × 104 TCID50/ml, and 100 μl of virus was added to wells in a 96-well incubation plate. Serial 10-fold dilution of test sera were prepared starting from 1:10, and 100-μl aliquots were added to virus wells. The sera/virus mixture was incubated for 60 min at 37°C. The medium was discarded from the cell monolayers, and four 50-μl replicates of the preincubated virus/sera mix were transferred from the incubation plate to appropriate wells in the test plate. At the same time, 12 virus control wells were prepared by adding 103 TCID50 per well from the above diluted virus solution, and the plates were incubated for 30 min at 37°C. The inoculum was discarded and the cells were washed three times with 200 μl of cold PBS, followed by the addition of 100 μl of fresh media. After incubation at 37°C for 20 h, the medium was discarded, the cells were fixed in 100% ice-cold methanol for 15 min and air-dried, and the wells were blocked with 200 μl of 1% BSA/PBS. The neutralization of MRV was monitored by immunofluorescent microscopy with rabbit anti-MRV antibodies and goat anti-rabbit conjugated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA). Fluorescence was observed by using an Olympus fluorescent microscope as described above.
Sequence and Phylogenetic Analysis.
Extraction and purification of dsRNA and synthesis of randomly primed cDNA were carried out as described (23, 37). Primers (primer sequences will be supplied upon request) designed by using PulV and NBV small genome segment sequences were used for PCR amplification and sequencing of the MelV small genome segments. Genome segment terminal sequences were obtained by using a two-step PCR amplification procedure, first by the single primer amplification technique (SPAT) (37), then by a seminested PCR using the combination of MelV genome segment-specific primers and the adaptor-specific primer used in the single primer amplification technique. Phylogenetic trees were constructed by using the neighbor-joining algorithm with bootstrap values determined by 1,000 replicates in the MEGA3 software package (38).
Serological Survey.
The panel of human sera collected during an investigation of the potential risk of bat-to-human transmission of NiV (25) was used in this study. The original panel consisted of 153 serum samples collected from adult residents (mean age 38 ± 15 years) of Tioman Island, which represented 8% of the total adult population on the island. Due to the supply shortage and poor quality of some serum samples, only 109 sera were tested in this study. The sera were heat-inactivated by incubation at 56°C for 30 min before being tested for MelV- or PulV-specific antibodies by using the virus-neutralization test described above.
Supplementary Material
Acknowledgments
We thank C. T. Tan, V. H. T. Chong, K. T. Wong, and K. J. Goh for their permission to use the panel of human sera collected from Tioman Island; G. Smith for providing the MRV prototype viruses; K. McPhie for providing MRV sera; K. Selleck for technical assistance; and T. Pye and E. Hansson for help with DNA sequencing.
Abbreviations
- CPE
cytopathic effect
- MDCK
Madin–Darby canine kidney
- MelV
Melaka virus
- MRV
mammalian orthoreoviruses
- NBV
Nelson Bay orthoreovirus
- NiV
Nipah virus
- PulV
Pulau virus
- RTI
respiratory tract illness
- SARS
severe acute respiratory syndrome
- TCID50
tissue culture 50% infective dose.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession nos. EF026043–EF026046).
This article contains supporting information online at www.pnas.org/cgi/content/full/0701372104/DC1.
References
- 1.Peiris JSM, Guan Y, Yuen KY. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B, et al. Nat Med. 2004;28:444–445. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BH, Poon RW, Cai JJ, Luk WK, et al. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nibert ML, Schiff LA. In: Fields Virology. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1679–1728. [Google Scholar]
- 5.Mertens PPC, Duncan R, Attoui H, Dermody TS. In: Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. San Diego: Elsevier Academic; 2005. pp. 447–454. [Google Scholar]
- 6.Duncan R. Virology. 1999;260:316–328. doi: 10.1006/viro.1999.9832. [DOI] [PubMed] [Google Scholar]
- 7.Chappell JD, Duncan R, Mertens PPC, Dermody TS. In: Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. San Diego: Elsevier Academic; 2005. pp. 455–465. [Google Scholar]
- 8.Tyler KL. In: Fields Virology. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1729–1745. [Google Scholar]
- 9.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Clin Microbiol Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, et al. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 11.Eaton BT, Broder CC, Middleton D, Wang L-F. Nat Rev Microbiol. 2006;4:23–35. doi: 10.1038/nrmicro1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, et al. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 13.Philbey AW, Kirkland PD, Ross AD, Davis RJ, Gleeson AB, Love RJ, Daniels PW, Gould AR, Hyatt AD. Emerg Infect Dis. 1998;4:269–271. doi: 10.3201/eid0402.980214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chant K, Chan R, Smith M, Dwyer DE, Kirkland P. Emerg Infect Dis. 1998;4:273–275. doi: 10.3201/eid0402.980215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chua KB, Wang L-F, Lam SK, Crameri G, Yu M, Wise T, Boyle D, Hyatt AD, Eaton BT. Virology. 2001;283:215–229. doi: 10.1006/viro.2000.0882. [DOI] [PubMed] [Google Scholar]
- 16.Speare R, Skerratt L, Foster R, Berger L, Hooper P, Lunt R, Blair D, Hansman D, Goulet M, Cooper S. Commun Dis Intell. 1997;21:117–120. doi: 10.33321/cdi.1997.21.25. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, et al. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 18.Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH, Wong SS, Leung SY, Chan KH, Yuen KY. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Delicat A, Paweska JT, Gonzalez JP, Swanepoel R. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 20.Gard GP, Marshall ID. Arch Virol. 1973;43:34–42. doi: 10.1007/BF01249346. [DOI] [PubMed] [Google Scholar]
- 21.Gard GP, Compans RW. J Virol. 1970;6:100–106. doi: 10.1128/jvi.6.1.100-106.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chua KB. Microbes Infect. 2003;5:487–490. doi: 10.1016/s1286-4579(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard LI, Chua KB, Cummins D, Hyatt AD, Crameri GS, Eaton BT, Wang L-F. Arch Virol. 2006;151:229–239. doi: 10.1007/s00705-005-0644-4. [DOI] [PubMed] [Google Scholar]
- 24.Duncan R, Corcoran J, Shou J, Stoltz D. Virology. 2004;319:131–140. doi: 10.1016/j.virol.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Chong HT, Tan CT, Goh KJ, Lam SK, Chua KB. Neurol J Southeast Asia. 2003;8:31–34. [Google Scholar]
- 26.Cleaveland S, Laurenson MK, Taylor LH. Philos Trans R Soc London B. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua KB, Chua BH, Wang CW. Malays J Pathol. 2002;24:15–21. [PubMed] [Google Scholar]
- 28.Childs JE. Arch Virol Suppl. 2004;18:1–11. doi: 10.1007/978-3-7091-0572-6_1. [DOI] [PubMed] [Google Scholar]
- 29.Woolhouse MEJ, Gowtage-Sequeria S. Emerg Infect Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negrete OA, Levroney EL, Aguilar HC, Bertolotti-Ciarlet A, Nazarian R, Tajyar S, Lee B. Nature. 2005;436:401–405. doi: 10.1038/nature03838. [DOI] [PubMed] [Google Scholar]
- 31.Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungall BA, Bishop KA, Choudhry V, Dimitrov DS, Wang L-F, Eaton BT, Broder CC. Proc Natl Acad Sci USA. 2005;102:10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drescher U. Curr Opin Genet Dev. 2002;12:397–402. doi: 10.1016/s0959-437x(02)00316-7. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 35.Wang L-F, Shi Z, Zhang S, Field H, Daszak P, Eaton BT. Emerg Infect Dis. 2006;12:1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King DA, Peckham C, Waage JK, Brownile J, Woolhouse MEJ. Science. 2006;313:1392–1393. doi: 10.1126/science.1129134. [DOI] [PubMed] [Google Scholar]
- 37.Attoui H, Billoir F, Cantaloube JF, Biagini P, de Micco P, de Lamballerie X. J Virol Methods. 2000;89:147–158. doi: 10.1016/s0166-0934(00)00212-3. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Tamura K, Nei M. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.