Abstract
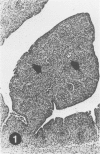
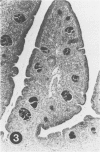
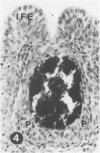
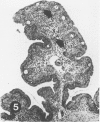
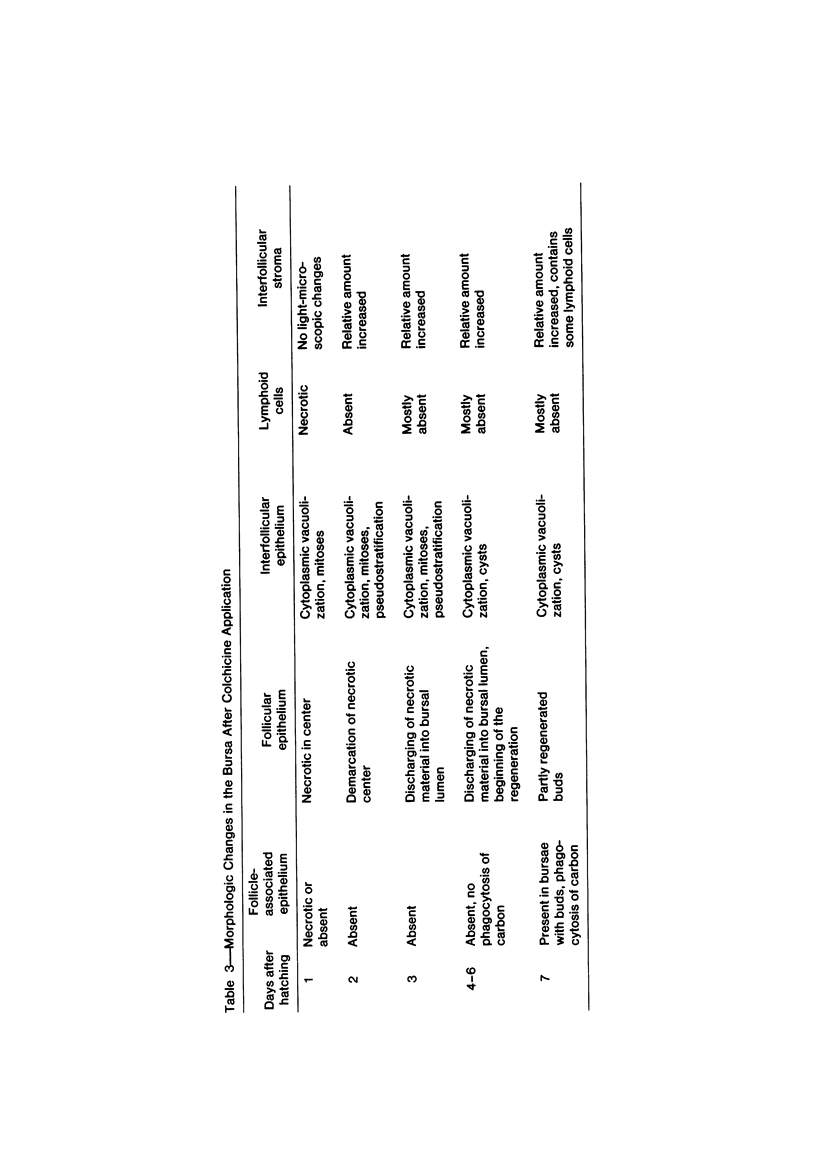
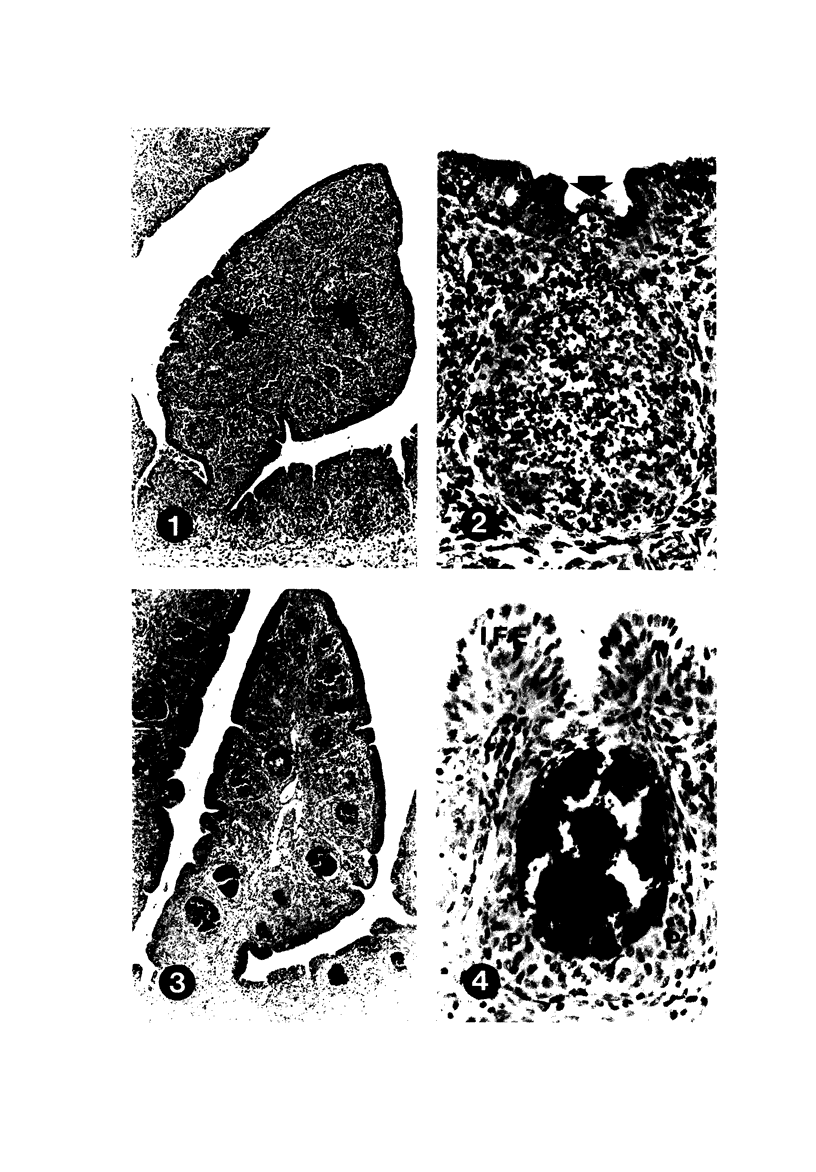
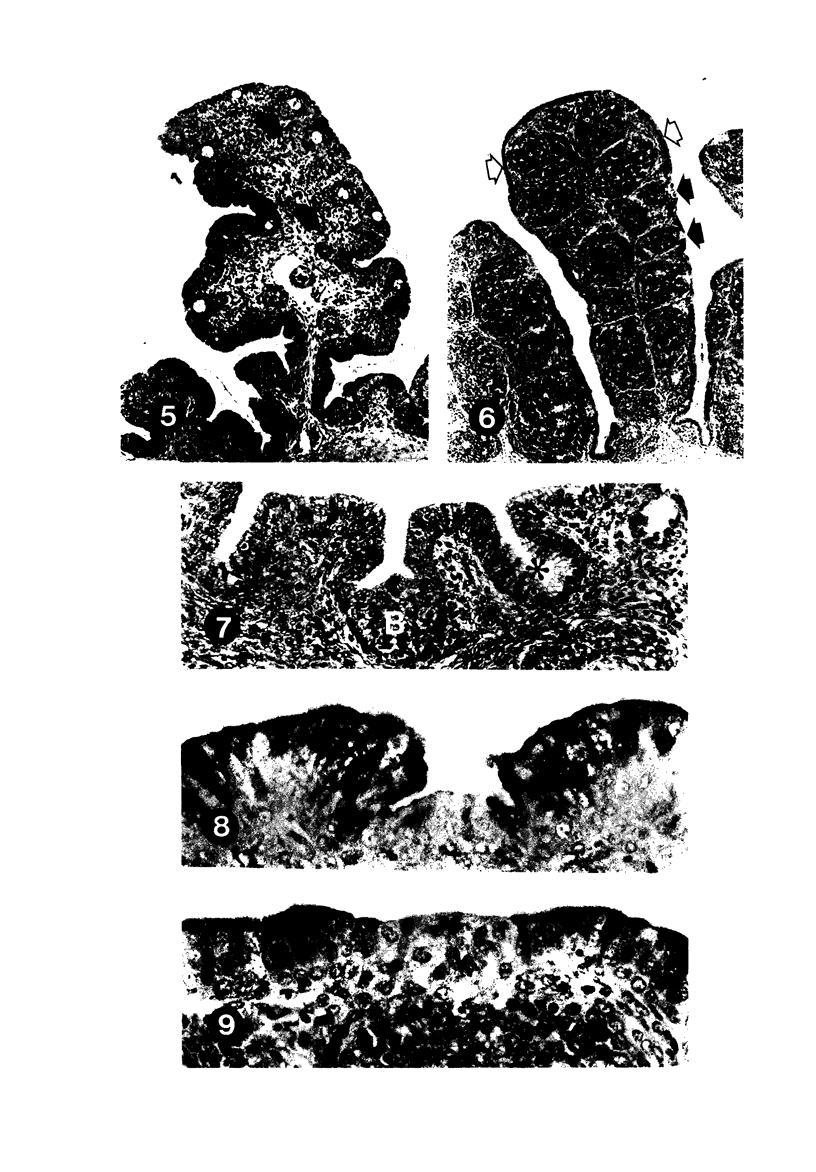
To induce chemical bursectomy, 30 microliter colchicine dissolved in saline solution (1 mg/ml) was applied on the anal lips of White Leghorn chickens once daily for four consecutive days after hatching. Histologic characteristics of the bursa of Fabricius, spleen, thymus, cecal tonsils, and rectal wall were studied 1-7 days after hatching. Total necrosis of the lymphoid cells and the follicle-associated epithelium in the bursa was observed during the four days of colchicine application. The bursal stroma remained unchanged, and only minor changes were found in the interfollicular surface epithelium. After colchicine application ceased, some regeneration of the epithelium, as evidenced by small epithelial buds, was found. At the end of the observation period the epithelial buds were often covered by the follicle-associated epithelium, which was capable of phagocytizing carbon. However, practically no lymphoid repopulation was seen in the buds. Since this method of colchicine application had no direct effect on other lymphoid organs or on the survival or weight of the chickens, this bursectomy model seems to be a new tool for use in studies of bursal function.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMAN G. A., KNOUFF R. A. Testosterone suppression of mesenchymal alkaline phosphatase activity and lympho-epithelial nodule formation in the bursa of Fabricius in the embryonic chick. Anat Rec. 1963 May;146:23–27. doi: 10.1002/ar.1091460105. [DOI] [PubMed] [Google Scholar]
- Ahlqvist J., Anderson L. Methyl green-pyronin staining: effects of fixation; use in routine pathology. Stain Technol. 1972 Jan;47(1):17–22. doi: 10.3109/10520297209116529. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Raymond D. A., Peterson R. D., South M. A., Good R. A. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966 Jan 1;123(1):75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskola J., Ruuskanen O., Fräki J. E., Viljanen M. K., Toivanen A. Alkaline phosphatase in the developing bursa of Fabricius. A comparative study of the cyclophosphamide- and testosterone-induced immunodeficiencies in the chick embryo. Scand J Immunol. 1977;6(3):185–194. doi: 10.1111/j.1365-3083.1977.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Eskola J., Toivanen A. Cell transplantation into immunodeficient chicken embryos: reconstituting capacity of cells from the bursa of fabricius, spleen, bone marrow, thymus, and liver of 18-day-old embryos. Cell Immunol. 1976 Sep;26(1):68–77. doi: 10.1016/0008-8749(76)90348-8. [DOI] [PubMed] [Google Scholar]
- Eskola J., Toivanen P. Effect of in ovo treatment with cyclophosphamide on lymphoid system in chicken. Cell Immunol. 1974 Sep;13(3):459–471. doi: 10.1016/0008-8749(74)90265-2. [DOI] [PubMed] [Google Scholar]
- Glick B. Immunity studies in testosterone propionate injected chicks. Int Arch Allergy Appl Immunol. 1970;38(1):93–103. doi: 10.1159/000230262. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Cooper M. D. Development and distribution of immunoglobulin-containing cells in the chicken. An immunofluorescent analysis using purified antibodies to mu, gamma and light chains. J Immunol. 1971 Feb;106(2):371–382. [PubMed] [Google Scholar]
- Kincade P. W., Lawton A. R., Bockman D. E., Cooper M. D. Suppression of immunoglobulin G synthesis as a result of antibody-mediated suppression of immunoglobulin M synthesis in chickens. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1918–1925. doi: 10.1073/pnas.67.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman S. P., Weidanz W. P. The effect of cyclophosphamide on the ontogeny of the humoral immune response in chickens. J Immunol. 1970 Sep;105(3):614–619. [PubMed] [Google Scholar]
- Linna T. J., Frommel D., Good R. A. Effects of early cyclophosphamide treatment on the development of lymphoid organs and immunological functions in the chickens. Int Arch Allergy Appl Immunol. 1972;42(1):20–39. doi: 10.1159/000230590. [DOI] [PubMed] [Google Scholar]
- MEYER R. K., RAO M. A., ASPINALL R. L. Inhibition of the development of the bursa of Fabricius in the embryos of the common fowl by 19-nortestosterone. Endocrinology. 1959 Jun;64(6):890–897. doi: 10.1210/endo-64-6-890. [DOI] [PubMed] [Google Scholar]
- Naukkarinen A., Arstila A. U., Sorvari T. E. Morphological and functional differentiation of the surface epithelium of the bursa Fabricii in chicken. Anat Rec. 1978 Aug;191(4):415–432. doi: 10.1002/ar.1091910403. [DOI] [PubMed] [Google Scholar]
- Olah I., Glick B., McCorkle F., Stinson R. Light and electron microscope structure of secretory cells in the medulla of bursal follicles of normal and cyclophosphamide treated chickens. Dev Comp Immunol. 1979 Winter;3(1):101–115. doi: 10.1016/s0145-305x(79)80010-5. [DOI] [PubMed] [Google Scholar]
- Olah I., Glick B. Secretory cell in the medulla of the bursa of Fabricius. Experientia. 1978 Dec 15;34(12):1642–1643. doi: 10.1007/BF02034727. [DOI] [PubMed] [Google Scholar]
- Schaffner T., Herring J., Gerber H., Cottier H. Bursa of Fabricus: uptake of radioactive particles and radiotoxic "sealing" of bursal follicles. Adv Exp Med Biol. 1976;66:33–39. doi: 10.1007/978-1-4613-4355-4_5. [DOI] [PubMed] [Google Scholar]
- Schaffner T., Mueller J., Hess M. W., Cottier H., Sordat B., Ropke C. The bursa of Fabricius: a central organ providing for contact between the lymphoid system and intestinal content. Cell Immunol. 1974 Aug;13(2):304–312. doi: 10.1016/0008-8749(74)90247-0. [DOI] [PubMed] [Google Scholar]
- Sorvari R., Naukkarinen A., Sorvari T. E. Anal sucking-like movements in the chicken and chick embryo followed by the transportation of environmental material to the bursa of Fabricius, caeca and caecal tonsils. Poult Sci. 1977 Sep;56(5):1426–1429. doi: 10.3382/ps.0561426. [DOI] [PubMed] [Google Scholar]
- Sorvari R., Sorvari T. E. Bursa Fabricii as a peripheral lymphoid organ. Transport of various materials from the anal lips to the bursal lymphoid follicles with reference to its immunological importance. Immunology. 1977 Apr;32(4):499–505. [PubMed] [Google Scholar]
- Sorvari T., Sorvari R., Ruotsalainen P., Toivanen A., Toivanen P. Uptake of environmental antigens by the bursa of Fabricius. Nature. 1975 Jan 17;253(5488):217–219. doi: 10.1038/253217a0. [DOI] [PubMed] [Google Scholar]
- Sorvari T., Toivanen A., Toivanen P. Transplantation of bursal stem cells into cyclophosphamide-treated chicks. Redevelopment of bursal follicles. Transplantation. 1974 Jun;17(6):584–592. doi: 10.1097/00007890-197406000-00007. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A. Bursal and postbursal stem cells in chicken. Functional characteristics. Eur J Immunol. 1973 Sep;3(9):585–595. doi: 10.1002/eji.1830030912. [DOI] [PubMed] [Google Scholar]
- WARNER N. L., SZENBERG A., BURNET F. M. The immunological role of different lymphoid organs in the chicken. I. Dissociation of immunological responsiveness. Aust J Exp Biol Med Sci. 1962 Oct;40:373–387. doi: 10.1038/icb.1962.42. [DOI] [PubMed] [Google Scholar]