Abstract
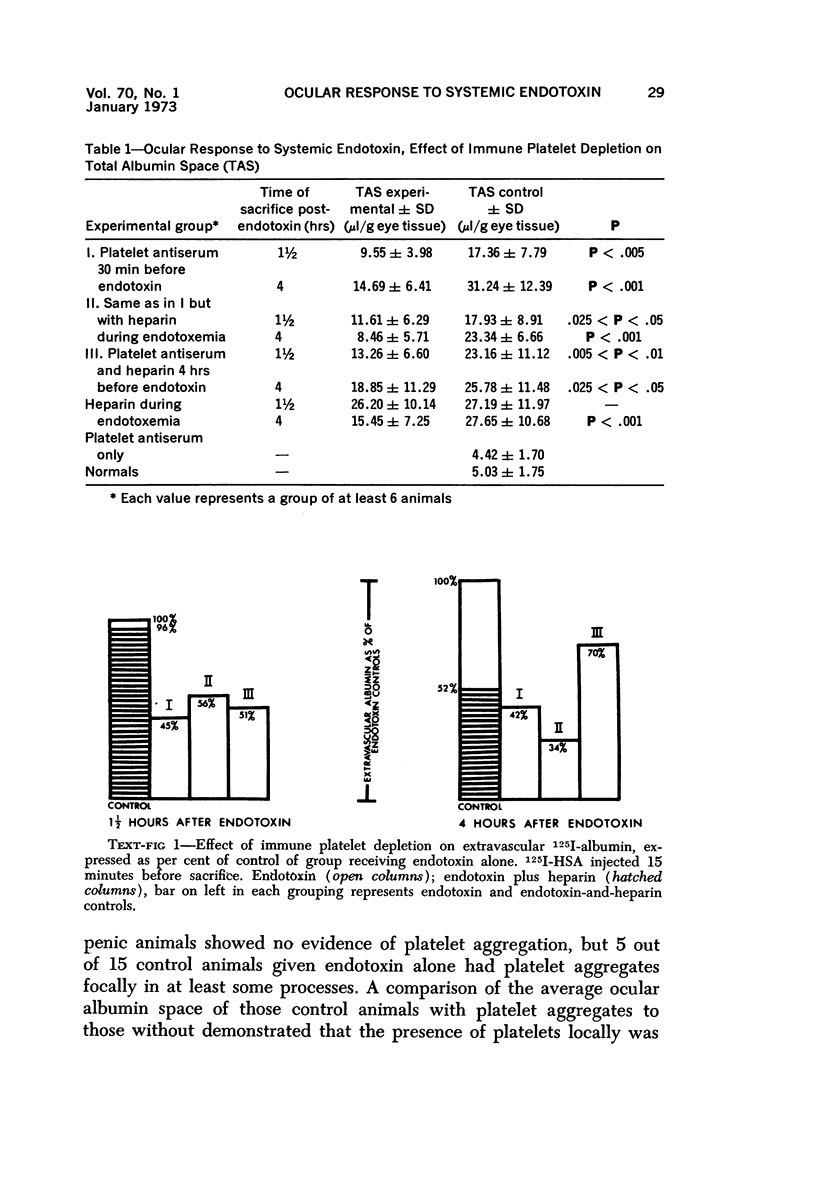
Platelet depletion by a specific goat antirabbit platelet antiserum profoundly affected the response of the ocular blood vessels to intravenous endotoxin. The altered permeability of the vessels of the iridial portion of the ciliary processes in the thrombocytopenic animals was reduced by 50% at 1½ hours and by 30 to 58% at 4 hours after endotoxin administration. Intravascular fibrin at the 4-hour period could be eliminated by a properly timed platelet depletion. By preventing platelet aggregations and fibrin formation, the permeability alteration could be reduced by 66% and the usual stromal hemorrhages practically eliminated. Underlying these effects of the platelet was evidence of a vascular trauma unrelated to platelet activity and probably dependent to some extent on adrenergic stimulation. Platelet aggregation and/or fibrin formation had to be superimposed on the underlying vascular trauma to produce endothelial denudation.
Full text
PDF












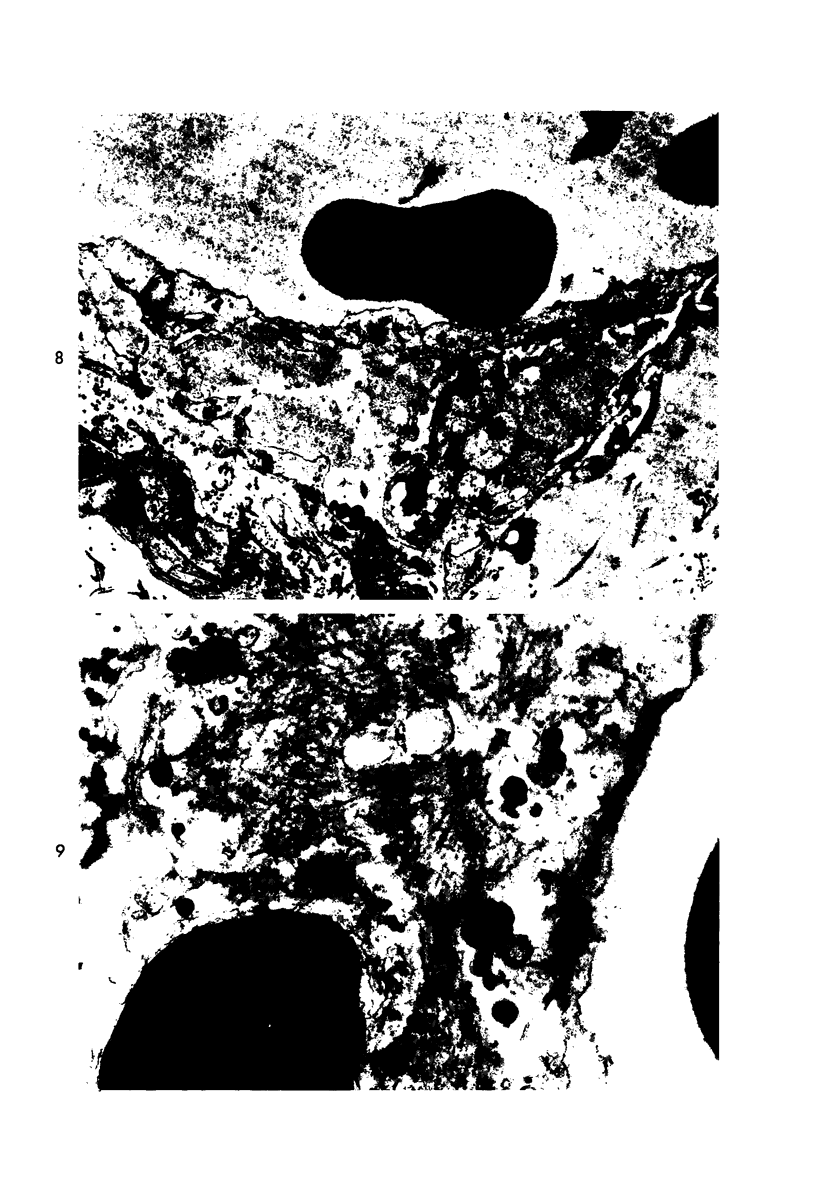
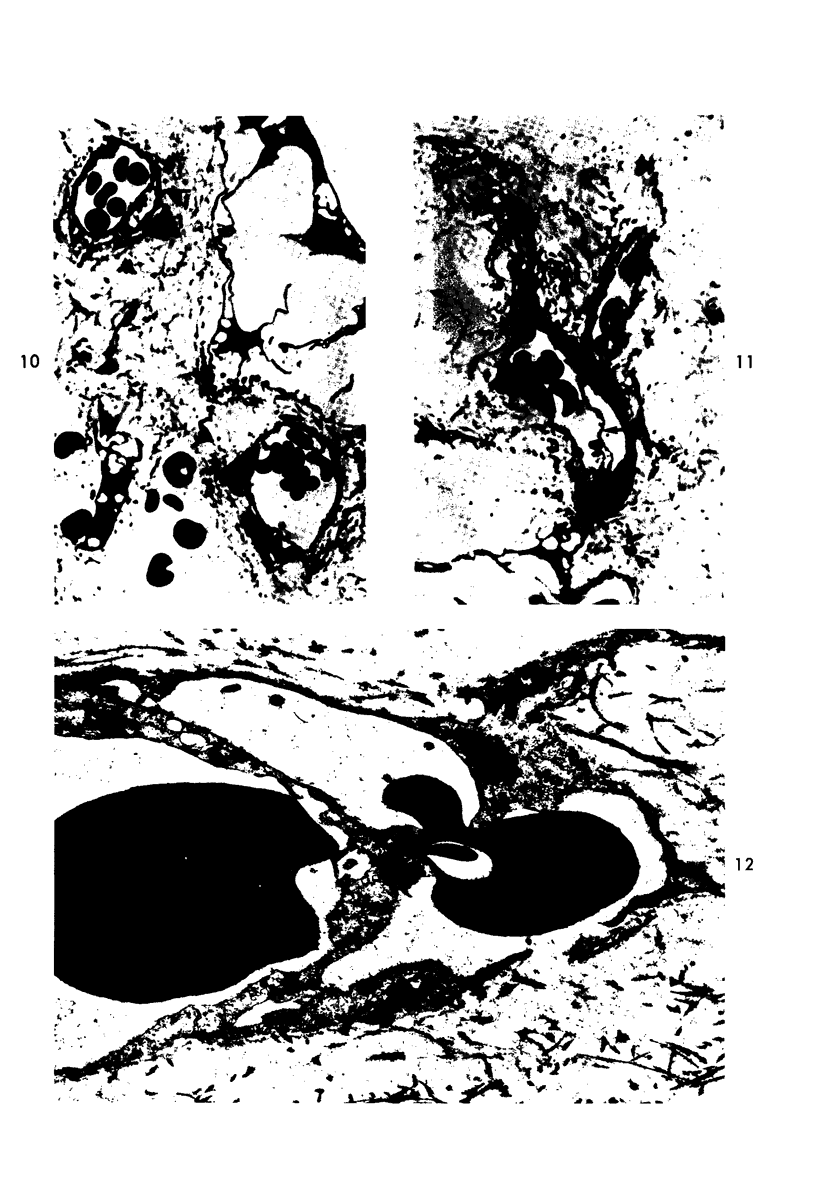

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane C. G. Mechanisms involved in the deposition of immune complexes in tissues. J Exp Med. 1971 Sep 1;134(3 Pt 2):75s–89s. [PubMed] [Google Scholar]
- DAVIS R. B., MEEKER W. R., Jr, BAILEY W. L. Serotonin release by bacterial endotoxin. Proc Soc Exp Biol Med. 1961 Dec;108:774–776. doi: 10.3181/00379727-108-27063. [DOI] [PubMed] [Google Scholar]
- DES PREZ R. M., HOROWITZ H. I., HOOK E. W. Effects of bacterial endotoxin on rabbit platelets. I. Platelet aggregation and release of platelet factors in vitro. J Exp Med. 1961 Dec 1;114:857–874. doi: 10.1084/jem.114.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Prez R. M. The effects of bacterial endotoxin on rabbit platelets. V. Heat labile plasma factor requirements of endotoxin-induced platelet injury. J Immunol. 1967 Nov;99(5):966–973. [PubMed] [Google Scholar]
- Fish M. B., Aronson S. B., Pollycove M., Coon M. A. Ocular blood volume. Arch Ophthalmol. 1969 Sep;82(3):377–380. doi: 10.1001/archopht.1969.00990020379016. [DOI] [PubMed] [Google Scholar]
- Fong J. S., Good R. A. Prevention of the localized and generalized Shwartzman reactions by an anticomplementary agent, cobra venom factor. J Exp Med. 1971 Sep 1;134(3 Pt 1):642–655. doi: 10.1084/jem.134.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E. Increased mitotic activity in rabbit endothelium after endotoxin. An autoradiographic study. Lab Invest. 1971 Apr;24(4):318–320. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- HOROWITZ H. I., DES PREZ R. M., HOOK E. W. Effects of bacterial endotoxin on rabbit platelets. II. Enhancement of platelet factor 3 activity in vitro and in vivo. J Exp Med. 1962 Nov 1;116:619–633. doi: 10.1084/jem.116.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes E. L., Jr, Aronson S. B., McKay D. G. Ocular vascular permeability. Effect of systemic administration of bacterial endotoxin. Arch Ophthalmol. 1970 Sep;84(3):360–367. doi: 10.1001/archopht.1970.00990040362017. [DOI] [PubMed] [Google Scholar]
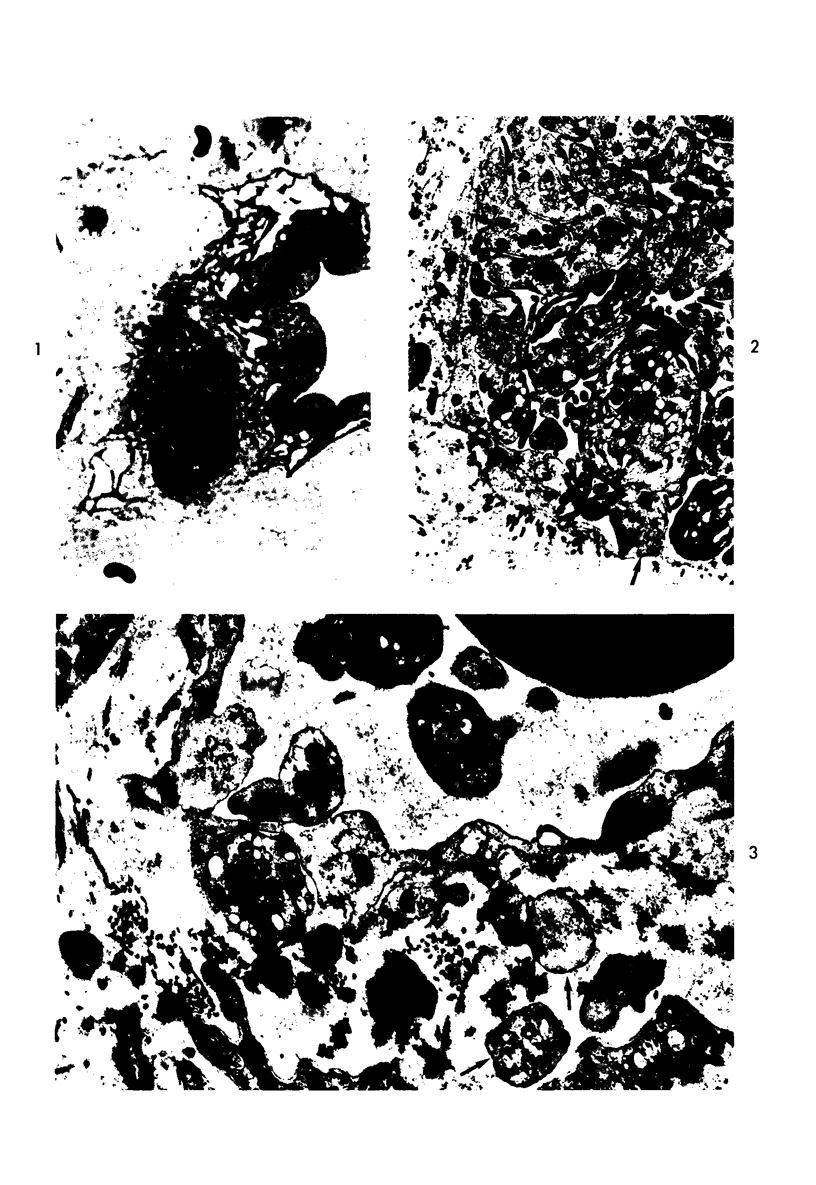
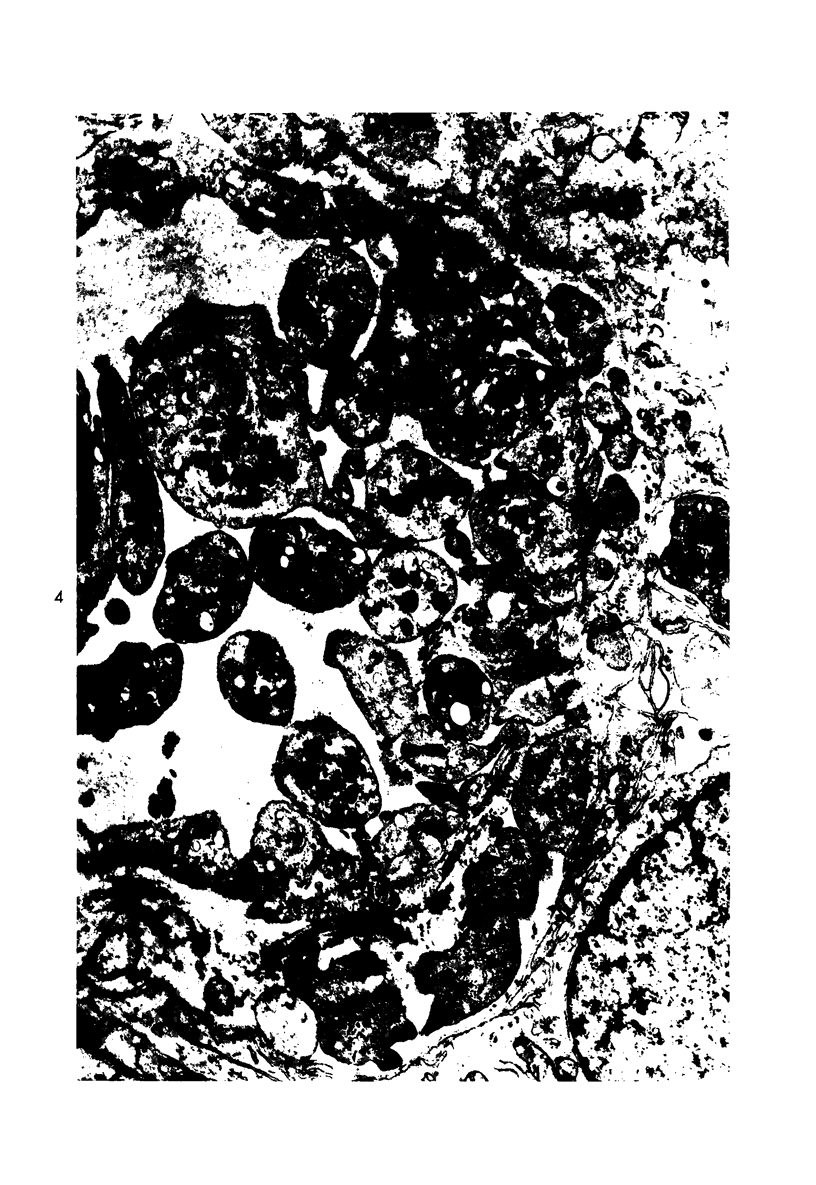
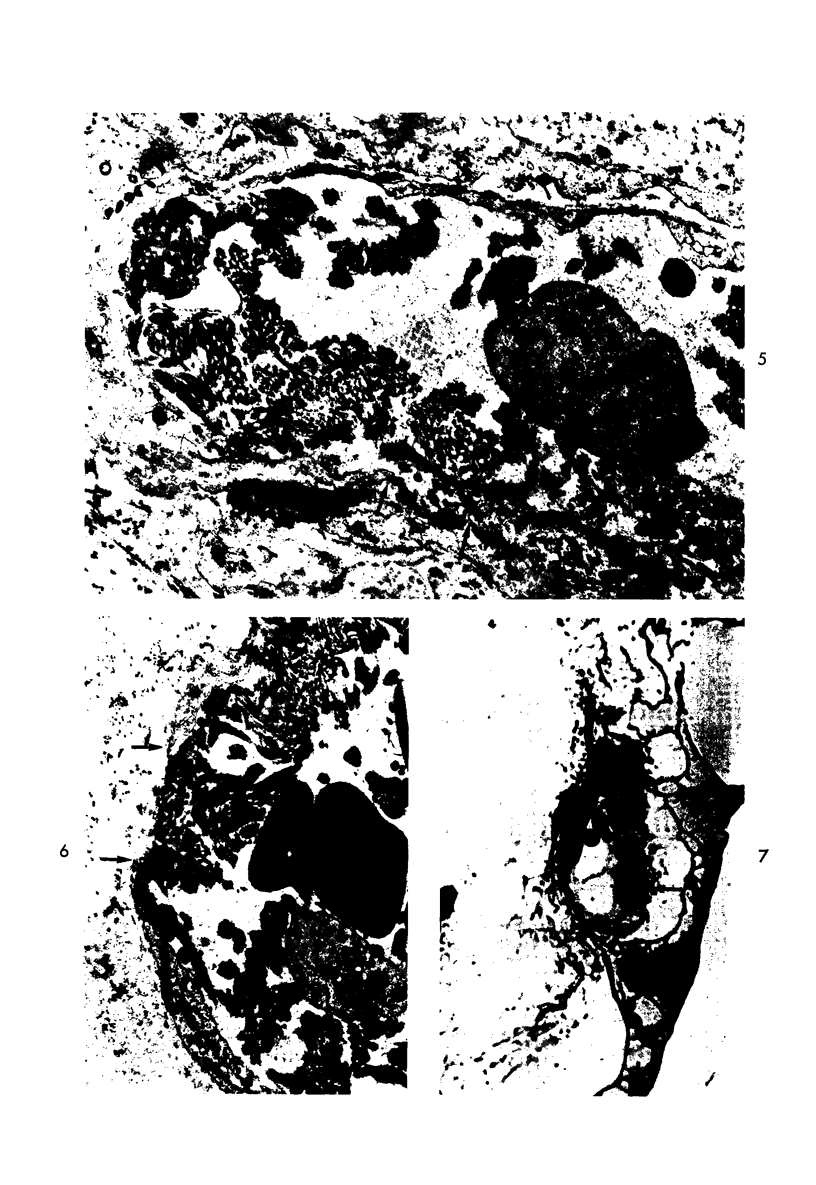
- Howes E. L., Jr, McKay D. G., Aronson S. B. An ultrastructural study of the ciliary process in the rabbit following systemic administration of bacterial endotoxin. Lab Invest. 1971 Mar;24(3):217–228. [PubMed] [Google Scholar]
- Howes E. L., Jr, McKay D. G. Effect of cervical sympathectomy on the ocular response to systemic endotoxin. Proc Soc Exp Biol Med. 1972 Mar;139(3):839–844. doi: 10.3181/00379727-139-36249. [DOI] [PubMed] [Google Scholar]
- Jorgensen L., Hovig T., Rowsell H. C., Mustard J. F. Adenosine diphosphate-induced platelet aggregation and vascular injury in swine and rabbits. Am J Pathol. 1970 Nov;61(2):161–176. [PMC free article] [PubMed] [Google Scholar]
- Kozart D. M. Light and electron microscopic study of regional morphologic differences in the processes of the ciliary body in the rabbit. Invest Ophthalmol. 1968 Feb;7(1):15–33. [PubMed] [Google Scholar]
- LEVIN J., CLUFF L. E. PLATELETS AND THE SHWARTZMAN PHENOMENON. J Exp Med. 1965 Feb 1;121:235–246. doi: 10.1084/jem.121.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour J. G., McKay D. G. Requirement of the adrenal glands for provocation of the generalized Shwartzman reaction. Lab Invest. 1970 Apr;22(4):281–285. [PubMed] [Google Scholar]
- Margaretten W., McKay D. G. The effect of leukocyte antiserum on the generalized Shwartzman reaction. Am J Pathol. 1969 Nov;57(2):299–305. [PMC free article] [PubMed] [Google Scholar]
- Margaretten W., McKay D. G. The requirement for platelets in the active Arthus reaction. Am J Pathol. 1971 Aug;64(2):257–270. [PMC free article] [PubMed] [Google Scholar]
- Margaretten W., McKay D. G. The role of the platelet in the generalized Shwartzman reaction. J Exp Med. 1969 Mar 1;129(3):585–590. doi: 10.1084/jem.129.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. M., Stewart G. J. The effects of endotoxin on vascular endothelium. J Exp Med. 1969 May 1;129(5):833–848. doi: 10.1084/jem.129.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. G., Margaretten W., Csavossy I. An electron microscope study of the effects of bacterial endotoxin on the blood-vascular system. Lab Invest. 1966 Dec;15(12):1815–1829. [PubMed] [Google Scholar]
- McKay D. G., Müller-Berghaus G., Cruse V. Activation of Hageman factor by ellagic acid and the generalized Shwartzman reaction. Am J Pathol. 1969 Mar;54(3):393–420. [PMC free article] [PubMed] [Google Scholar]
- Moore S., Lough J. Lipid accumulation in renal arterioles due to platelet aggregate embolism. Am J Pathol. 1970 Feb;58(2):283–293. [PMC free article] [PubMed] [Google Scholar]
- Müller-Berghaus G., McKay D. G. Prevention of the generalized Shwartzman reaction in pregnant rats by alpha-adrenergic blocking agents. Lab Invest. 1967 Sep;17(3):276–280. [PubMed] [Google Scholar]
- Nachman R. L., Weksler B., Ferris B. Increased vascular permeability produced by human platelet granule cationic extract. J Clin Invest. 1970 Feb;49(2):274–281. doi: 10.1172/JCI106237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med. 1951 May;93(5):489–504. doi: 10.1084/jem.93.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H. M., Moore S., Merrick H. W., Smith M. R. Platelets in early hyperacute allograft rejection in kidneys and their modification by sulfinpyrazone (Anturan) therapy. An experimental study. Am J Pathol. 1972 Mar;66(3):445–460. [PMC free article] [PubMed] [Google Scholar]
- Silver M. D., Stehbens W. E. The behaviour of platelets in vivo. Q J Exp Physiol Cogn Med Sci. 1965 Jul;50(3):241–247. doi: 10.1113/expphysiol.1965.sp001788. [DOI] [PubMed] [Google Scholar]
- Spielvogel A. R. An ultrastructural study of the mechanisms of platelet-endotoxin interaction. J Exp Med. 1967 Aug 1;126(2):235–250. doi: 10.1084/jem.126.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]