Abstract
Studies in humans suggest that allo-immunization induces CC-chemokines, CD8-suppressor factors (SF) and anti-HIV immunity. Here we report that allo-immunization with unmatched leucocytes from partners of women with recurrent spontaneous abortion elicits specific antibodies to the CCR5 receptor. Such antibodies inhibit replication of M-tropic HIV-1 (R5) and MIP-1β-mediated chemotaxis. These CCR5 antibodies were also found in the sera of multiparous women that were naturally immunized by semi-allogeneic fetal antigens. The specificity of these antibodies was demonstrated by adsorption with CCR5 transfected HEK-293 cells, a baculovirus CCR5 preparation and a peptide of the 2nd extra-cellular loop of CCR5. Allo-immunization also stimulated increased concentrations of the CXC chemokine, SDF-1α and CD8-SF that inhibit T-tropic HIV-1 (X4) replication. We suggest that allo- immunization may elicit (a) CC chemokines, CCR5 antibodies and CD8-SF that inhibit M-tropic HIV-1 infection and (b) the CXC chemokine SDF-1α and CD8-SF that inhibit T-tropic HIV-1 infection
Keywords: allo-immunization, HIV-1, chemokines, CCR5 antibodies, recurrent spontaneous abortion
INTRODUCTION
The CCR5 molecule, expressed on the cell surface of T cells, monocytes and immature dendritic cells (DC), has been subverted by R5 HIV to serve as a major coreceptor in initiating HIV infection [1,2]. CC chemokines bind and downmodulate CCR5 both in vitro[3] and in vivo[4]. Antibodies to the CCR5 receptor have been reported in macaques following immunization with either human cells [5,6], a CCR5 preparation or extracellular peptides of CCR5 [7] or DNA of CCR5 [8]. It is of interest that CCR5 antibodies have been reported in 12·5% of HIV exposed but uninfected women [9]. CCR5 antibodies have also been found in 2 subjects who have a homozygous 32 b.p. deletion in CCR5 and do not express cell surface CCR5 [10]. Thus, protection from HIV infection has been associated with CCR5 antibodies in (a) humans with a homozygous CCR5 mutation (b) women who remain sero-negative, though exposed to HIV through sexual contact, and (c) in xenoimmunized macaques challenged by SIV (simian immuno-deficiency virus). In all 3 instances there are additional protective mechanisms, but especially significant is a rise in the levels of 3 CC chemokines – RANTES, MIP-1α and MIP-1β which bind CCR5 [11–13]. Furthermore, a complementary inhibitory effect on HIV replication has been demonstrated between CC chemokines and antibodies to the extracellular domains of CCR5 in vitro and in vivo[7].
In our earlier investigation we found increased concentrations of the CC chemokines RANTES, MIP-1α and MIP-1β and downmodulation of cell-surface expression of CCR5 and CXCR4 (14). There was also a significant decrease in vitro in both R5 and X4 HIV infectivity of CD4+ T cells and an increase in CD8+ T cell-derived HIV suppressor factors (SF) after allo-immunization in vivo (14). The objectives of this investigation were to find out if allo-immunization in women with recurrent spontaneous abortion (RSA) stimulates the production of stromal derived factor-1α (SDF-1α) which would account for the downmodulation of cell-surface CXCR4, as has been demonstrated in vitro[3,15]. Furthermore, the relative ease of eliciting antibodies to CCR5 raised the possibility that direct immunization with unmatched leucocytes or by semi-allogeneic fetal antigens in multiparous women might break tolerance and elicit autoantibodies to CCR5. Indeed, a significant increase in CD8+ T-cell-derived SDF-1α and serum antibodies to the extracellular domains of CCR5 were found in women alloimmunized with their partner's mononuclear cells. An increase in antibodies to CCR5 was also found in HLA alloimmune typing sera collected from multiparous women.
MATERIALS AND METHODS
Collection of sera from 3 cohorts
Sera were collected from 3 cohorts: (a) women with recurrent spontaneous abortion (RSA) were from the series reported before [14]. Blood was taken from seven of these women before and after allo-immunization with about 370 × 106 PBMC from their partners; (b) Seven selected allo-antisera used for HLA typing were made available by courtesy of the UK Transplant Support Services Authority; (c) Control sera were collected from 13 healthy non-parous and 6 multiparous age and sex matched subjects.
Preparation of CCR5 in baculovirus
Baculovirus (Autographa californica) nuclear polyhedrosis virus was used to express the protein in an insect cell line –Spondoptera frugiperda[16]. Briefly, high titre (109 PFU per ml) baculovirus stock expressing CCR5 was used to infect insect cells for 2 days, at 28°C. A recombinant baculovirus expressing CCR5 was prepared by using the full length gene of human CCR5 which was PCR cloned from pDNA 3·1 CCR5 DNA (kindly provided by Dr John Moore) into the baculovirus transfer vector pAChis. The DNA construct was then used to form baculovirus expressing CCR5+ 6 HIS as described earlier for SIV gp120 [17]. Expression of the gene was confirmed by PCR and then by immunostaining of the infected cells. A cell lysate of this preparation and a control baculovirus lysate were used.
Preparation of peptides derived from the sequence of the four extracellular domains of CCR5
The four peptides and a random unrelated 20mer were purchased from Neosystem Laboratories (Strasbourg, France). The sequences of the peptides are shown in Table 1.
Table 1.
Peptides derived from the extracellular domains of CCR5.
| N-terminal | (aa 1–20): | Met – Asp – Tyr – Gln – Val – Ser – Ser – Pro – Ile – Tyr – Asp – Ile – Asp – Tyr – Tyr – Thr – Ser – Glu – Pro – Cys |
| 1st loop | (aa 89–102): | His – Tyr – Ala – Ala – Ala – Gln – Trp – Asp – Phe – Gly – Asn – Thr – Met – Cys – Gln |
| 2nd loop | (aa178–197): | Cys – Ser – Ser – His – Phe – Pro – Tyr – Ser – Gln – Tyr – Gln – Phe – Trp – Lys – Asn – Phe – Gln – Thr – Leu – Lys |
| 3rd loop | (aa258–279): | Asn – Thr – Phe – Gln – Glu – Phe – Phe – Gly – Leu – Asn – Asn – Cys – Ser – Ser – Asn – Arg – Leu – Asp – Gln |
The overlapping N terminal peptide (11–31) and 2nd loop peptide (168–187) were also synthesized and used in the inhibition studies. The results were similar to the two overlapping peptides but the potency of inhibition was less marked and the results are not presented.
Culture of CCR5 transfected HEK-293 cells
Culture of HEK-293 cell lines with or without the transfected CCR5 was carried out as described elsewhere [18]. In brief, the FLAG-CCR5 sequence was subcloned into pcDNA3 (Invitrogen) and transfected into HEK-293 cells using Lipofectamine according to the manufacturer's instructions. Cells were expanded in the presence of 400 μg/ml G418 in MEM (Gibco, Paisley, UK), supplemented with 10% FCS, 100 μg/ml penicillin and streptomycin and 2 mm glutamine. The expression of CCR5 on HEK-293 was determined by flow cytometry with moAb to CCR5 (2D7).
ELISA for CCR5 antibodies and inhibition assay
Serum IgG was separated by ion exchange chromatography. Serum IgG antibodies to CCR5 and its extracellular peptides were assayed by ELISA as described previously [6]. Briefly plates were coated with a predetermined optimal concentration of antigen (1 μg/ml) and with a random 20-amino acid peptide (R20) as a control antigen. They were then incubated with doubling dilutions of test samples. Bound antibody was detected by incubation with rabbit IgG antihuman IgG (2 μg; Sigma, Oxford, UK), followed by affinity-purified goat antirabbit IgG-alkaline phosphatase conjugate (Sigma). The IgG antibody titres are presented as reciprocals before and after each immunization. For the competitive inhibition assay the test sera were preincubated with 0, 25, 50 and 100 μg/ml of the CCR5 lysate, baculovirus lysate or peptides in a total volume of 100 μl and applied to the CCR5 coated plates. The results are presented as percent inhibition of antibody binding, with increasing concentrations of inhibitor and a range of antibody titres from 1:800 to 1:25.
Inhibition of HIV replication with serum antibodies
To assay the inhibition of replication by serum, PHA-stimulated human CD4+ cells were incubated with the R5 strain HIV-1BaL (MRC, NIBSC, Potters Bar, UK) in the presence of IgG antibodies at dilutions of 1:10 and 1:100. The cells were then plated in 96-well plates (2 × 105 cells/well) and cultured in 20% IL-2 medium (Lymphocult-T-LF, Biotest, Solihul, UK) containing the serum. The cultures were re-fed at day 2 and day 5 with the same medium, and on day 7 the supernatant of the culture was used in the reverse transcriptase (RT) activity assay. The RT activity was determined by using a Quan-T-RT assay kit (Amersham, UK). The results are presented as the proportion (%) of the control (untreated) HIV culture RT activity of the CD4+ cells.
Inhibition of chemotaxis with serum antibodies
The chemotaxis assay [19] was carried out by using a 6·5-mm diameter, 5 μm pore size polycarbonate Transwell culture insert (Costar, Cambridge, MA, USA) as described before [20]. Briefly, PBMC were suspended at 5 × 106/ml in the assay medium consisting of a 1:1 mixture of RPMI and M199, supplemented with 0·5% of human serum albumin (Sigma); 5 × 105 cells in 100 μl of the medium were added to the filter inserts (top chamber). The chemokine MIP-1β (R & D Systems, Oxford, UK) was diluted in the assay medium, and 600 μl per well of MIP-1β was distributed, in duplicate, into 24-well cluster plates (bottom chamber). The plates were incubated at 37°C in 5% CO2 for 4 h. Following chemotaxis, the cells in the bottom chamber were collected and counted by flow cytometry (Epics Profile II, Coulter, High Wycombe, UK). The effect of antibodies to CCR5 on chemotaxis was determined by incubating PBMC with IgG antibodies at dilutions of 1:10 and 1:100 for 30 min, before adding the cells into the inserts.
Generation of CD8-SF
Generation of CD8-SF from a CD8+ T cell enriched population was carried out according to the method previously described [21,22]. Human PBMC were prepared from heparinized blood by gradient centrifugation on Lympho-Prep (NYCOMED, Oslo, Norway). CD8+ cell populations were enriched by panning, using negative selection. CD4+ cells were removed with anti-CD4 MAb (OKT4 hybridoma culture supernatant), monocytes and B cells with anti-immunoglobulin antibodies (Serotec, Oxford, UK). The enriched CD8+ cells were further separated into CD28+ depleted or CD28− enriched populations by antibodies to CD28 (Cymbus Biotech. UK). CD8+ T cells, CD28+ or CD28− T cells were then stimulated for 3 days with 10 μg/ml PHA (Sigma) in 10% FCS-RPMI medium supplemented with 2 mm glutamine, 100 μg/ml of penicillin and streptomycin. PHA stimulated cells were washed and resuspended at 2 × 106/ml in the 10% FCS-RPMI medium containing 20% IL-2 (Biotest, Solihull, UK). After 2 days incubation at 37°C in 5% CO2, the culture supernatant was collected and the cells were replenished with fresh medium. This procedure was repeated up to 3 times. The collected supernatants were filtered through a 0·45-μm filter and stored at −70°C for use in the CD8-SF activity assay.
Mixed lymphocyte reaction
In vitro stimulation by mixed lymphocyte reaction (MLR) was carried out by culturing PBMC from women before they were immunized with an equal number of irradiated (2500 rads) male partner's PMBC at a concentration of 106/ml in 10% autologous serum, 2 mm glutamine and 100 μg/ml of penicillin and streptomycin. After 7 days of culture the viable cells were separated by density gradient centrifugation by Lympho-Prep, and CD8+ cells were enriched for generation of CD8-SF as described.
Assay for CD8-SF
CD8-SF activity was assayed by inhibition of HIV replication in HIV acutely infected CD4+ cells, infected either with the R5 strain HIV-1Ba-L or the X4 strain HIV-1LAI as described as above. To assay the activity of CD8-SF, 100 μl of CD8+ cell culture supernatant diluted at 1:2 and 1:5 was added at the start of the culture to HIV infected CD4+ cells. As a control CD4+ cells were cultured in medium alone. After incubation for 2 days, 100 μl per well of the culture fluid was removed and replaced with 100 μl per well of diluted CD8+ cell supernatant (1:2 or 1:5) or control medium. On Day 7 the RT activity was determined by Quan-T-RT kits.
Assay for the chemokine SDF-1α
SDF-1α was assayed in the culture supernatants generated by PHA stimulation of CD8+ T cells before and after allo- immunization and from 6 multiparous and 13 non-parous women. Specific ELISA kits (R & D System, Oxon, UK) were used for SDF-1α measurement and the results were expressed in pg/ml.
RESULTS
IgG antibodies to CCR5 detected by ELISA
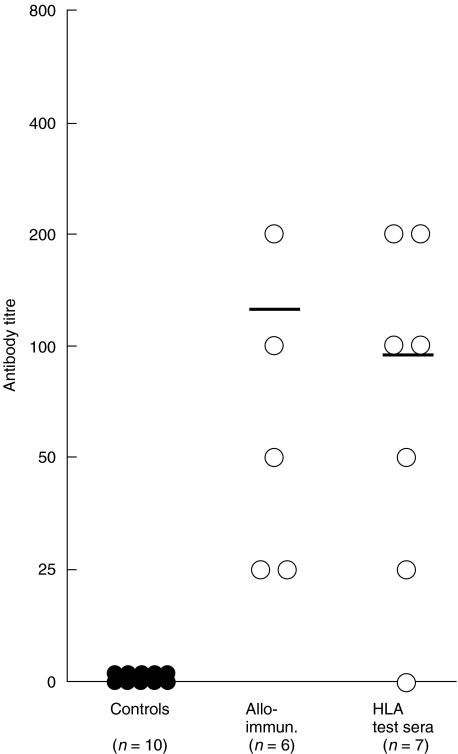
Antibodies to CCR5 were assayed in 7 HLA typing sera, in 6 sera from women allo-immunized with PBMC from their partners as a therapeutic measure for RSA and in sera from 10 healthy control females. IgG antibody titres to CCR5 between 1:25 and 1:400 were found by ELISA in 6 out of 7 allo-immune HLA typing sera selected from multiparous women (Fig. 1). Similar titres of CCR5 antibodies were found after alloimmunization in all 6 women who showed no detectable antibodies before alloimmunization (Fig. 1). No antibodies to CCR5 were found in the 10 healthy controls. Thus, antibodies to CCR5 were detected by virtue of repeated immunization with fetal allo-antigens or after allo-immunization with unmatched PBMC.
Fig. 1.
IgG antibodies to CCR5 in sera from normal controls (n = 10), allo-immunized women (n = 6) and HLA typing sera (n = 7). Serum antibodies were assayed by ELISA. Doubling dilutions of test samples were applied to plates coated with a predetermined optimal concentration of CCR5 antigen preparation (1 μg/ml) and the bound antibodies to CCR5 were detected by secondary rabbit antibody to human IgG, followed by affinity-purified goat antirabbit IgG-alkaline phosphatase conjugate.
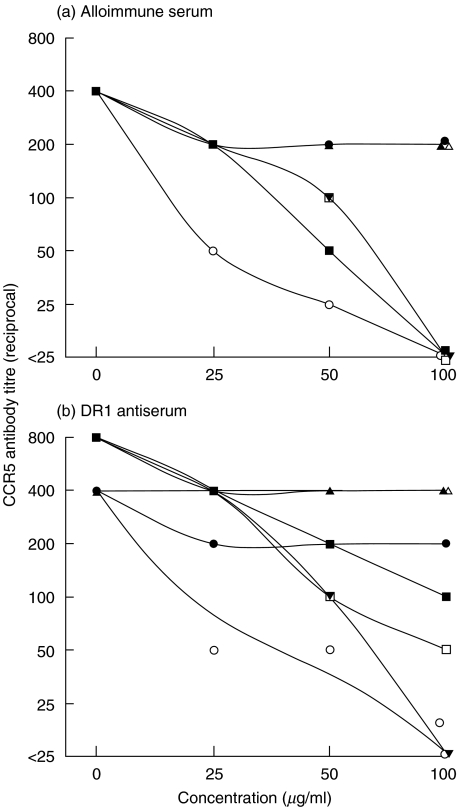
Specificity studies of the CCR5 antibodies were then carried out by inhibition with either CCR5 lysates or peptides from sequences of the extracellular domains of CCR5. Sera from allo-immunized women were adsorbed (from 1:400 to <1:25) with the CCR5 lysate, with the N terminal, 1st and 2nd loop but not with the 3rd loop peptide, an unrelated peptide (R20) nor the control baculovirus lysate (Fig. 2a). The DR1 antiserum from the allo-immune typing sera was tested and this showed a similar pattern of adsorption to that seen with the allo-immune sera i.e. adsorption with the CCR5 lysate and 2nd loop peptide, but only partial adsorption with the N terminal and 1st loop peptides (Fig. 2b). These results suggest that CCR5 auto-antibodies in women allo-immunized directly with unmatched leucocytes recognize CCR5 and the N terminal, 1st and 2nd extracellular loops of CCR5, whilst those allo-immunized during pregnancy recognize predominantly native CCR5, the 2nd extracellular loop and to a lesser degree the N terminal of CCR5.
Fig. 2.
Dose-dependent inhibition of serum antibodies to CCR5 from a representative (a) alloimmune serum and (b) DR1 antiserum, by CCR5 and the peptides derived from its 4 extracellular domains. The test sera were preincubated with 0, 25, 50 and 100 μg/ml of the CCR5 lysate, the control baculovirus lysate, 4 CCR5 or control peptide and applied to the CCR5 coated plates. The results are presented as percent of inhibition of antibody binding, with increasing concentrations of inhibitor. ○ CCR5, • baculovirus, □ N terminal, ▪ 1st loop, ▾ 2nd loop, ▵ 3rd loop, ▴ control.
Functional antibodies to CCR5
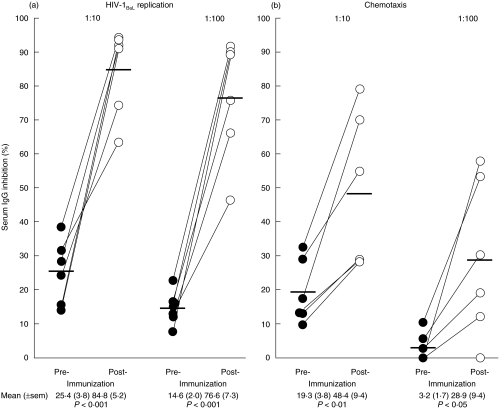
IgG antibodies inhibiting HIV replication (Fig. 3a) were increased in sera (at 1:10) after allo-immunization (mean ± sem, 84·8 ± 5·2%), when compared with sera before immunization (25·4 ± 3·8%) (t = 8·31, P < 0·001, n = 6). Significant inhibition was also detected at a serum dilution of 1:100 (t = 8·80, P < 0·01, n = 6). The specificity of the inhibitory antibodies to CCR5 was tested by inhibition with HEK-293 cells transfected with CCR5, as compared with untransfected HEK-293 cells (Table 2). A decrease in R5 HIV replication was found from a mean (±sem) of 72·0 (±6·2)% in the untreated to 30·8 (±5·4)% in the HEK-293-CCR5 adsorbed sera (Table 2), compared with the HEK-293 cells (64·3 ± 3·8%). Control sera from non-parous women (n = 3) showed no inhibiting antibodies.
Fig. 3.
Inhibition of (a) HIV-1BaL replication and (b) MIP-1β mediated chemotaxis by serum IgG collected before and after allo-immunization. Serum IgG was prepared from 6 subjects before and one month after allo-immunization. The serum IgG titres were tested at 1:10 and 1:100 dilutions for suppression of R5 HIV-1BaL replication in CD4+ cells and inhibition of PBMC chemotaxis mediated by MIP-1β. The results are presented as percent inhibition of viral replication and chemotaxis.
Table 2.
Functional assays of human serum IgG antibodies to CCR5 adsorbed with CCR5 transfected or untransfected HEK-293 cells
| % Inhibition of HIV replication | % Inhibition of chemotaxis | |||||
|---|---|---|---|---|---|---|
| Subject no. | Untreated | 293 | CCR5-293 | Untreated | 293 | CCR5-293 |
| 3637 | 75.9 | 65.7 | 23.1 | 72 | 91 | 52.5 |
| 3590 | 46.4 | 56.6 | 33.6 | 79.3 | 61.4 | 27.8 |
| 3690 | 66.3 | 77.4 | 13.8 | 70.2 | 51.1 | 50.3 |
| 3703 | 64 | 48.6 | 28.0 | ND | ND | ND |
| 3584 | 90.1 | 67.8 | 29.3 | 86.0 | 87.8 | 48.1 |
| 3595 | 89.3 | 69.9 | 56.7 | 55 | 78.6 | 16.9 |
| Mean (+sem) | 72.0 (6.2) | 64.3 (3.8) | 30.8 (5.4) | 72.5(4.6) | 73.9 (6.9) | 39.1 (6.3) |
| 1* | 8.3 | 12.9 | 8.1 | 3 | 6.4 | 36.2 (24.5) |
| 2* | 11.4 | 1.8 | 6.5 | 25.9 | 22.5 | 29.8 |
| 3* | 12.6 | 0 | 16.1 | 32.5 | 31.9 | 33.4 |
| Mean (+sem) | 10.8 (1.3) | 4.9 (4.0) | 10.2 (2.9) | 31.6 (3.1) | 30.2 (4.0) | 29.2 (2.6) |
Control sera from non-parous women showed no inhibiting antibodies.
IgG antibodies inhibiting MIP-1β specific chemotaxis of CCR5+ cells were found in 6 sera from allo-immunized women; 19·3 ± 3·8% before increased to 48·4 ±9·4 % after allo-immunization (t = 4·36, P < 0·01, n = 6) at 1:10 dilution (Fig. 3b). A significant difference was also evident at a dilution of 1:100 (t = 2·96, P < 0·05, n = 6). As with specific inhibition of R5 HIV replication, a decrease in chemotaxis was found from 75·5 ± 5·3 % in the untreated to 39·1 ± 6·3 % in the HEK-293-CCR5 adsorbed sera but not with the HEK-293 treated sera (72·5 ± 4·6%) or the sera from control subjects (Table 2). Control sera from non-parous women (n = 3) showed no inhibitory antibodies. Thus, both R5 HIV replication and chemotaxis inhibition antibodies show specific adsorption with CCR5.
Allo-immune HLA typing sera from multiparous women
The 7 HLA typing sera derived from multiparous women and 5 normal control sera were tested for inhibition of R5 HIV replication and MIP-1β generated chemotaxis (Table 3). They were arranged in a decreasing sequence from the most potent to the least potent inhibitory antibodies of R5 HIV replication and chemotaxis. The results suggest that the DR1 antiserum (at 1:10 dilution) is most effective at inhibiting R5 HIV replication (97·5%) and chemotaxis (63·1%). The mean inhibition of R5 HIV replication by the allo-sera (at 1:10) was significantly greater than that found in the 5 controls (t = 2·46, P < 0·05). However, the difference in chemotaxis failed to reach the 5% level of significance.
Table 3.
Inhibition of HIV replication (HIV-BaL) and chemokines of PBMC by 7 sera selected for HLA typing in the past and 5 control sera
| HIV Replication | Chemotaxis | ||||
|---|---|---|---|---|---|
| HLA | Serum no. | 1:10 | 1:100 | 1:10 | 1:100 |
| DR1 | 6084 | 97.5 | 67.1 | 63.1 | 26.5 |
| B17, DR7, 9 | 3103 | 66.5 | 7.2 | 44.9 | 28.3 |
| A3, DR3 | 7502 | 28.4 | 24.3 | 41.4 | 37.0 |
| DR4, DR5 | 9861 | 28.5 | 10.7 | 32.9 | 0 |
| DR3, DW6 | 5072 | 44.1 | 33.4 | 32.3 | 21.7 |
| B27 | 6135 | 48.0 | 27.7 | 29.2 | 15.1 |
| DQ1, DR4 | 11212 | 24.9 | 13.9 | 26.5 | 8.8 |
| Controls | |||||
| 1 | 33.5 | 23.9 | 17.4 | 21.3 | |
| 2 | 22.2 | 2.9 | 36.0 | 3.4 | |
| 3 | 12.6 | 0 | 32.5 | 10.6 | |
| 4 | 11.4 | 0 | 25.9 | ND | |
| 5 | 8.3 | 0 | 36.0 | ND | |
| Mean | 17.6 | 5.4 | 29.6 | 11.7 | |
| (±sem) | (4.6) | (4.7) | (3.6) | (4.2) | |
Stimulation of CD8+ T cells to produce SDF-1α
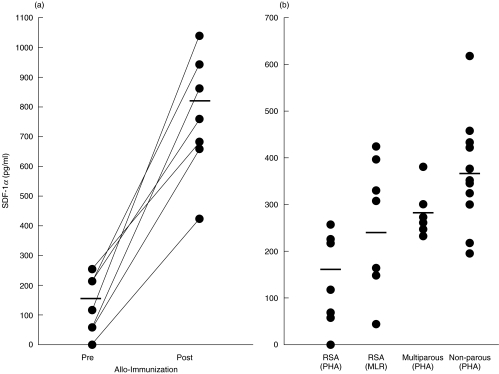
CD8+ T cells collected before and about 1 month after allo- immunization showed a significant increase in SDF-1α produced by stimulation with PHA in all 7 subjects tested (Fig. 4a); mean (±sem), SDF-1α before 130·1 ± 34·0 pg/ml compared with 779 ± 97 pg/ml after immunization (t = 6·61, P < 0·001, n = 7). MLR with preimmunized T cells (Fig. 4b), using irradiated partner's cells in vitro also stimulated a significant increase in the concentration of SDF-1α (234·1 ± 58·6 pg/ml), as compared with the PHA stimulated cells (t = 2·82, P < 0·05, n = 7). It is of interest that MLR stimulation of T cells generates higher concentrations of SDF-1α (234·1 ± 58·6 pg/ml) than mitogenic stimulation with PHA (130·1 ± 34 pg/ml). Furthermore, whilst in vivo alloimmunization significantly increased the concentration of SDF-1α about 6-fold, as assayed by in vitro maximal (PHA) stimulation (130·1 and 779 pg/ml), in vitro MLR stimulated only a slight increase. However, SDF-1α production by stimulation of MLR in vitro, before and after allo-immunization in vivo was not examined.
Fig. 4.
The concentration of CD8+ T cell-derived SDF-1α (a) in women with RSA before and after allo-immunization and (b) in non-parous and multiparous controls. CD8+ enriched populations derived from RSA, controls and mutiparous women were stimulated with 10 μg/ml of PHA. One-way MLR was carried out in RSA subjects with their partners PBMC before allo-immunization. The supernatant collected from cultures of CD8+ enriched cells were assayed for SDF-1 by ELISA and the results are presented as pg/ml.
We then tested women who have not been known to be pregnant and multiparous women matched for age (Fig. 4b). These results showed that non-parous control women had a higher concentration of CD8+ T cell-derived SDF-1 (372·9 ± 30·1 pg/ml; n = 13), as compared with that found in multiparous women (261·1 ± 8·7 pg/ml; n = 6) (Fig. 4b). This difference was significant (t = 2·37, P < 0·05). The concentration of SDF-1α in preimmunized women with RSA (130·1 ± 34 pg/ml; n = 7) was also significantly lower than that found in the non-parous controls (t = 5·03, P < 0·001). These results raise the possibility that RSA may be associated with a low SDF-1α concentration and this might be rectified by allo-immunization.
Up-regulation of CD8-SF by allo-immunization in vitro and MLR in vitro
CD8+ T-cell-derived M-tropic HIV-SF were up-regulated in all 7 subjects from a mean (±sem) of 32·7 ± 3·4% before to 70·6 ± 2·5% after allo-immunization or a net mean increase of 37·9% (Table 4). The increase in X4 HIV-SF was comparable; 20·2 ± 1·3% before to 60·4 ± 9·8% after immunization, with a net mean increase of 40·2%. It is noteworthy that the one-way MLR, using the male partner's irradiated leucocytes to stimulate the female's cells before allo-immunization showed that in vitro MLR elicited comparable R5 HIV-SF (71 ± 4·6%) as those induced by allo-immunization in vivo (70·6 ± 2·5%) (Table 4). The corresponding results for T-tropic HIV-SF were similar; 64·7 ± 6·8% for the MLR and 60·4 ± 9·8% for the post allo-immunization CD8-SF. The post allo-immunization PBMC were then separated into CD28+ and CD28− T cells by negative selection with moAb to CD28. Significantly greater levels of R5 HIV-SF were found with CD28+ cells in all subjects (mean 73 ± 4·4%) than with CD28− cells (29·7 ± 6·8%) (t = 5·431, P < 0·01). However, only 3 of the 6 subjects examined showed greater X4 HIV-SF in the CD28+ (49·8 ± 9·7%), compared with the CD28− cells (35·9 ± 11·4%) and this was not significant.
Table 4.
R5 and X4 HIV suppressor factors (SF), derived from CD8+ T cells pre- and post-alloimmunization.
| R5 HIV | X4 HIV (LAI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (BaL) Immunization | Post-immunization | Immunization | Post-immunization | |||||||
| Pre- | Post- | CD28+ | CD28− | MLR* | Pre- | Post- | CD28+ | CD28− | MLR* | |
| F5 | 35.7 | 76.7 | 74.7 | 54.2 | 71.7 | 18.3 | 67.1 | 59.5 | 35.9 | 79.7 |
| F9 | 41.6 | 74.4 | 86.5 | 0 | 65.8 | 22.8 | 91.5 | 85.5 | 71.4 | 90.6 |
| F10 | 46.6 | 78.6 | 73.2 | 42.2 | 68.2 | 18.7 | 22.2 | 53.7 | ND | 50.2 |
| F13 | 20.8 | 59.6 | 64.3 | 8.7 | 64.5 | 22.8 | 91.5 | 74.2 | 73.8 | 72.4 |
| F15 | 33.8 | 68.5 | 76.3 | 32.7 | 87.8 | 26.1 | 24.8 | 21.3 | 19.5 | 35.8 |
| F17 | 22.1 | 74.0 | 85.9 | 43.4 | 88.0 | 18.0 | 65.0 | 7.1 | 12.4 | 74.0 |
| F18 | 28.4 | 62.7 | 50.3 | 26.9 | 51.6 | 14.9 | 61.1 | 47.6 | 2.3 | 50.4 |
| Mean % | 32.7 | 70.6 | 73.0 | 29.7 | 71.0 | 20.2 | 60.4 | 49.8 | 35.9 | 64.7 |
| (±sem) | (3.4) | (2.5) | (4.4) | (6.8) | (4.6) | (1.3) | (9.8) | (9.7) | (11.4) | (6.8) |
| Difference | 37.9% | 43.3% | 40.2% | 13.9% | ||||||
The pre-immunization T cells were stimulated with the partner's cells in a one way MLR and the CD8-SF was determined and expressed as a percent inhibition of R5 and X4 HIV.
DISCUSSION
Antibodies to CCR5 were first demonstrated in rhesus monkeys immunized with a human CD4+ T cell line (C8166) [5,6]. Although the antibodies were induced by xeno-immunization, there is 97·7% identity between human and rhesus monkey CCR5 [23]. We demonstrate here that autoantibodies to CCR5 were generated in women allo-immunized with their partners’ PBMC. Antibodies to CCR5 were also found in 6 out of 7 tissue typing sera, originally selected as allo-antisera from the blood of multiparous women. Antibodies to CCR5 were demonstrated by 3 methods: ELISA, inhibition of CCR5-dependent chemotaxis and inhibition of HIV replication. The specificity of the CCR5 antibodies was demonstrated by absorption with CCR5 but not control baculovirus lysate and by using a CCR5-specific chemotaxis assay. Thus, allo-immunization in women may induce CCR5 autoantibodies when carried out by direct immunization with PBMC or under physiological conditions of multiple pregnancies, with foetal semi-allogeneic paternal haplotype.
The specificity of serum antibodies to CCR5 was demonstrated by ELISA with dose-dependent inhibition occuring with the CCR5 lysate (but not the baculovirus control lysate) and with peptides derived from the sequence of the N terminal (aa1–20) 1st (aa 89–102) and 2nd loops (aa 178–197) but not the 3rd loop of CCR5. Furthermore, the two functional assays for CCR5 antibodies were also examined for specificity by adsorption with HEK 293 cells transfected with CCR5, as compared with untransfected HEK 293 cells. Both HIV replication and MIP-1β specific chemotaxis of CCR5+ cells showed significant inhibition with the HEK 293 cells transfected with CCR5, but negligible inhibition with the control cells, confirming the specificity of these two functional assays for antibodies to CCR5.
The mechanism responsible for breaking tolerance to CCR5 is not clear. In the homozygous 32 bp deletion of CCR5 there is complete absence of cell surface expression of CCR5 [9] and this has also been found in a few sero-negative subjects exposed to HIV [8]. Thus, an encounter with CCR5+ cells, such as is found in ejaculates, by individuals lacking cell surface CCR5, would be treated as foreign and so elicit antibodies to CCR5. However, this interpretation would not apply to our cohort of allo-immunized women, all of whom expressed cell surface CCR5, though at a lower molecular density [14]. We suggest that tolerance to the CCR5 self antigen may be broken under powerful allo-immune stimulation which releases numerous cytokines and chemokines. Another possibility is the involvement of an unrelated viral agent, since autoantibodies to CCR5 were induced in C57 BL/6 mice by immunization with a papilloma virus that expressed CCR5 peptides [24].
Antibodies to CCR5 elicited by allo-immunization inhibit MIP-1β-mediated chemotaxis of CCR5+ cells and HIV replication. Blocking CCR5 with antibodies may also inhibit CCR5 mediated migration of Th1 cells, immature dendritic cells and monocytes [25]. This mechanism might prevent migration of harmful effector cells and offers a rationale for the clinical practice of allo-immunization of women with spontaneous recurrent abortions, by blocking the CCR5+ Th1 cells and switching to the Th2 type immune response which appears to be required for preventing rejection of a semi-allogeneic fetus [26,27].
Women immunized with unmatched PBMC from their partners showed raised concentrations of the CXC chemokine SDF-1α which is known to down-regulate cell-surface CXCR4 [3,15]. The potency of allo-immunization in vivo was much greater than the in vitro stimulated MLR, as the concentration of SDF-1α increased about 6-fold in the former, compared with a 2-fold increase in the latter. Whereas allo-immunization with a single large dose of unmatched cells up-regulated the concentration of SDF-1 produced by CD8+ T cells, such SDF-1 production was decreased, in multiparous compared with non-parous women. However, artificially allo-immunized women are not comparable with women presumably naturally allo-immunized by fetal semi-allogeneic cells, though in both cohorts PHA was used to stimulate production of SDF-1. An evaluation of the baseline levels of SDF-1α showed that the mean concentration was lowest in women with RSA (130·1 ± 34·0 pg/ml) and was increased in both multiparous women (261·1 ± 8·7 pg/ml) and non-parous control women (372·9 ± 30·1 pg/ml). This raises the possibility that a low concentration of SDF-1α in women with RSA is one of the factors associated with recurrent abortion and that allo-immunization might rectify this deficiency.
In a previous study we found that allo-immunization increased CC chemokines, CD8+ T cell derived SF, decreased cell surface expression of CCR5, and decreased CD4+ T cell infectivity with the R5 HIV [14]. Here we demonstrated that SDF-1, the only known ligand for CXCR4 was also up-regulated by allo-immunization which could account for the significant decrease in the cell surface expression of CXCR4 and the associated decrease in CD4+ T cell infectivity with the X4 HIV [14]. Furthermore, the concern that blocking the CCR5-mode of HIV transmission may enhance the CXCR4-dependent X4 HIV switch and thereby accelerate the onset of AIDS is unlikely to happen, as we have demonstrated here that allo-immunization up-regulates SDF-1α, downmodulates CXCR4 and induces CD8-SF that prevents X4 virus infection.
We next addressed the involvement of the CD28+ subset of cells in allogeneic stimulation of CD8-SF production. This was based on the report that the human CD8+CD28+ subset of cells generates CD8-SF [28,29]. Indeed, a significant increase in CD8-SF activity against the R5 and X4 HIV-1 strains was found with the CD28-enriched cells, as compared with the CD28-depleted cells. Generation of CD8-SF by CD28+ cells was dependent on allo-immunization, as in unimmunized control subjects neither CD28+ nor CD28− subsets elicited CD8-SF greater than 20% against R5 or X4 HIV-1 strains (data not presented).
CC chemokines bind and downmodulate CCR5 in vitro[3] and in vivo[4]. Antibodies to CCR5 may have a similar effect and may induce dimerization of CCR5 that results in internalization of CCR5 [30]. This is consistent with inhibition by CCR5 and the 2nd extracellular loop of both the alloimmune and DR1 sera, as CC chemokines also preferentially bind the 2nd extracellular loop of CCR5. Thus, an HIV noncognate chemokine-mediated mechanism, targeting the CCR5 coreceptor can be harnessed to protect against primary HIV infection by immunization which elicits anti-CCR5 antibodies and up-regulates CC chemokines [7]. This type of HIV noncognate protection against HIV is found in nature, with the homozygous 32 bp mutation in the 2nd extracellular loop of CCR5, which prevents cell surface expression of CCR5, up-regulates the 3 CC chemokines [11] and induces autoantibodies to CCR5 [10]. Indeed, a complementary inhibition of HIV replication has been demonstrated between suboptimal concentrations of the 3 CC chemokines and CCR5 antibodies in vitro[7]. A dual vaccination strategy will now be pursued that will utilize an HIV noncognate mechanism targeting the CCR5 coreceptors and cognate HIV immunity.
We suggest an overall scheme that may account for protection against HIV infection during natural allo-immunization, as occurs in multiparous women or in some sex workers, and that is induced by artificial allo-immunization [31]. Allo-immunization elicits up-regulation of CD8+ T-cell-derived CC chemokines and CCR5 antibodies that block and downmodulate cell surface CCR5. The concentration of the CXC chemokine, SDF-1α is also increased which blocks and downmodulates cell-surface CXCR4. The combined effect of the CC and CXC chemokines will inhibit transmission of the R5 and X4 strains of HIV, aided by generation of CD8+ T-cell-derived R5 and X4 types of HIV SF. This mechanism may not only prevent initial CCR5-utilizing HIV transmission, but also a later switch to CXCR4-utilizing HIV infection which is associated with the development of AIDS.
Acknowledgments
This work was supported partly by the European Community Biomed Grant (BMH4 CT97-2345), the National Institute of Allergy and Infectious Diseases, National Institute of Health, USA and the Guy's and St Thomas’ Charitable Trust.
REFERENCES
- 1.Deng H, Liu R, Ellmeier W, et al. Identification of a major co- receptor for primary isolates of HIV-1. Nature. 1996;381:661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 2.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 3.Amara A, Gall SL, Schwarts O, et al. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–46. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehner T, Wang Y, Cranage M, et al. Upregulation of β-chemokines and downmodulation of CCR5 coreceptors inhibit simian immunodeficiency virus transmission in non-human primates. Immunology. 2000;99:569–77. doi: 10.1046/j.1365-2567.2000.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehner T, Wang Y, Bravery CA, et al. National Cooperative Vaccine Development Grant Meeting. Bethesda: NIH; May 1997. Antibodies to CCR5 receptors and b-chemokines, generated by xenogeneic immunization, in the prevention of SIV infection in macaques. [Google Scholar]
- 6.Lehner T, Wang Y, Doyle C, et al. Induction of inhibitory antibodies to the CCR5 chemokine receptor and their complementary role in preventing SIV infection in macaques. Eur J Immunol. 1999;29:2427–35. doi: 10.1002/(SICI)1521-4141(199908)29:08<2427::AID-IMMU2427>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Lehner T, Doyle C, Wang Y, et al. Immunogenicity of the extracellular domains of the CC chemokine receptor 5 and the effects of SIV or HIV infectivity. J Immunol. 2001;166:7446–55. doi: 10.4049/jimmunol.166.12.7446. [DOI] [PubMed] [Google Scholar]
- 8.Zuber B, Hinkula J, Vadros D, et al. Induction of immune responses and break of tolerance by DNA against the HIV-1 coreceptor CCR5 but no protection from SIVsm challenge. Virology. 2000;278:400–11. doi: 10.1006/viro.2000.0633. [DOI] [PubMed] [Google Scholar]
- 9.Lopalco L, Barassi C, Pastori C, et al. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 in vivo. J Immunol. 2000;164:3426–33. doi: 10.4049/jimmunol.164.6.3426. [DOI] [PubMed] [Google Scholar]
- 10.Ditzel HJ, Rosenkilde MM, Garred P, et al. The CCR5 receptor acts as an alloantigen in CCR5 delta 32 homozyqous individuals: identification of chemokines and HIV-1-blocking human antibodies. Proc Natl Acad Sci USA. 1998;95:5241–5. doi: 10.1073/pnas.95.9.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paxton WA, Martin SR, Tse D, et al. Relative resistant to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nature (Med) 1996;2:412–7. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 12.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 co-receptor accounts for resistance of some multiply-exposed individuals to HIV infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 13.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Tao L, Mitchell E, et al. Allo-immunization in women elicits CD8 suppressor factors, β-chemokines and resistance of CD4+ cells to HIV infection. Nature (Medicine) 1999;5:1004–9. doi: 10.1038/12440. [DOI] [PubMed] [Google Scholar]
- 15.Endres MJ, Clapham PR, Marsh M, et al. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–56. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 16.Doyle CB, Bhattacharyya U, Kent KA, et al. Regions required for CD4 binding in the external glycoprotein gp120 of simian immunodeficiency virus. J Virol. 1995;69:1256–60. doi: 10.1128/jvi.69.2.1256-1260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehner T, Bergmeier L, Tao L, et al. Targeted lymph node immunization with simian immunodeficiency virus p27 antigen to elicit genital, rectal and urinary immune responses in nonhuman primates. J Immunol. 1994;153:1858–68. [PubMed] [Google Scholar]
- 18.Raport CJ, Gosling J, Schweickart VL, et al. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β and MIP-1α. J Biol Chem. 1996;271:17161–6. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 19.Bleul CC, Fuhlbrigge RC, Casanovas JM, et al. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Tao L, Mitchell E, Bergmeier L, et al. The effect of immunization on chemokines and CCR5 and CXCR4 coreceptor functions in SIV binding and chemotaxis. Vaccine. 1999;17:1826–36. doi: 10.1016/s0264-410x(98)00482-4. [DOI] [PubMed] [Google Scholar]
- 21.Mackewicz CE, Levy JA. CD8+ cell anti-HIV activity: nonlytic suppresion of virus replication. AIDS Res Hum Retroviruses. 1992;8:629–40. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 22.Lehner T, Wang Y, Cranage M, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–75. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Zhou P, Ho DD, et al. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–14. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chackerian C, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci USA. 1999;96:2373–8. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Mossmann TR, Guilbert L, et al. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J Immunol. 1993;151:4562–73. [PubMed] [Google Scholar]
- 27.Wegmann TG, Lin H, Guilbert L, Mossmann TR. Bidirctional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon. Immunol Today. 1994;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 28.Landay AL, Mackewicz CE, Levy JA. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymtomatic clinical status. Clin Immuno Immunopath. 1993;69:106–16. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 29.Barker E, Bossart KN, Fujimura SH, Levy JA. CD28 costimulation increases CD8+ cell suspension of HIV replication. J Immunol. 1997;159:5123–31. [PubMed] [Google Scholar]
- 30.Vila-Coro AJ, Mellado M, Martin de Ana A, et al. HIV-1 infection through the CCR5 receptor is blocked by receptor dimerization. Proc Natl Acad Sci USA. 2000;97:3388–93. doi: 10.1073/pnas.050457797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehner T, Shearer GM, Hackett CJ, et al. Allo-immunization as a strategy for vaccine design against HIV/AIDS. AIDS Res Hum Retroviruses. 2000;116:309–13. doi: 10.1089/088922200309188. [DOI] [PubMed] [Google Scholar]