Abstract
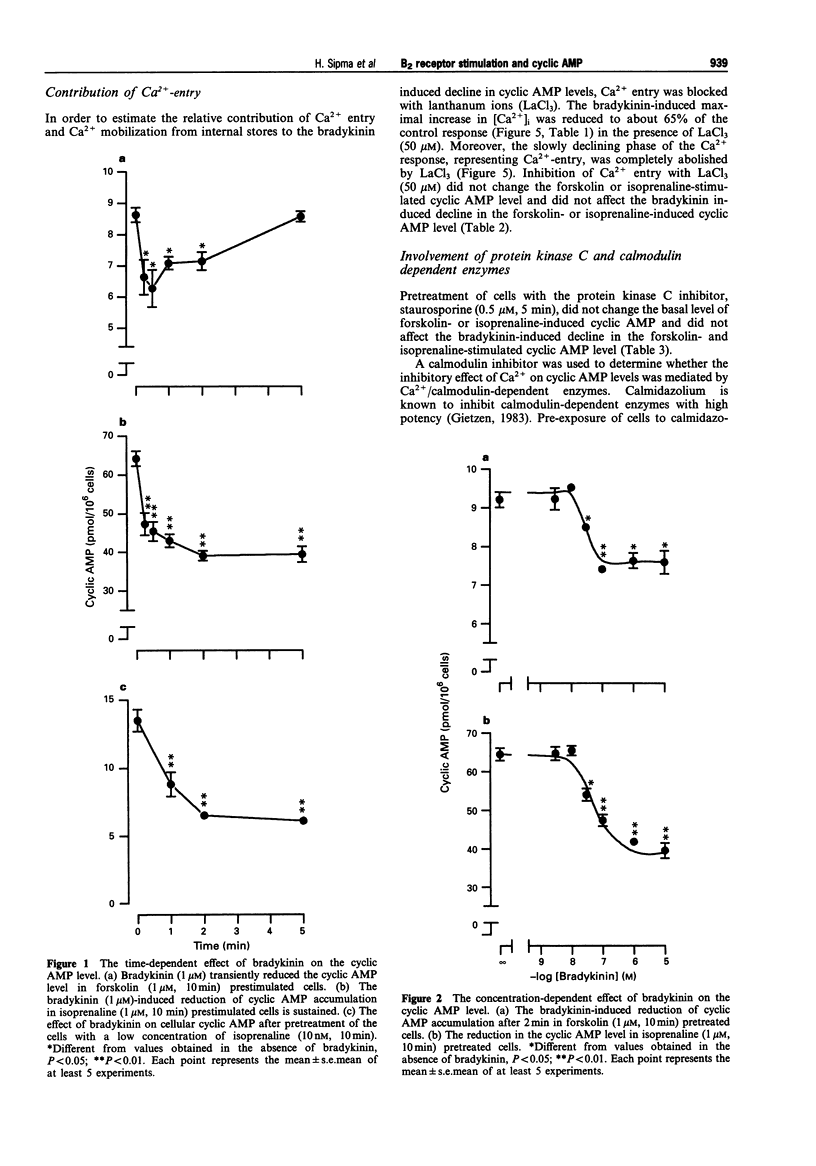
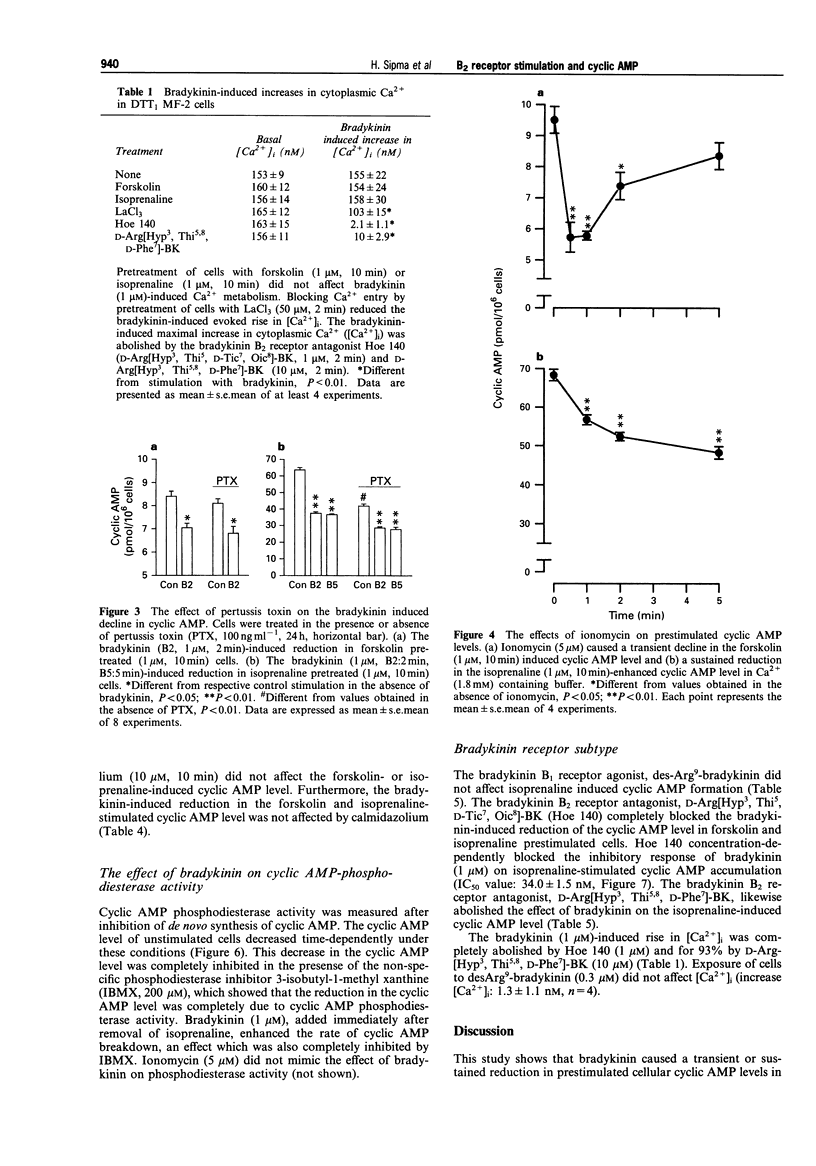
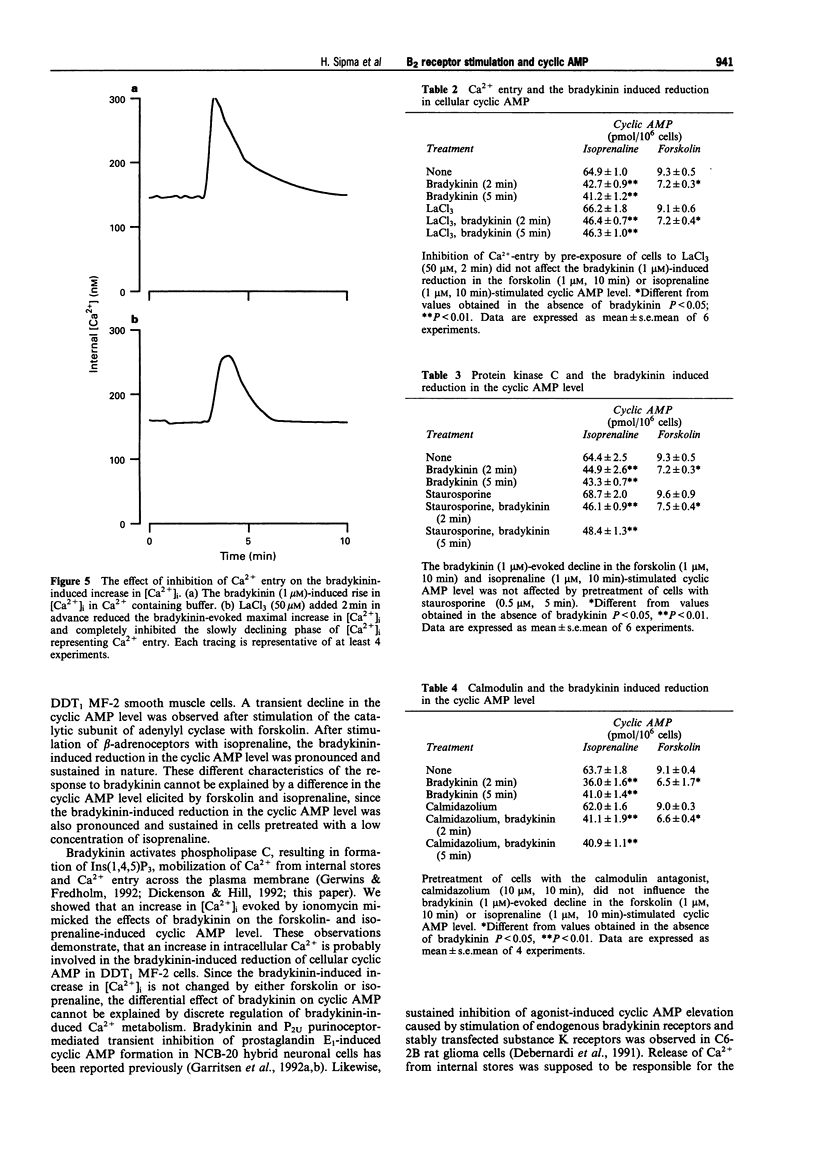
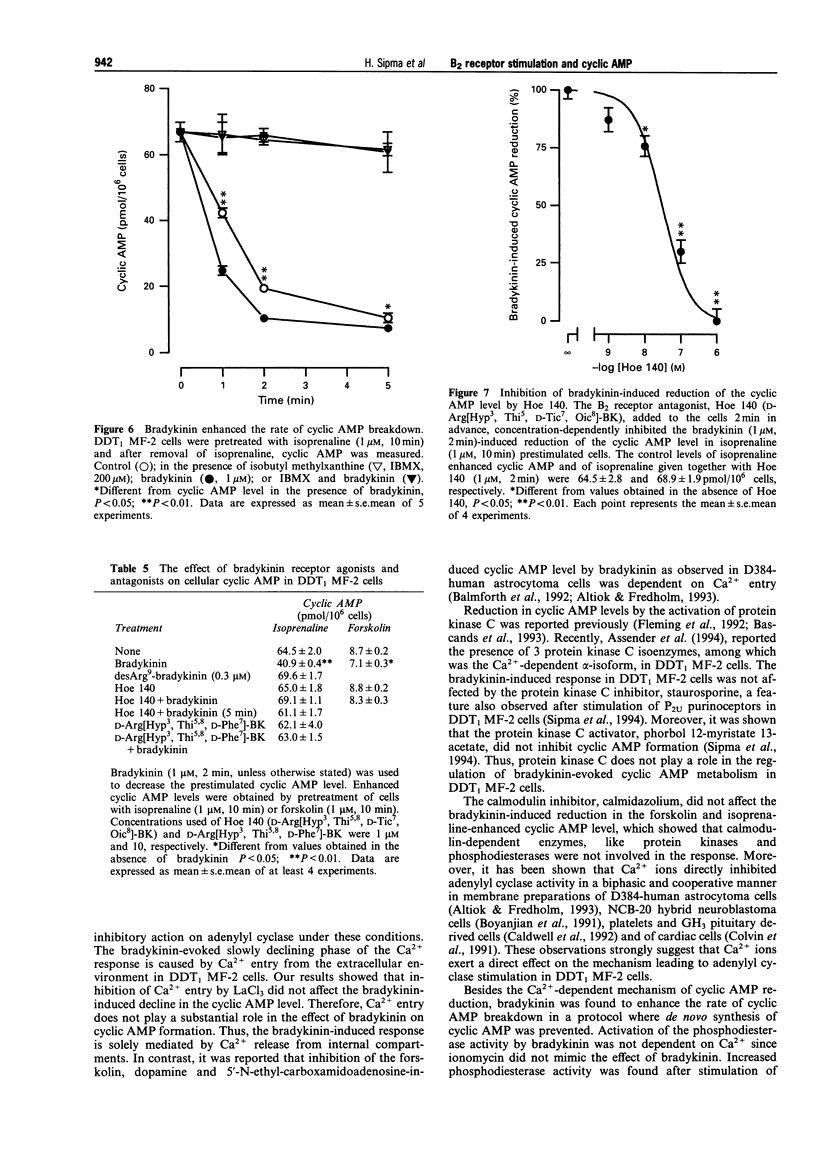
1. Bradykinin caused a transient reduction of about 25% in the cyclic AMP level in forskolin prestimulated DDT1 MF-2 smooth muscle cells (IC50: 36.4 +/- 4.9 nM) and a pronounced, sustained inhibition (40%) of the isoprenaline-stimulated cyclic AMP level (IC50: 37.5 +/- 1.1 nM). 2. The Ca2+ ionophore, ionomycin, mimicked both the bradykinin-induced transient reduction in the forskolin-stimulated cyclic AMP level and the sustained reduction in the isoprenaline-stimulated cyclic AMP level. 3. The Ca(2+)-dependent effect on cyclic AMP induced by bradykinin was mediated solely by Ca2+ release from internal stores, since inhibition of Ca2+ entry with LaCl3 did not reduce the response to bradykinin. 4. The involvement of calmodulin-dependent enzyme activities, protein kinase C or an inhibitory GTP binding protein in the bradykinin-induced responses was excluded since a calmodulin inhibitor, calmidazolium, a PKC inhibitor, staurosporine and pertussis toxin, respectively did not affect the decline in the cyclic AMP level. 5. Bradykinin enhanced the rate of cyclic AMP breakdown in intact cells, which effect was not mimicked by ionomycin. This suggested a Ca(2+)-independent activation of phosphodiesterase activity by bradykinin in DDT1 MF-2 cells. 6. The bradykinin B1 receptor agonist, desArg9-bradykinin, did not affect cyclic AMP formation in isoprenaline prestimulated cells, while the bradykinin B2 receptor antagonists, Hoe 140 (D-Arg[Hyp3, Thi5, D-Tic7, Oic8]-BK) and D-Arg[Hyp3, Thi5,8, D-Phe7]-BK completely abolished the bradykinin response in both forskolin and isoprenaline prestimulated cells. 7. Bradykinin caused an increase in intracellular Ca2+, which was antagonized by the bradykinin B2 receptor antagonists, Hoe 140 and D-Arg[Hyp3, Thi5,8, D-Phe7]-BK.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altiok N., Fredholm B. B. Bradykinin inhibits cyclic AMP accumulation in D384-human astrocytoma cells via a calcium-dependent inhibition of adenylyl cyclase. Cell Signal. 1993 May;5(3):279–288. doi: 10.1016/0898-6568(93)90018-h. [DOI] [PubMed] [Google Scholar]
- Assender J. W., Kontny E., Fredholm B. B. Expression of protein kinase C isoforms in smooth muscle cells in various states of differentiation. FEBS Lett. 1994 Mar 28;342(1):76–80. doi: 10.1016/0014-5793(94)80588-1. [DOI] [PubMed] [Google Scholar]
- Balmforth A. J., Parkinson F. E., Altiok N., Fredholm B. B. Identification of a B2-bradykinin receptor linked to phospholipase C and inhibition of dopamine stimulated cyclic AMP accumulation in the human astrocytoma cell line D384. Naunyn Schmiedebergs Arch Pharmacol. 1992 Sep;346(3):303–310. doi: 10.1007/BF00173543. [DOI] [PubMed] [Google Scholar]
- Bascands J. L., Pecher C., Girolami J. P. Indirect inhibition by bradykinin of cyclic AMP generation in isolated rat glomeruli and mesangial cells. Mol Pharmacol. 1993 Oct;44(4):818–826. [PubMed] [Google Scholar]
- Boyajian C. L., Garritsen A., Cooper D. M. Bradykinin stimulates Ca2+ mobilization in NCB-20 cells leading to direct inhibition of adenylylcyclase. A novel mechanism for inhibition of cAMP production. J Biol Chem. 1991 Mar 15;266(8):4995–5003. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell K. K., Boyajian C. L., Cooper D. M. The effects of Ca2+ and calmodulin on adenylyl cyclase activity in plasma membranes derived from neural and non-neural cells. Cell Calcium. 1992 Feb;13(2):107–121. doi: 10.1016/0143-4160(92)90004-c. [DOI] [PubMed] [Google Scholar]
- Colvin R. A., Oibo J. A., Allen R. A. Calcium inhibition of cardiac adenylyl cyclase. Evidence for two distinct sites of inhibition. Cell Calcium. 1991 Jan;12(1):19–27. doi: 10.1016/0143-4160(91)90081-o. [DOI] [PubMed] [Google Scholar]
- DeBernardi M. A., Seki T., Brooker G. Inhibition of cAMP accumulation by intracellular calcium mobilization in C6-2B cells stably transfected with substance K receptor cDNA. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9257–9261. doi: 10.1073/pnas.88.20.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the alpha-action of catecholamines on guinea-pig taenia caeci. J Physiol. 1981 Jul;316:109–125. doi: 10.1113/jphysiol.1981.sp013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Histamine H1-receptor-mediated calcium influx in DDT1MF-2 cells. Biochem J. 1992 Jun 1;284(Pt 2):425–431. doi: 10.1042/bj2840425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., White T. E., Hill S. J. The effects of elevated cyclic AMP levels on histamine-H1-receptor-stimulated inositol phospholipid hydrolysis and calcium mobilization in the smooth-muscle cell line DDT1MF-2. Biochem J. 1993 Jun 1;292(Pt 2):409–417. doi: 10.1042/bj2920409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming N., Mellow L., Bhullar D. Regulation of the cAMP signal transduction pathway by protein kinase C in rat submandibular cells. Pflugers Arch. 1992 May;421(1):82–89. doi: 10.1007/BF00374737. [DOI] [PubMed] [Google Scholar]
- Garritsen A., Zhang Y., Cooper D. M. Purinergic receptor regulation of signal transduction in NCB-20 cells. Mol Pharmacol. 1992 Apr;41(4):743–749. [PubMed] [Google Scholar]
- Gerwins P., Fredholm B. B. Stimulation of adenosine A1 receptors and bradykinin receptors, which act via different G proteins, synergistically raises inositol 1,4,5-trisphosphate and intracellular free calcium in DDT1 MF-2 smooth muscle cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7330–7334. doi: 10.1073/pnas.89.16.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen K. Comparison of the calmodulin antagonists compound 48/80 and calmidazolium. Biochem J. 1983 Dec 15;216(3):611–616. doi: 10.1042/bj2160611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goureau O., Tanfin Z., Harbon S. Prostaglandins and muscarinic agonists induce cyclic AMP attenuation by two distinct mechanisms in the pregnant-rat myometrium. Interaction between cyclic AMP and Ca2+ signals. Biochem J. 1990 Nov 1;271(3):667–673. doi: 10.1042/bj2710667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992 Nov;56(2):131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Hoiting B., Molleman A., Duin M., den Hertog A., Nelemans A. P2 purinoceptor-mediated inositol phosphate formation in relation to cytoplasmic calcium in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol. 1990 Jul 31;189(1):31–39. doi: 10.1016/0922-4106(90)90227-o. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Griesbacher T., Eckhardt M., Henke S., Breipohl G., Knolle J. New, long-acting, potent bradykinin antagonists. Br J Pharmacol. 1991 Feb;102(2):297–304. doi: 10.1111/j.1476-5381.1991.tb12169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman A., Nelemans A., Den Hertog A. P2-purinoceptor-mediated membrane currents in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol. 1989 Oct 4;169(1):167–174. doi: 10.1016/0014-2999(89)90829-7. [DOI] [PubMed] [Google Scholar]
- Norris J. S., Gorski J., Kohler P. O. Androgen receptors in a Syrian hamster ductus deferens tumour cell line. Nature. 1974 Mar 29;248(447):422–424. doi: 10.1038/248422a0. [DOI] [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Pyne S., Pyne N. J. Bradykinin-stimulated phosphatidate and 1,2-diacylglycerol accumulation in guinea-pig airway smooth muscle: evidence for regulation 'down-stream' of phospholipases. Cell Signal. 1994 Mar;6(3):269–277. doi: 10.1016/0898-6568(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Pyne S., Pyne N. J. Differential effects of B2 receptor antagonists upon bradykinin-stimulated phospholipase C and D in guinea-pig cultured tracheal smooth muscle. Br J Pharmacol. 1993 Sep;110(1):477–481. doi: 10.1111/j.1476-5381.1993.tb13835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipma H., Duin M., Hoiting B., den Hertog A., Nelemans A. Regulation of histamine- and UTP-induced increases in Ins(1,4,5)P3, Ins (1,3,4,5)P4 and Ca2+ by cyclic AMP in DDT1 MF-2 cells. Br J Pharmacol. 1995 Jan;114(2):383–390. doi: 10.1111/j.1476-5381.1995.tb13238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipma H., den Hertog A., Nelemans A. The phospholipase C activating P2U purinoceptor also inhibits cyclicAMP formation in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol. 1994 Aug 16;268(3):431–437. doi: 10.1016/0922-4106(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Stevens P. A., Pyne S., Grady M., Pyne N. J. Bradykinin-dependent activation of adenylate cyclase activity and cyclic AMP accumulation in tracheal smooth muscle occurs via protein kinase C-dependent and -independent pathways. Biochem J. 1994 Jan 1;297(Pt 1):233–239. doi: 10.1042/bj2970233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner L. I., Harden T. K., Wells J. N., Martin M. W. Identification of the phosphodiesterase regulated by muscarinic cholinergic receptors of 1321N1 human astrocytoma cells. Mol Pharmacol. 1986 May;29(5):455–460. [PubMed] [Google Scholar]
- Yang C. M., Hsia H. C., Chou S. P., Ong R., Hsieh J. T., Luo S. F. Bradykinin-stimulated phosphoinositide metabolism in cultured canine tracheal smooth muscle cells. Br J Pharmacol. 1994 Jan;111(1):21–28. doi: 10.1111/j.1476-5381.1994.tb14018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


