Abstract
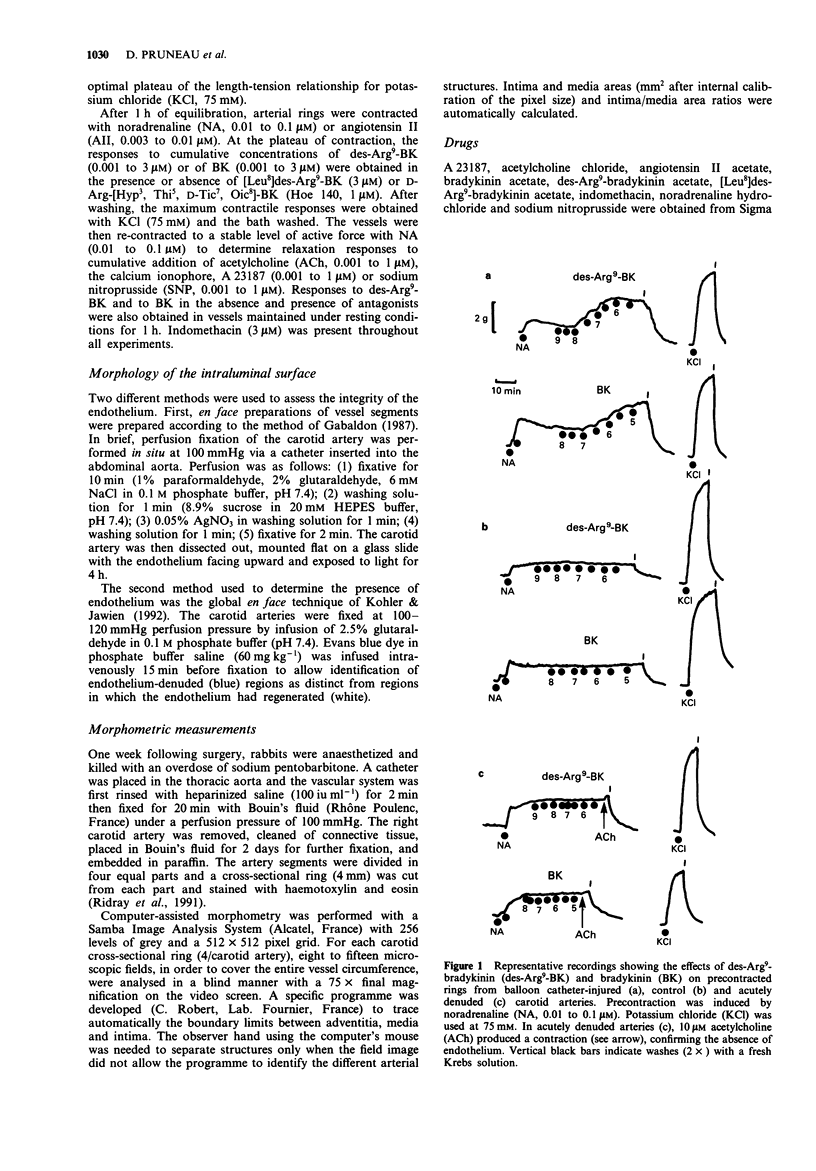
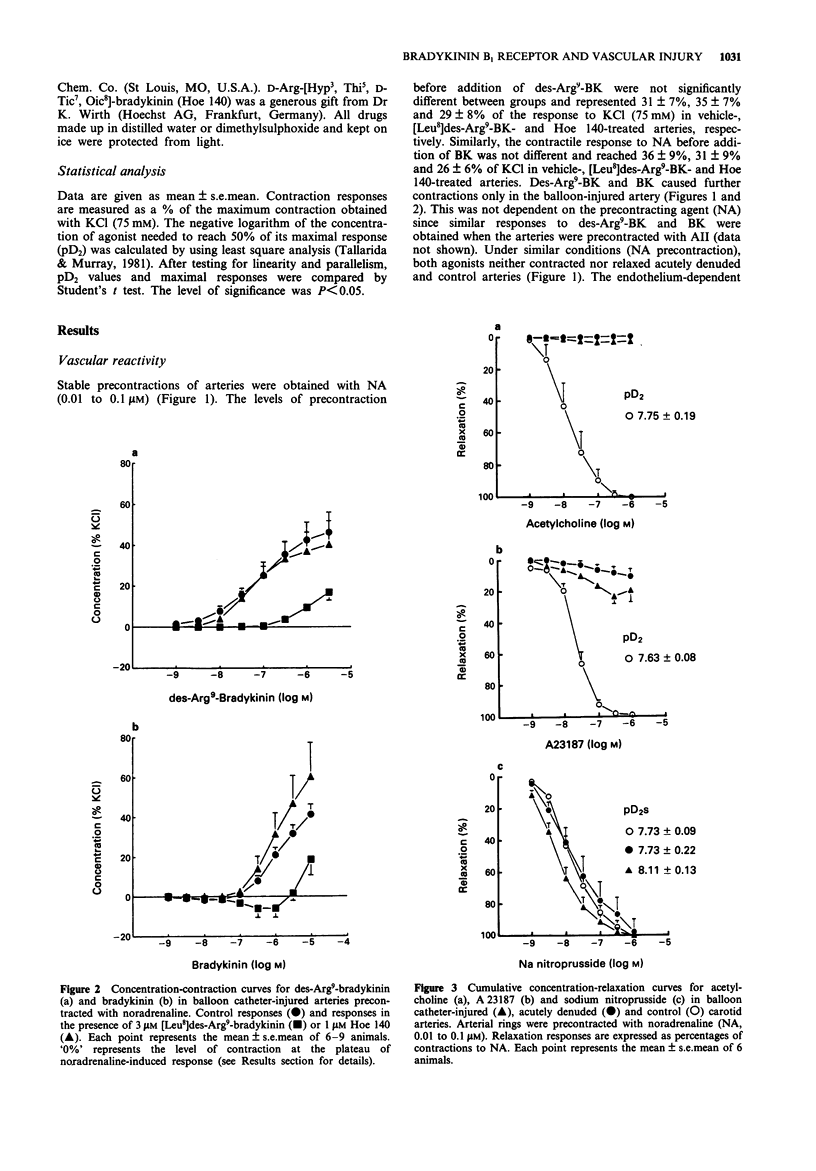
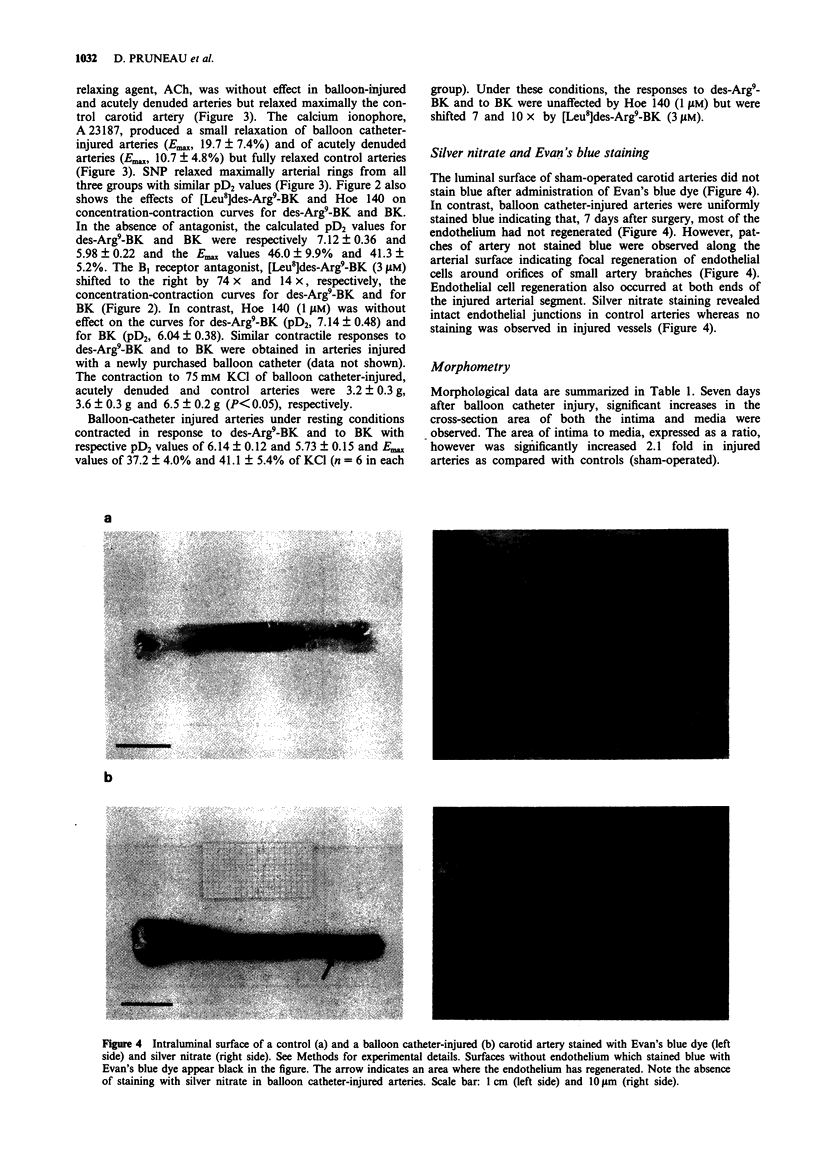
1. Balloon catheter injury to the rabbit carotid artery damaged the endothelium and induced neointima formation over 7 days. The area of intima, expressed as a percentage of the media, was 16.2 +/- 4.2% and 8.2 +/- 0.1% in balloon catheter-injured and sham-operated arteries. 2. Seven days after arterial injury, carotid arteries were isolated and set up as ring preparations in organ baths for isometric tension measurements. Balloon catheter-injured arteries first contracted with noradrenaline (0.01-0.1 microM), contracted further in a concentration-dependent manner to bradykinin (BK; pD2, 5.98 +/- 0.22; Emax, 41.3 +/- 5.2% of KCl) and to des-Arg9-BK (pD2, 7.12 +/- 0.36; Emax, 46.0 +/- 9.9% of KCl). In contrast, vessel segments with endothelium either intact or acutely removed were unresponsive to both BK receptor agonists. 3. The concentration-contraction curves for BK and for des-Arg9-BK were shifted to the right by the B1 receptor antagonist, [Leu8]des-Arg9-BK (3 microM), but not by the selective B2 receptor antagonist, Hoe 140 (1 microM). 4. Thus, BK and its metabolite, des-Arg9-BK act as vasoconstrictor agents following balloon catheter injury. These effects appear to be mediated by activation of B1 receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Niimi Y., Hamasaki H. Prevention of intimal thickening after endothelial removal by a nonpeptide angiotensin II receptor antagonist, losartan. Br J Pharmacol. 1992 Jul;106(3):665–671. doi: 10.1111/j.1476-5381.1992.tb14392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk B. C., Gordon J. B., Alexander R. W. Pharmacologic roles of heparin and glucocorticoids to prevent restenosis after coronary angioplasty. J Am Coll Cardiol. 1991 May;17(6 Suppl B):111B–117B. doi: 10.1016/0735-1097(91)90946-7. [DOI] [PubMed] [Google Scholar]
- Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- Briner V. A., Tsai P., Schrier R. W. Bradykinin: potential for vascular constriction in the presence of endothelial injury. Am J Physiol. 1993 Feb;264(2 Pt 2):F322–F327. doi: 10.1152/ajprenal.1993.264.2.F322. [DOI] [PubMed] [Google Scholar]
- Bönner G., Schunk U. Hypotensive effect of bradykinin in normotensives and patients with renovascular hypertension. Adv Exp Med Biol. 1986;198(Pt B):321–327. doi: 10.1007/978-1-4757-0154-8_40. [DOI] [PubMed] [Google Scholar]
- Campbell D. J., Kladis A., Duncan A. M. Bradykinin peptides in kidney, blood, and other tissues of the rat. Hypertension. 1993 Feb;21(2):155–165. doi: 10.1161/01.hyp.21.2.155. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., Kemp B. K., Pruneau D., Angus J. A. Comparison of contractile responses to 5-hydroxytryptamine and sumatriptan in human isolated coronary artery: synergy with the thromboxane A2-receptor agonist, U46619. Br J Pharmacol. 1993 Sep;110(1):360–368. doi: 10.1111/j.1476-5381.1993.tb13818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois D., Marceau F. The ability of des-Arg9-bradykinin to relax rabbit isolated mesenteric arteries is acquired during in vitro incubation. Eur J Pharmacol. 1987 Oct 6;142(1):141–144. doi: 10.1016/0014-2999(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Gabaldón M. Methodological approaches for the study of the aortic endothelium of the rat. Atherosclerosis. 1987 May;65(1-2):139–149. doi: 10.1016/0021-9150(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Goldstein R. H., Wall M. Activation of protein formation and cell division by bradykinin and des-Arg9-bradykinin. J Biol Chem. 1984 Jul 25;259(14):9263–9268. [PubMed] [Google Scholar]
- Kohler T. R., Jawien A. Flow affects development of intimal hyperplasia after arterial injury in rats. Arterioscler Thromb. 1992 Aug;12(8):963–971. doi: 10.1161/01.atv.12.8.963. [DOI] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Marceau F., Lussier A., Regoli D., Giroud J. P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen Pharmacol. 1983;14(2):209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Nwator I. A., Whalley E. T. Angiotensin converting enzyme inhibitors and expression of des-Arg9-BK (kinin B1) receptors in vivo. Eur J Pharmacol. 1989 Jan 24;160(1):125–132. doi: 10.1016/0014-2999(89)90661-4. [DOI] [PubMed] [Google Scholar]
- Pelc L. R., Gross G. J., Warltier D. C. Mechanism of coronary vasodilation produced by bradykinin. Circulation. 1991 Jun;83(6):2048–2056. doi: 10.1161/01.cir.83.6.2048. [DOI] [PubMed] [Google Scholar]
- Pruneau D., Bélichard P. Induction of bradykinin B1 receptor-mediated relaxation in the isolated rabbit carotid artery. Eur J Pharmacol. 1993 Aug 3;239(1-3):63–67. doi: 10.1016/0014-2999(93)90976-o. [DOI] [PubMed] [Google Scholar]
- Regoli D. C., Marceau F., Lavigne J. Induction of beta 1-receptors for kinins in the rabbit by a bacterial lipopolysaccharide. Eur J Pharmacol. 1981 Apr 24;71(1):105–115. doi: 10.1016/0014-2999(81)90391-5. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Regoli D., Rhaleb N. E., Drapeau G., Dion S. Kinin receptor subtypes. J Cardiovasc Pharmacol. 1990;15 (Suppl 6):S30–S38. [PubMed] [Google Scholar]
- Ridray S., Capron L., Heudes D., Picon L., Ktorza A. Effects of fasting and refeeding on the proliferative response of rat aorta to injury. Am J Physiol. 1991 Jul;261(1 Pt 2):H190–H195. doi: 10.1152/ajpheart.1991.261.1.H190. [DOI] [PubMed] [Google Scholar]
- Roberts R. A., Gullick W. J. Bradykinin receptor number and sensitivity to ligand stimulation of mitogenesis is increased by expression of a mutant ras oncogene. J Cell Sci. 1989 Nov;94(Pt 3):527–535. doi: 10.1242/jcs.94.3.527. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Holmes D. R., Jr, Topol E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992 Nov 1;20(5):1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A. Bradykinin-degrading enzymes: structure, function, distribution, and potential roles in cardiovascular pharmacology. J Cardiovasc Pharmacol. 1992;20 (Suppl 9):S4–S9. doi: 10.1097/00005344-199200209-00003. [DOI] [PubMed] [Google Scholar]



