Abstract
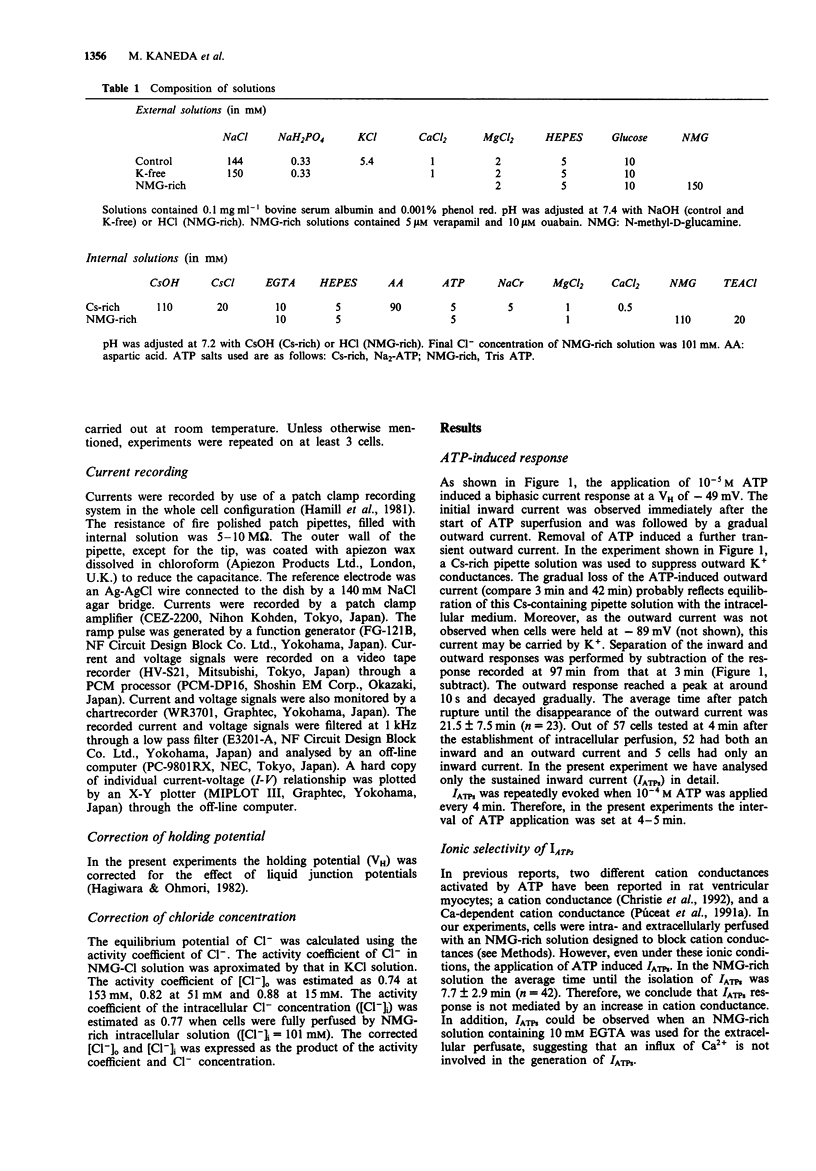
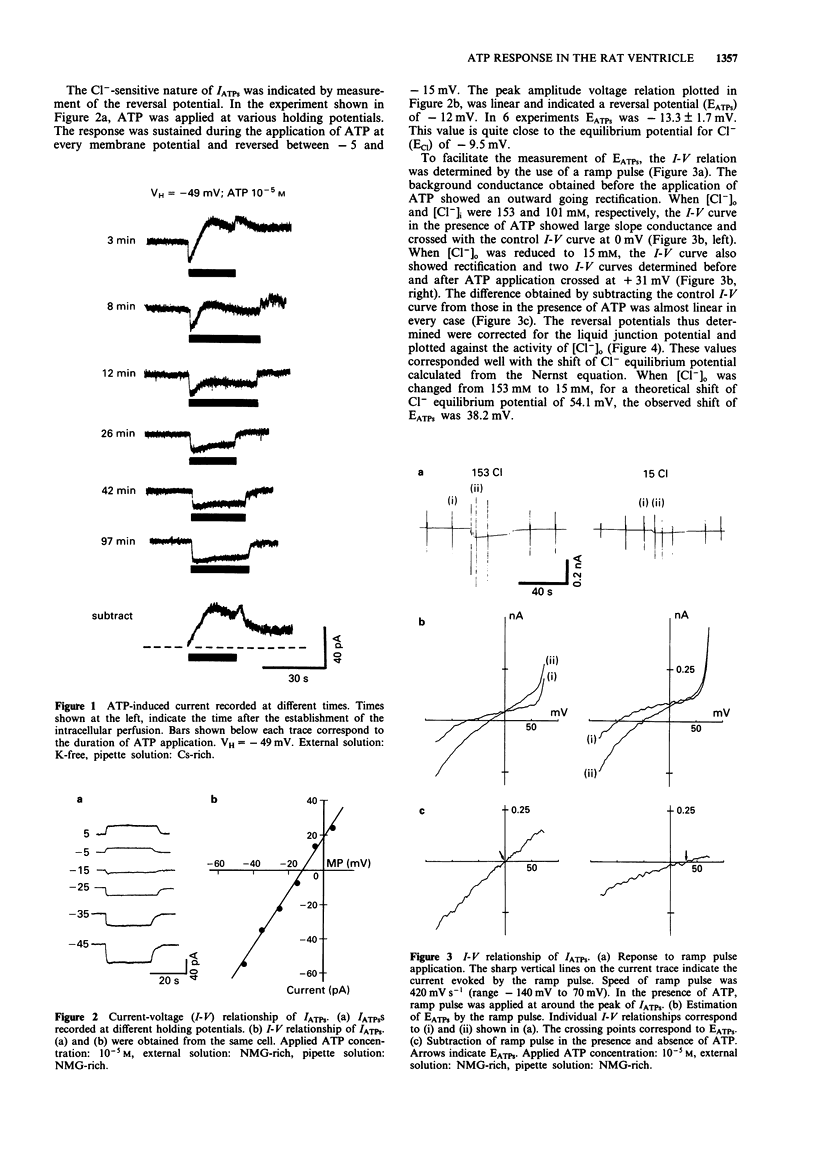
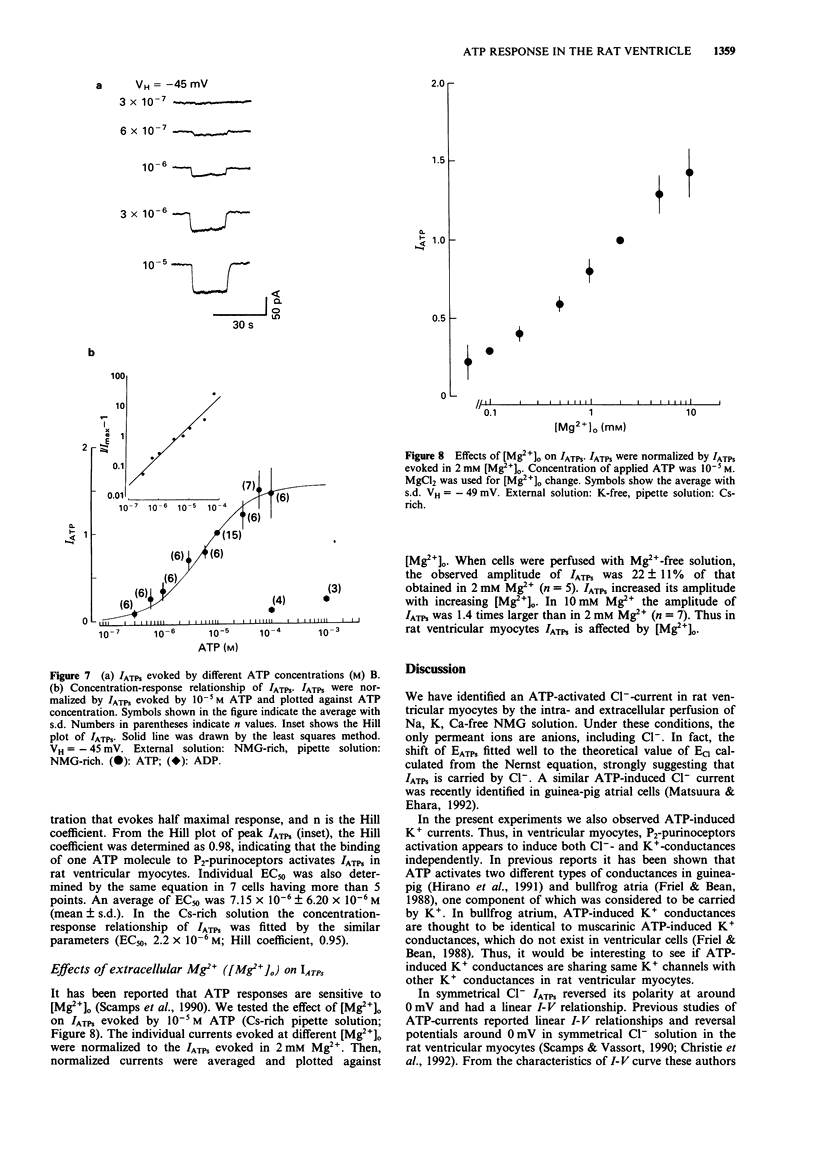
1. Rat ventricular myocytes were dissociated and their responses to extracellularly applied ATP were recorded using patch pipettes under the whole cell configuration. 2. ATP initially induced an inward current followed by an outward current at -50 mV. With a Cs-rich pipette solution the late outward current was blocked, leaving a sustained inward current (IATPs) suggesting that a K+ conductance underlies the late response. 3. When the extracellular Cl- concentration was changed, the reversal potential of IATPs corresponded well to the shift of the Cl- equilibrium potential. IATPs was reversibly blocked by the chloride channel blocker, 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS). 4. The concentration-response curve of IATPs had a Hill coefficient of 0.98 and an EC50 value of 5.2 x 10(-6) M. 5. ATP was more potent than ADP, while AMP and adenosine were ineffective, suggesting that P2-purinoceptor activation induced IATPs. 6. The activation of IATPs was depressed by depleting the extracellular Mg2+ and increased by adding Mg2+. 7. Our results strongly suggest that P2-purinoceptor activation by ATP induces both a Cl(-)-conductance (IATPs) and a K(+)-conductance in rat ventricular myocytes.
Full text
PDF
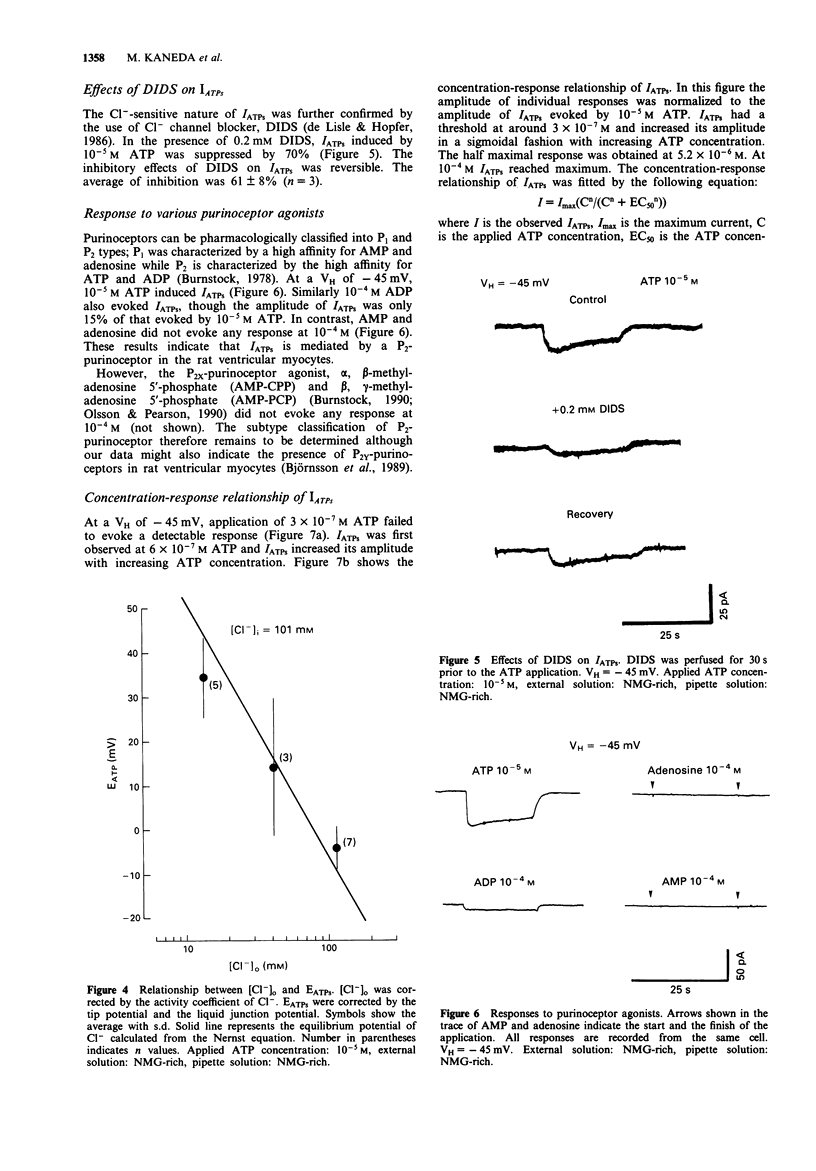

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björnsson O. G., Monck J. R., Williamson J. R. Identification of P2Y purinoceptors associated with voltage-activated cation channels in cardiac ventricular myocytes of the rat. Eur J Biochem. 1989 Dec 8;186(1-2):395–404. doi: 10.1111/j.1432-1033.1989.tb15222.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Christie A., Sharma V. K., Sheu S. S. Mechanism of extracellular ATP-induced increase of cytosolic Ca2+ concentration in isolated rat ventricular myocytes. J Physiol. 1992 Jan;445:369–388. doi: 10.1113/jphysiol.1992.sp018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Danziger R. S., Raffaeli S., Moreno-Sanchez R., Sakai M., Capogrossi M. C., Spurgeon H. A., Hansford R. G., Lakatta E. G. Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Calcium. 1988 Aug;9(4):193–199. doi: 10.1016/0143-4160(88)90023-1. [DOI] [PubMed] [Google Scholar]
- De Lisle R. C., Hopfer U. Electrolyte permeabilities of pancreatic zymogen granules: implications for pancreatic secretion. Am J Physiol. 1986 Apr;250(4 Pt 1):G489–G496. doi: 10.1152/ajpgi.1986.250.4.G489. [DOI] [PubMed] [Google Scholar]
- De Young M. B., Scarpa A. ATP receptor-induced Ca2+ transients in cardiac myocytes: sources of mobilized Ca2+. Am J Physiol. 1989 Oct;257(4 Pt 1):C750–C758. doi: 10.1152/ajpcell.1989.257.4.C750. [DOI] [PubMed] [Google Scholar]
- De Young M. B., Scarpa A. Extracellular ATP induces Ca2+ transients in cardiac myocytes which are potentiated by norepinephrine. FEBS Lett. 1987 Oct 19;223(1):53–58. doi: 10.1016/0014-5793(87)80508-2. [DOI] [PubMed] [Google Scholar]
- Fleetwood G., Gordon J. L. Purinoceptors in the rat heart. Br J Pharmacol. 1987 Jan;90(1):219–227. doi: 10.1111/j.1476-5381.1987.tb16843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kasanuki H., Hosoda S. Background current in sino-atrial node cells of the rabbit heart. J Physiol. 1992 Mar;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Abe S., Sawanobori T., Hiraoka M. External ATP-induced changes in [Ca2+]i and membrane currents in mammalian atrial myocytes. Am J Physiol. 1991 Apr;260(4 Pt 1):C673–C680. doi: 10.1152/ajpcell.1991.260.4.C673. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Evidence that ATP is a neurotransmitter in the frog heart. Eur J Pharmacol. 1986 May 27;124(3):285–289. doi: 10.1016/0014-2999(86)90229-3. [DOI] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T. Activation of chloride current by purinergic stimulation in guinea pig heart cells. Circ Res. 1992 Apr;70(4):851–855. doi: 10.1161/01.res.70.4.851. [DOI] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Pucéat M., Clément O., Scamps F., Vassort G. Extracellular ATP-induced acidification leads to cytosolic calcium transient rise in single rat cardiac myocytes. Biochem J. 1991 Feb 15;274(Pt 1):55–62. doi: 10.1042/bj2740055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M., Clément O., Vassort G. Extracellular MgATP activates the Cl-/HCO3- exchanger in single rat cardiac cells. J Physiol. 1991 Dec;444:241–256. doi: 10.1113/jphysiol.1991.sp018875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F., Legssyer A., Mayoux E., Vassort G. The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ Res. 1990 Oct;67(4):1007–1016. doi: 10.1161/01.res.67.4.1007. [DOI] [PubMed] [Google Scholar]
- Scamps F., Vassort G. Mechanism of extracellular ATP-induced depolarization in rat isolated ventricular cardiomyocytes. Pflugers Arch. 1990 Nov;417(3):309–316. doi: 10.1007/BF00370997. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Tachibana M., Kaneko A. Effects of glycine and GABA on isolated bipolar cells of the mouse retina. J Physiol. 1990 Feb;421:645–662. doi: 10.1113/jphysiol.1990.sp017967. [DOI] [PMC free article] [PubMed] [Google Scholar]


