Abstract
The lutein-5,6-epoxide (Lx) cycle operates in some plants between lutein (L) and its monoepoxide, Lx. Whereas recent studies have established the photoprotective roles of the analogous violaxanthin cycle, physiological functions of the Lx cycle are still unknown. In this article, we investigated the operation of the Lx cycle in light-harvesting antenna complexes (Lhcs) of Inga sapindoides Willd, a tropical tree legume accumulating substantial Lx in shade leaves, to identify the xanthophyll-binding sites involved in short- and long-term responses of the Lx cycle and to analyze the effects on light-harvesting efficiency. In shade leaves, Lx was converted into L upon light exposure, which then replaced Lx in the peripheral V1 site in trimeric Lhcs and the internal L2 site in both monomeric and trimeric Lhcs, leading to xanthophyll composition resembling sun-type Lhcs. Similar to the violaxanthin cycle, the Lx cycle was operating in both photosystems, yet the light-induced Lx → L conversion was not reversible overnight. Interestingly, the experiments using recombinant Lhcb5 reconstituted with different Lx and/or L levels showed that reconstitution with Lx results in a significantly higher fluorescence yield due to higher energy transfer efficiencies among chlorophyll (Chl) a molecules, as well as from xanthophylls to Chl a. Furthermore, the spectroscopic analyses of photosystem I-LHCI from I. sapindoides revealed prominent red-most Chl forms, having the lowest energy level thus far reported for higher plants, along with reduced energy transfer efficiency from antenna pigments to Chl a. These results are discussed in the context of photoacclimation and shade adaptation.
Plants must cope with contrasting light environments throughout their life. Variations in light intensity, spectral composition, and spatiotemporal patterns of fluctuations induce a variety of responses in plants, from the level of the photosynthetic apparatus to whole-plant architecture (Schurr et al., 2006). Species are sometimes classified into sun and shade plants according to the typical light conditions in their habitats. However, many species are in fact able to acclimate and both sun- and shade-plant phenotypes often appear in different individuals of a species or genotype, depending on growth light environments. Furthermore, it is well known that such sun-shade acclimation can be observed in different leaves of a single plant (Björkman, 1981) or even in chloroplasts of different mesophyll cells within a single leaf (Bassi, 1986; Terashima et al., 1986; Vogelmann and Evans, 2002). This remarkable photoacclimatory plasticity supposedly provides a competitive advantage under heterogeneous or fluctuating environments that occur in nature (Walters, 2005).
Photosynthetic performance under strong light is largely determined by the capacity for photosynthetic electron transport, photophosphorylation, CO2 conductance, and CO2 fixation, whereas success under weak light is associated with respiratory down-regulation and augmentation of light capture efficiency (Björkman, 1981; Anderson, 1986). Sun acclimation, for example, leads to increased activity of PSII, ATPase, and Rubisco, whereas shade acclimation enhances accumulation of the major light-harvesting antenna complex (Lhc) of PSII (LHCII) containing chlorophyll (Chl) a, Chl b, and xanthophylls (Anderson et al., 1988; Bailey et al., 2001; Ballottari et al., 2007). Photoacclimation thus engages coordinated resource allocation between the components of these processes (Osmond et al., 1999), which become rate-limiting factors under the corresponding light environments, to achieve a proper balance between energy input and output.
The complement of this photosynthetic adjustment is the operation of photoprotection and damage repair (Osmond et al., 1999), whose importance is most evident in plants exposed to unfavorable conditions. The regulatory mechanisms involved in this side of photosynthesis have also been a focus of thorough investigation (Demmig-Adams and Adams, 1992a; Niyogi, 1999; Osmond et al., 1999; Chow and Aro, 2005). Profound knowledge has been gained about the mechanisms of thermal energy dissipation through physiological, biochemical, biophysical, and genetic approaches (Demmig-Adams and Adams, 1992a; Formaggio et al., 2001; Gilmore, 2001; Morosinotto et al., 2003; Holt et al., 2004, 2005; Horton et al., 2005), as well as recent progress in crystallography of the Lhcs (Liu et al., 2004; Pascal et al., 2005).
One of the constituents of the regulatory mechanism for energy dissipation in Lhcs is the violaxanthin (V) cycle in which V deepoxidase (VDE) and zeaxanthin (Z) epoxidase (ZE) catalyze the interconversions between V, antheraxanthin (A), and Z in a light-dependent manner (Demmig-Adams and Adams, 1992a). Under illumination, V molecules released from Lhcs into the lipid phase of thylakoid membrane are converted to A and Z by the activity of VDE (Yamamoto and Higashi, 1978; Yamamoto, 2006), which reverses the flux direction in the β,β-carotenoid biosynthetic pathway downstream of β-carotene (β-Car). Some of these deepoxidized xanthophylls (Z in particular) then replace V in the peripheral xanthophyll-binding site (V1 site; Caffarri et al., 2001) and one of the two internal binding sites (L2 site) of Lhcs (V → Z exchange; Färber et al., 1997; Verhoeven et al., 1999; Jahns et al., 2001; Morosinotto et al., 2002a; Wehner et al., 2004, 2006). Binding of Z to the site L2 brings about energy dissipation (Wentworth et al., 2000; Frank et al., 2001; Dall'Osto et al., 2005), which is associated with conformational changes in Lhcs (Horton et al., 1991, 1996, 2005; Moya et al., 2001; Morosinotto et al., 2003; Dall'Osto et al., 2005). Alternatively or additionally, it has been suggested that a few Z molecules may bind to PsbS protein, an essential component for energy dissipation in higher plants (Li et al., 2000), and thereby induce strong dissipation (Aspinall-O'Dea et al., 2002; Hieber et al., 2004; Holt et al., 2004). Besides, accumulation of Z in thylakoid membranes can also mitigate lipid peroxidation caused by formation of reactive oxygen species (Havaux and Niyogi, 1999; Havaux et al., 2004). Although the physiological role of the V cycle for PSI is still unknown (Thayer and Björkman, 1992; Lee and Thornber, 1995; Färber et al., 1997; Verhoeven et al., 1999), pronounced increase in the pool size of the V-cycle pigments often found in sun plants or sun leaves indicates the up-regulation of V-cycle-dependent photoprotection in both PSII and PSI during sun acclimation (e.g. Thayer and Björkman, 1990; Demmig-Adams and Adams, 1992b; Verhoeven et al., 1999).
Analogous to the V cycle in the β,β-carotenoid biosynthetic pathway, some species possess another xanthophyll cycle operating in the β,ɛ-carotenoid biosynthetic pathway downstream of α-Car (Bungard et al., 1999; Matsubara et al., 2001, 2003, 2005; García-Plazaola et al., 2002, 2004). This xanthophyll cycle involves part of the pool of lutein (L), the most abundant xanthophyll bound in higher plant Lhcs (Kühlbrandt et al., 1994; Liu et al., 2004), and its monoepoxidized form lutein-5,6-epoxide (Lx). The conversions between L and Lx are presumably catalyzed by the same enzymes as in the V cycle, VDE and ZE. However, the fact that many plants do not contain more than a trace of Lx (Young, 1993) suggests altered substrate specificity or affinity of ZE to L in plants having the Lx cycle (Matsubara et al., 2003). In fact, the two xanthophyll cycles differ in the epoxidation kinetics in the dark, with recovery of Lx being much slower than that of V, whereas light-induced deepoxidation of Lx and V proceeds in much the same way (Matsubara et al., 2001, 2005; García-Plazaola et al., 2002; Snyder et al., 2005). This slow epoxidation in the Lx cycle seems to result in pronounced sun-shade characteristics in Lx concentration (Matsubara et al., 2001, 2002), with a striking example reported for a tropical tree legume Inga sapindoides Willd (Matsubara et al., 2005). In marked contrast to extremely high Lx content in deeply shaded leaves, sun leaves of I. sapindoides typically contain low levels of Lx. Based on the resemblance in chemical structures of these xanthophylls, the parallel deepoxidation kinetics in the two cycles (Matsubara et al., 2001, 2003; García-Plazaola et al., 2002, 2003), as well as the similar distribution patterns of Lx and V within the thylakoids (Matsubara et al., 2003, 2005), a distinctive role of the Lx cycle in photoacclimation has been proposed. It has been suggested that the light-harvesting centers (containing Lx, the dominant form in deeply shaded leaves) are converted into photoprotective centers (containing L, dominant form in sun-exposed leaves) via slowly reversible Lx → L exchange in the internal L2 site (“lock in”) upon illumination (Matsubara et al., 2005). If so, this slowly reversible Lx → L conversion may represent short-term, early steps in the long-term process of shade-to-sun acclimation. However, neither the occurrence of such Lx → L exchange in Lhcs nor its effect on light energy transfer has been demonstrated yet.
In this study, we therefore examined whether slowly reversible Lx → L exchange occurs in Lhcs upon light-induced rapid deepoxidation in shade leaves and whether the relative levels of these xanthophylls shift in a similar manner in Lhcs during shade-to-sun acclimation. As our results clearly showed both short-term Lx → L exchange and long-term Lx → L shift in vivo, we further investigated the effects on the excitation energy transfer in an in vitro system by using monomeric recombinant Lhcs (Lhcb1 and Lhcb5) and native trimeric LHCIIs. Here, we report an indication that the Lx → L exchange can induce protein conformational changes to modulate the light-harvesting efficiency in some antenna complexes. Furthermore, spectroscopic analyses of PSI-LHCI holocomplexes from I. sapindoides revealed conspicuous red Chl forms that have the lowest energy levels so far found in higher plants. The ability of I. sapindoides to accumulate large amounts of Lx in the light-harvesting antennae and the presence of prominent red Chl forms in PSI-LHCI are discussed in the context of photoacclimation and shade adaptation.
RESULTS
Pigment Composition and Distribution in Sun and Shade Thylakoids
Dark-adapted sun and shade leaves of I. sapindoides were collected for thylakoid isolation. Some of the shade leaves were exposed to a light intensity of approximately 200 μmol m−2 s−1 for 30 min prior to dark adaptation (shadeL). The effective PSII efficiency measured at the end of this light treatment was 0.18 (±0.06 sd, n = 8). The maximal PSII efficiency (Fv/Fm) of these preilluminated shadeL leaves did not fully recover during the subsequent 24-h dark adaptation and remained slightly, but not significantly, lower than the Fv/Fm values of other dark-adapted leaves (Table I), suggesting that the light treatment caused little photoinhibition.
Table I.
Pigment composition of thylakoid membranes isolated from dark-adapted sun and shade leaves of I. sapindoides
The maximal PSII efficiency (Fv/Fm) was measured in dark-adapted leaves (n = 8–11, ±sd). Some of the shade leaves were exposed to a light intensity of approximately 200 μmol m−2 s−1 for 30 min to induce xanthophyll deepoxidation (shadeL) before dark adaptation. Concentrations of carotenoid pigments are given on a Chl basis (mmol mol−1 Chl a + b). None of the samples contained detectable levels of Z or A. For pigments, n = 3 (±sd).
| Fv/Fm | Chl a/b | N | V | Lx | L | Lx + L | Lx/L | α-Car | β-Car | α + β-Car | α/β-Car | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shade | 0.79 | 2.6 | 58.0 | 25.1 | 67.9 | 82.1 | 150.0 | 0.83 | 58.5 | 37.0 | 95.4 | 1.58 | 328.6 |
| (0.01) | (0.1) | (0.9) | (0.4) | (1.2) | (4.9) | (5.1) | (0.05) | (0.9) | (5.1) | (5.2) | (0.22) | (7.3) | |
| ShadeL | 0.68 | 2.7 | 53.5 | 20.9 | 26.0 | 106.5 | 132.5 | 0.24 | 53.0 | 38.5 | 91.5 | 1.38 | 298.3 |
| (0.09) | (0.1) | (0.6) | (0.8) | (2.6) | (2.9) | (3.9) | (0.03) | (1.1) | (1.1) | (1.6) | (0.05) | (4.3) | |
| Sun | 0.78 | 3.2 | 49.2 | 32.7 | 25.3 | 87.1 | 112.4 | 0.29 | 48.6 | 53.0 | 101.6 | 0.92 | 295.9 |
| (0.01) | (0.1) | (0.9) | (1.6) | (0.9) | (3.1) | (3.3) | (0.01) | (4.3) | (2.5) | (5.0) | (0.09) | (6.3) |
Higher Chl a-to-Chl b ratios (Chl a/b), indicative of a smaller PSII light-harvesting antenna size, were found in the pigment extracts of thylakoids isolated from sun leaves (Table I). As previously reported (Matsubara et al., 2005), pronounced Lx accumulation was to be seen in thylakoids from shade leaves, whereas the levels of Lx were much lower in sun leaves (67.9 versus 25.3 mmol mol−1 Chl a + b). Although total carotenoids declined from shade to shadeL, due presumably to photooxidation, the decrease in Lx was accompanied by a marked increase in L, the only carotenoid that exhibited an increase after the light treatment, manifesting the light-induced deepoxidation of Lx to L and the slow epoxidation of L back to Lx during the subsequent dark adaptation (Matsubara et al., 2005). The fact that neither Z nor A was detected in these dark-adapted samples, including shadeL, indicates that ZE was active during the dark adaptation. As for the carotenes, sun leaves had much higher levels of β-Car with lower levels of α-Car compared to shade leaves. Accordingly, the balance between these two carotenes (α/β-Car) was >1 in shade and shadeL compared to <1 in sun (Table I).
It has been shown that the distribution pattern of Lx within the thylakoid membrane resembles that of V, indicating similar binding sites for these two xanthophylls in Lhcs (Matsubara et al., 2003, 2005). Then, deepoxidation of Lx to L could induce Lx → L exchange in Lhcs in much the same way as has been demonstrated for V → Z exchange (Bassi et al., 1993; Färber et al., 1997; Jahns et al., 2001; Morosinotto et al., 2002a; Wehner et al., 2004, 2006). To pinpoint the xanthophyll-binding sites for Lx → L exchange and to identify the effects of short- and long-term light exposure on the carotenoid distribution within the thylakoid membrane of Lx-accumulating plants, different pigment-protein complexes were isolated from shade, shadeL, and sun thylakoids by solubilization with 0.6% dodecyl-α-d-maltoside (α-DM) followed by a Suc density gradient.
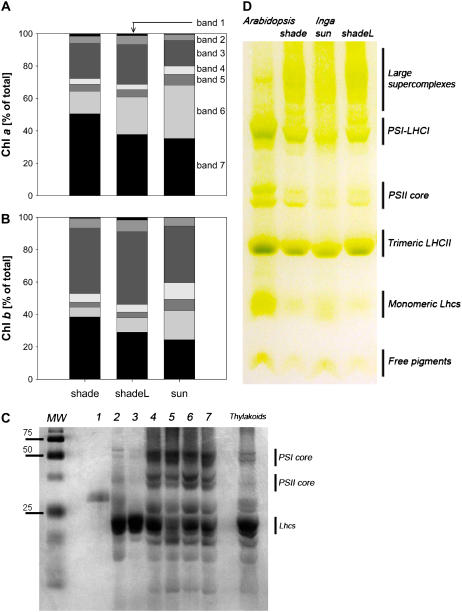
Seven fractions were isolated from each sample. Distribution patterns of Chl a and Chl b among these seven bands are illustrated in Figure 1, A and B, respectively. The bands were identified with the SDS-PAGE profiles (Fig. 1C), as well as absorption spectra (see below). Whereas the mild detergent treatment with 0.6% α-DM allowed isolation of Lhc monomers (band 2) and LHCII trimers (band 3), it did not allow the complete separation of the PSII core and the PSI-LHCI of I. sapindoides (Fig. 1C). The thylakoids of I. sapindoides are further characterized by the prominent band 7, containing large supercomplexes, which also appeared in the native green gel (Fig. 1D). This supercomplex fraction was also observed in our previous study with this species, although it was then regarded as an unsolubilized fraction and hence not included in the analysis (Matsubara et al., 2005). As can be seen in Figure 1D, the same mild treatment allows isolation of the PSII core and PSI-LHCI and yields fewer supercomplexes in Arabidopsis (Arabidopsis thaliana). Here, thylakoids from Arabidopsis and I. sapindoides were solubilized and fractionated under the very same condition and yet the levels of complexes with large Mrs were far greater in the latter.
Figure 1.
Suc density gradient profiles of thylakoids from dark-adapted sun and shade leaves of I. sapindoides. Some of the shade leaves were exposed to approximately 200 μmol m−2 s−1 for 30 min prior to the dark adaptation (shadeL). Distribution profiles of Chl a (A) and Chl b (B) among the seven fractions obtained (bands 1–7, from the lowest to the highest density) are shown. Concentrations of the pigments are given relative to the total (=100%). Polypeptide compositions of the seven bands were then analyzed by denaturing SDS-PAGE (C) and identified by western blotting using specific antibodies (data not shown). Solubilized thylakoids of I. sapindoides were also separated by nondenaturing Deriphat-PAGE and compared with thylakoids from Arabidopsis under the same condition (D).
Generally, only small amounts of free Chls (0.2%–1.7%, band 1) were found in the thylakoid samples of I. sapindoides (Fig. 1, A and B). The carotenoid composition in band 1 (Supplemental Table S1) reflected that of the thylakoids (Table I), although with much higher proportions of Lx, L, and V. More than 60% of Chl a was distributed in the three fractions containing PSII and PSI core complexes (Fig. 1A, bands 5–7). In contrast, more than one-half of Chl b was recovered in the three fractions with high concentrations of Lhcs (Fig. 1B, bands 2–4). The two dominant fractions in shade leaves were bands 3 and 7, both containing LHCIIs. The relative contribution of these two bands was lower in sun leaves, in which other fractions, especially band 6, comprised a greater portion than in shade. These features in the relative abundance of pigment-protein complexes in sun and shade thylakoids are consistent with acclimatory modification of PSII antenna size (Anderson, 1986; Anderson et al., 1988), which is also evident in the Chl a/b ratios (Table I). The distribution patterns of total xanthophylls resembled those of Chl b in all samples (data not shown). Conversely, a large part of carotenes (>95% and >90% for β-Car and α-Car, respectively) were found in bands 5 to 7 containing most of the PSI-LHCI and PSII core complexes.
Short- and Long-Term Effects of Light on the Carotenoid Composition of Lhcs
Concentrations of the major carotenoid species were determined in each Suc gradient band obtained from dark-adapted shade, shadeL, and sun leaves. The carotenoid concentrations of the two Lhc-only bands (bands 2 and 3) are summarized in Table II. For comparison, pigment composition of band 3 from Arabidopsis is also shown. Short-term light exposure caused a decrease in Lx with a simultaneous, and nearly stoichiometric, increase in L in both monomeric and trimeric Lhcs of shadeL. Consequently, the levels of these xanthophylls in shadeL approached those of sun. Still, the number of L molecules found in trimeric LHCIIs of sun was lower than that of Arabidopsis, a non-Lx species. In addition to the light-induced changes in Lx and L, trimeric LHCIIs contained higher levels of V in sun than in shade or shadeL, whereas monomeric Lhcs did not exhibit such a sun-shade difference in V content.
Table II.
Pigment composition of the Suc gradient bands containing Lhcs
Carotenoid concentrations are normalized to 100 Chl molecules to facilitate comparison of different samples. Carotenes were not detected in these bands (nd). Pigment composition of band 3 from Arabidopsis is also shown for comparison. sd < ±10%.
| Chl a/b | Carotenoid per 100 Chls
|
||||||
|---|---|---|---|---|---|---|---|
| N | V | Lx | L | α-Car | β-Car | ||
| Band 2 (monomeric Lhcs) | |||||||
| Shade | 1.8 | 6.1 | 3.7 | 7.8 | 10.5 | nd | nd |
| ShadeL | 1.9 | 8.0 | 2.2 | 2.5 | 14.1 | nd | nd |
| Sun | 2.2 | 6.8 | 3.8 | 2.5 | 12.0 | nd | nd |
| Band 3 (trimeric LHCIIs) | |||||||
| Shade | 1.3 | 9.1 | 1.4 | 7.4 | 10.5 | nd | nd |
| ShadeL | 1.3 | 9.0 | 1.0 | 2.6 | 15.3 | nd | nd |
| Sun | 1.5 | 8.2 | 3.1 | 3.3 | 13.8 | nd | nd |
| Arabidopsis | 1.4 | 9.2 | 2.5 | nd | 16.8 | nd | nd |
Thus, illumination seems to convert Lhc proteins from the Lx-rich type (shade) into the L-rich type (shadeL) via light-dependent deepoxidation and concomitant Lx → L exchange. Unlike the V cycle, the conversion and exchange of Lx → L was not fully reversible during 24-h dark adaptation. We note that the occurrence of Lx → L exchange was also confirmed in other Suc-gradient bands containing Lhcs of PSII and PSI (see below), suggesting that the Lx → L exchange happens in both PSII and PSI, as has been shown for the V → Z exchange (Thayer and Björkman, 1992; Lee and Thornber, 1995; Färber et al., 1997; Verhoeven et al., 1999). During long-term sun acclimation and down-sizing of LHCIIs (Table I), V seems to replace some of the Lx and L molecules in these complexes.
Sites of the Lx → L Exchange in Trimeric LHCII
The major Lhc, trimeric LHCII, has four xanthophyll-binding sites: three internal sites, termed L1, L2, and N1, and a peripheral site, termed V1 (Kühlbrandt et al., 1994; Croce et al., 1999; Liu et al., 2004). Because the data in Table II indicated Lx → L exchange in both monomeric and trimeric Lhcs upon deepoxidation, analogous to V → Z exchange during the operation of the V cycle, we examined the xanthophyll-binding sites involved in Lx → L exchange by isolating band 3 with a mild (0.6% α-DM) and a more stringent (1.0% β-DM) detergent. Because loosely bound xanthophylls in V1 are removed by harsh treatments (Ruban et al., 1999; Verhoeven et al., 1999; Caffarri et al., 2001), the difference in xanthophyll composition found between the samples isolated with α- and β-DM should reflect the xanthophyll species in the V1 site.
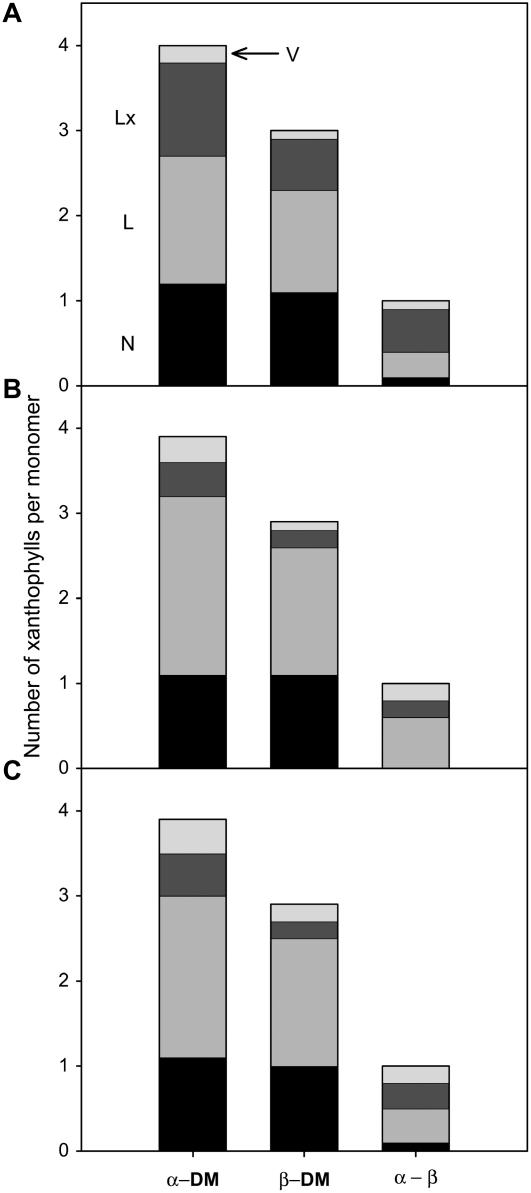
Assuming 14 and 12 Chl molecules per monomer for trimeric LHCIIs isolated with α- and β-DM, respectively (Dainese and Bassi, 1991; Liu et al., 2004), we estimated the number of xanthophyll pigments per monomer (Fig. 2). In accordance with the assumption, four xanthophylls were found in the α-DM samples, whereas only three were recovered in the β-DM samples. One molecule of neoxanthin (N) was present in all samples regardless of the detergent used or the light conditions of the leaves. Given the high specificity of the N1 site to N (Croce et al., 1999; Ruban et al., 1999; Caffarri et al., 2001), the recovery of almost exactly one N molecule in all samples verifies our assumption of Chl-binding stoichiometries. At least one L molecule per monomer was always present, very likely L in the site L1, which is conserved among all Lhcs and essential for protein folding and stability (Bassi et al., 1999; Formaggio et al., 2001). These two xanthophyll molecules, N in N1 and L in L1, seemed to be conserved in most LHCIIs of I. sapindoides, as is also the case in other higher plant species.
Figure 2.
Estimated number of xanthophylls in native LHCII complexes isolated from shade (A), shadeL (B), and sun leaves (C) of I. sapindoides. Band 3 from the Suc density gradient was isolated by a mild (0.6% α-DM) and a more stringent (1.0% β-DM) detergent treatment to identify the loosely bound xanthophylls in the peripheral V1 site, which are represented by the difference between the samples isolated with α- and β-DM (α−β). Xanthophyll levels are given per monomer assuming 14 and 12 Chl molecules per monomer of the trimeric LHCII complex isolated with α- and β-DM, respectively.
The difference between the samples solubilized with α- and β-DM (α−β) was largely attributable to Lx in shade (Fig. 2A), suggesting that Lx occupies more than one-half of the V1 sites in trimeric LHCIIs of shade leaves. The light treatment altered the dominant pigment species in V1 sites, from Lx in shade to L in shadeL (Fig. 2B). On the other hand, LHCIIs from sun leaves contained similar levels of V, Lx, and L in V1 (Fig. 2C). We note that the xanthophyll composition in the peripheral V1 sites is in line with the relative abundance of these pigments in the free pigment fraction (Supplemental Table S1). The composition of the third pigment in the β-DM samples, in addition to the common N in N1 and L in L1, likely represents the different xanthophyll species in the site L2. The main xanthophyll in the third pigment fraction was Lx in shade (Fig. 2A), whereas it was mostly replaced by L in shadeL and sun (Fig. 2, B and C). Hence, Lx → L exchange seems to take place in V1 as well as L2 in LHCIIs of I. sapindoides.
Carotenoid Composition and Spectroscopic Characteristics of the PSII Core and PSI-LHCI
Carotenoid composition was also analyzed in the Suc gradient bands containing PSII and PSI core complexes (Table III). Because solubilization with 0.6% α-DM did not allow complete separation of the PSII core and PSI-LHCI for I. sapindoides (Fig. 1), band 5 was reloaded onto another Suc gradient after pelleting and resuspending in 0.6% α-DM to obtain a PSII-enriched fraction (band 5b). Likewise, a second Suc gradient was run for band 6 to improve the purity of PSI-LHCI (band 6b), here albeit by resuspending in a stronger detergent (0.8% β-DM) because of the higher stability of PSI-LHCI (Ballottari et al., 2004).
Table III.
Pigment composition of the four Suc gradient bands containing core and antenna complexes
Bands 5 and 6 from the first Suc gradient were pelleted, resuspended in 0.6% α-DM or 0.8% β-DM, respectively, and loaded onto a second Suc gradient ultracentrifugation (bands 5b and 6b). Carotenoid concentrations are normalized to 100 or 180 (band 6b) Chls. N was not detected in band 6b (nd). sd was <±10%.
| Chl a/b | N | V | Lx | L | α-Car | β-Car | α/β-Car | |
|---|---|---|---|---|---|---|---|---|
| Band 4 | Carotenoid per 100 Chls | |||||||
| Shade | 1.6 | 6.8 | 2.3 | 6.9 | 9.4 | 1.8 | 0.7 | 2.6 |
| ShadeL | 1.7 | 6.9 | 1.8 | 2.4 | 12.9 | 1.7 | 1.1 | 1.6 |
| Sun | 1.6 | 7.0 | 3.3 | 2.7 | 12.1 | 0.8 | 0.8 | 1.0 |
| Band 5b (enriched in PSII core complexes) | Carotenoid per 100 Chls | |||||||
| Shade | 6.8 | 2.0 | 0.4 | 1.7 | 2.8 | 7.9 | 4.2 | 1.9 |
| ShadeL | 5.5 | 2.1 | 0.6 | 1.0 | 3.9 | 8.3 | 6.4 | 1.3 |
| Sun | 10.4 | 1.3 | 0.4 | 1.0 | 2.8 | 5.8 | 6.7 | 0.9 |
| Band 6b (PSI-LHCI complexes) | Carotenoid per 180 Chls | |||||||
| Shade | 10.2 | nd | 1.8 | 4.9 | 2.9 | 15.8 | 12.9 | 1.2 |
| ShadeL | 9.0 | nd | 1.9 | 2.6 | 4.1 | 14.3 | 13.3 | 1.1 |
| Sun | 9.5 | nd | 2.4 | 2.0 | 5.2 | 12.1 | 14.7 | 0.8 |
| Band 7 (enriched in PSII supercomplexes) | Carotenoid per 100 Chls | |||||||
| Shade | 3.2 | 3.0 | 0.9 | 4.3 | 5.4 | 6.2 | 3.7 | 1.7 |
| ShadeL | 3.2 | 2.0 | 1.9 | 1.9 | 9.6 | 7.6 | 6.4 | 1.2 |
| Sun | 4.6 | 2.7 | 0.8 | 1.4 | 4.9 | 4.9 | 6.8 | 0.7 |
The low Chl a/b and the presence of all major xanthophylls (Table III) indicate that band 5b still contained Lhcs together with PSII core complexes. Yet, it is clear that the pigment compositions of shade and shadeL were more similar to each other than to sun, which was characterized by higher Chl a/b. The resemblance of band 5b from shade and shadeL was further corroborated by the similarity in their absorption and fluorescence excitation spectra (Supplemental Fig. S1). In agreement with its higher Chl a/b, the PSII samples from sun leaves exhibited weaker absorption and fluorescence excitation than shade and shadeL in the spectral region of antenna pigments (>450 nm).
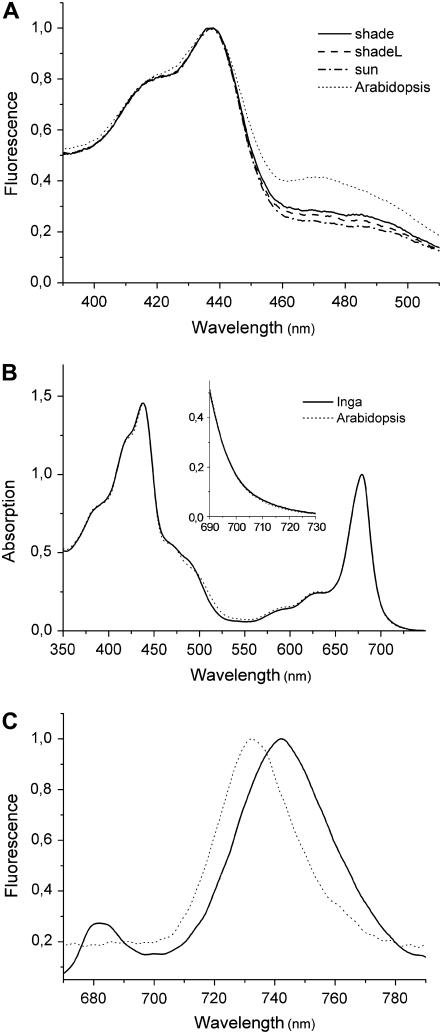
The second solubilization by using 0.8% β-DM allowed isolation of PSI-LHCI largely depleted of PSII particles (band 6b), as confirmed by the absence of N, which is specifically bound in the antenna complexes of PSII, but not in PSI-LHCI. Similar Chl a/b and the total carotenoid concentrations found in all band 6b samples (Table III) suggest that short- or long-term light exposure did not affect the antenna size of PSI in I. sapindoides. The illumination increased the L content in band 6b at the expense of Lx to bring the Lx:L ratio of shadeL close to sun, indicating the slowly reversible Lx → L exchange in the antenna complexes of PSI. However, these changes in carotenoid composition had little influence on the fluorescence excitation spectra of PSI-LHCI (Fig. 3A). Although small differences were found in the Soret region >440 nm, all Inga samples exhibited much lower fluorescence excitation in this region compared with Arabidopsis. The diminished fluorescence excitation in I. sapindoides was not due to lower light absorption because the absorption spectra of the two species were very similar in this spectral region (Fig. 3B). Importantly, measurements of low-temperature fluorescence emission spectra revealed that the prominent peak emanating from LHCI was red-shifted by about 10 nm in I. sapindoides compared to the corresponding peak in Arabidopsis (Fig. 3C), despite the fact that the two species did not show large differences in absorption in the red region at room temperature (Fig. 3B, inset) or low temperature (data not shown). These spectroscopic characteristics of PSI-LHCI were common to all Inga samples (data not shown) and thus seem to be species specific rather than an acclimatory response.
Figure 3.
Spectral characteristics of the PSI-LHCI fractions (band 6b). A, Comparison of the room-temperature fluorescence excitation spectra of shade (solid line), shadeL (dashed line), and sun (dash-dotted line) for I. sapindoides as well as Arabidopsis (dotted line). Fluorescence was detected at 685 nm. Data were normalized to the maximum. B, Room-temperature absorption spectra of I. sapindoides (solid line, only the data from shade are shown) and Arabidopsis (dotted line). The inset shows a closeup of the spectral region between 690 and 730 nm. Data were normalized to the maximum in the Qy region. C, Low-temperature fluorescence emission spectra of I. sapindoides (solid line) and Arabidopsis (dotted line). Spectra were measured with an excitation wavelength of 475 nm. Data were normalized to the maximum. Pigment composition of these samples (except for Arabidopsis) is given in Table III.
Comparison of Recombinant Lhc Proteins with Different Lx and L Content
Having found the Lx → L exchange in V1 and L2 sites of LHCII (Fig. 2), we then probed its effects on excitation energy transfer within individual Lhcs. It has been shown that binding of Z in L2 induces protein conformational changes to bring about energy dissipation (Moya et al., 2001). The observed Lx → L exchange in L2 raises an intriguing question about its physiological function. To address this question, we analyzed the spectroscopic characteristics of recombinant Lhcs containing different levels of Lx and/or L. Two proteins were used for this experiment as a model for the major and minor antenna complexes of PSII: Lhcb1, the most abundant LHCII polypeptide, and Lhcb5 (also called CP26) in which efficient V → Z exchange in L2 (Morosinotto et al., 2002a; Wehner et al., 2006) and strong fluorescence quenching upon Z binding (Wentworth et al., 2000; Frank et al., 2001; Dall'Osto et al., 2005) have been documented. The recombinant Lhcb1 (from Hordeum vulgare) and Lhcb5 (from Arabidopsis) were reconstituted with a pigment mix containing L and/or Lx in addition to Chl a and Chl b. For reconstitution of Lhcb1, which specifically binds one molecule of N in N1 (Fig. 2; Croce et al., 1999; Ruban et al., 1999; Caffarri et al., 2001), N was also added to the pigment mix. We note that the pigment sample of Lx used for the protein re constitution contained approximately 10% A. Accordingly, a small amount of A was found in all samples reconstituted with a pigment mix containing Lx, especially in Lhcb5, which has a relatively high affinity for A (Table IV).
Table IV.
Pigment composition of the recombinant Lhcb1 and Lhcb5
Recombinant Lhcb1 (420 μg of apoprotein) was reconstituted with 25 μg N, 65 μg L, Lx, or L:Lx (1:1), and 240 μg Chls (Chl a/b = 2.3). The same amount of recombinant Lhcb5 was reconstituted with 60 μg L, Lx, or L:Lx (1:1), and 240 μg Chls (Chl a/b = 3.0). The Lx pigment used for the reconstitution contained approximately 10% A. Samples were then separated from free pigments by Suc density gradient. Xanthophyll concentrations are normalized to 12 or nine Chl molecules for Lhcb1 and Lhcb5, respectively, according to the previous studies (see text). sd < ±0.1.
| Chl a/b | N | Lx | L | A | Total | |
|---|---|---|---|---|---|---|
| Lhcb1 | Xanthophyll per monomer (12 Chls) | |||||
| NL | 1.6 | 1.0 | – | 2.0 | – | 3.0 |
| NLx | 1.7 | 1.0 | 1.7 | – | 0.2 | 2.9 |
| NLLx | 1.6 | 0.9 | 0.7 | 1.4 | 0.1 | 3.1 |
| Lhcb5 (CP26) | Xanthophyll per monomer (9 Chls) | |||||
| L | 2.5 | – | – | 2.1 | – | 2.1 |
| Lx | 2.7 | – | 1.9 | – | 0.4 | 2.3 |
| LLx | 2.7 | – | 0.9 | 1.2 | 0.2 | 2.3 |
Regardless of the xanthophyll compositions added, Chl a/b of about 1.6 to 1.7 was obtained for Lhcb1 (Table IV), which is comparable with the values found in other studies (Croce et al., 1999; Jahns et al., 2001; Morosinotto et al., 2002a). The Chl a/b for Lhcb5 was about 2.5 to 2.7, being somewhat higher than the values reported for recombinant Lhcb5 from Zea mays (Frank et al., 2001; Croce et al., 2002; Morosinotto et al., 2002a). Based on the previous studies (Croce et al., 1999, 2002), we assumed binding of 12 and 9 Chls for a monomeric apoprotein of Lhcb1 and Lhcb5, respectively. Three xanthophyll molecules were found to be tightly incorporated into Lhcb1, whereas Lhcb5 had slightly more than two. Assuming that the site V1 is absent in monomers and N1 is either largely empty (Lhcb5) or occupied by N (Lhcb1), L1 and L2 are the two major binding sites to which L and Lx could bind in this experiment. Similar yields of refolded proteins were obtained for both Lhcb1 and Lhcb5 with and without L, suggesting that Lx bound in L1 can support efficient protein folding. When L and Lx were competing for binding sites, the binding affinity of L was twice as high as that of Lx for Lhcb1, whereas the difference was smaller for Lhcb5 (Table IV).
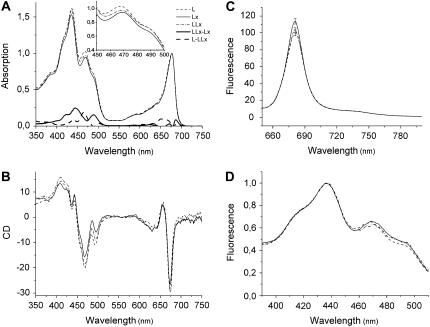
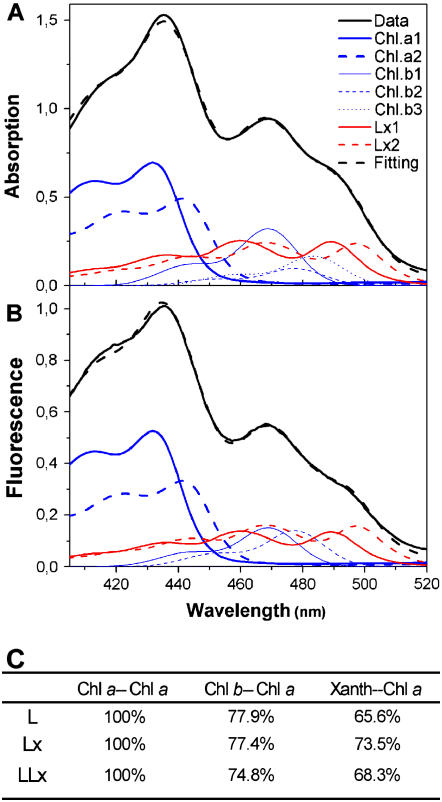
Spectroscopic analyses were performed with these recombinant proteins to characterize the effects of Lx and/or L binding. The absorption spectra of the three recombinant Lhcb5 samples revealed small, but conspicuous, differences in the Soret region (Fig. 4A, inset), as well as the Qy region. The difference spectra are shown for LLx − Lx (the difference between the samples reconstituted with L + Lx and Lx) and L − LLx. The sum of these two difference spectra corresponds to L − Lx (data not shown). To verify the consistency of these differences in the absorption spectra, circular dichroism spectra, which are sensitive to changes in organization and interactions of pigments in the protein matrix, were also examined in the same samples (Fig. 4B). The three samples were clearly distinguishable by the amplitudes and minima of the negative signals in the xanthophyll red-most transition (493, 494, and 496 nm for L, LLx, and Lx, respectively), as has been shown for recombinant Lhcb5 having different xanthophyll species (Croce et al., 2002). The two samples with Lx differed from the sample without Lx at 443 (+) and 630 (−) nm. Between the two Lx-containing samples, the one with L + Lx exhibited greater amplitude of the major signal at 673 (−) nm than the one with Lx only. Together with Figure 4A, these data indicate that Lx and L exert distinct effects on the environment of some Chl molecules in recombinant Lhcb5.
Figure 4.
Spectral properties of the recombinant Lhcb5 reconstituted with Lx (solid line), L (dashed line), or L + Lx (dash-dotted line). A, Room-temperature absorption spectra. Magnified difference spectra (×3) are also shown for the difference between the samples reconstituted with L + Lx and Lx (LLx − Lx, solid bold line) and with L and L + Lx (L − LLx, dashed bold line). Details of the spectral region between 450 and 500 nm can be seen in the inset. Data were normalized to Chl content. B, Circular dichroism spectra normalized to the Chl content. C, Comparison of fluorescence yield at room temperature. The error bar at the emission peak indicates sd (n = 3). Fluorescence emission was recorded with an excitation wavelength of 625 nm. D, Room-temperature fluorescence excitation spectra. Fluorescence was detected at 682 nm and data were normalized to the maximum. Pigment composition of these samples is given in Table IV.
Xanthophylls are known to modulate light-harvesting efficiency and energy dissipation in the antenna complexes. The changes in the Chl environment induced by binding of Lx and/or L (Fig. 4, A and B) may modify excitation energy transfer within Lhcb5. Thus, we analyzed energy transfer efficiency in the recombinant Lhcb5 samples by measuring fluorescence emission and excitation spectra (Fig. 4, C and D). Although the shape of the fluorescence emission spectra did not differ between the samples (Fig. 4C), direct excitation of Chl a at Qx transition (625 nm) gave rise to a significantly higher fluorescence yield in the sample with Lx (+13.6% compared with L and +10.6% compared with L + Lx; P < 0.015). There was no significant difference between the fluorescence yield of the samples with L and with L + Lx. Comparison of the fluorescence excitation spectra (Fig. 4D) and the absorption spectra (Fig. 4A) indicated the highest and the lowest energy transfer efficiency from the antenna pigments to Chl a in the sample with Lx and with L, respectively. The former had the highest fluorescence excitation in 460 to 510 nm, whereas absorption in this spectral region was higher in the latter.
For a more quantitative analysis of energy transfer efficiency from the antenna pigments to Chl a, both the absorption (Fig. 5A) and fluorescence excitation spectra (Fig. 5B) were deconvoluted in terms of the spectra of individual pigments in the protein matrix (Croce et al., 2000) by using the constraints described in “Materials and Methods.” With the energy transfer efficiency among Chl a molecules (Chl a → Chl a) being fixed to be 100%, energy transfer efficiency of Chl b → Chl a and xanthophyll → Chl a was calculated from the deconvoluted models of the absorption and fluorescence excitation spectra (Fig. 5C). The highest increase in xanthophyll → Chl a energy transfer efficiency was found in the sample reconstituted with Lx (+12% compared with L). In contrast to the variations in xanthophyll → Chl a efficiency, different Lx and L levels had little effect on Chl b → Chl a energy transfer efficiency in Lhcb5.
Figure 5.
Fitting of the room-temperature absorption (A) and fluorescence excitation spectra (B) of recombinant Lhcb5 with the spectra of individual pigments in the protein matrix. Only the data from the sample reconstituted with Lx are shown. C, Energy transfer efficiency calculated for the transfer from Chl b to Chl a (Chl b → Chl a) and from xanthophylls to Chl a (Xanth → Chl a) based on the deconvoluted models of the absorption and the fluorescence excitation spectra. The energy transfer efficiency among Chl a molecules (Chl a → Chl a) was fixed to be 100%.
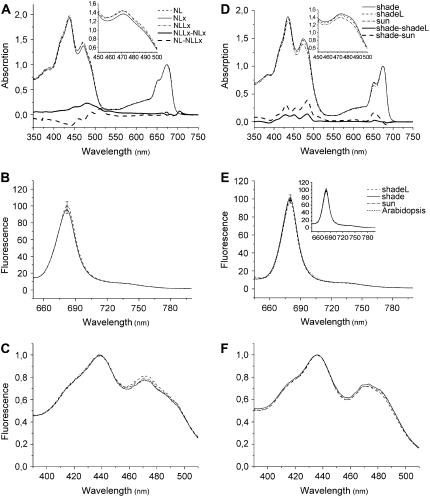
A contrasting picture was obtained for the recombinant Lhcb1 (Fig. 6, A–C). Unlike in Lhcb5, the differential effects of Lx and L on the absorption spectra of Lhcb1 were mainly restricted to the spectral region associated with these xanthophylls (Fig. 6A). There was no significant change in fluorescence yield (<5%; Fig. 6B) between the samples having different Lx:L ratios and the fluorescence excitation spectra largely reflected the differences in the absorption spectra (Fig. 6C). These findings in recombinant Lhcb1 were confirmed by the experiment with native trimeric LHCIIs (band 3) isolated from shade, shadeL, and sun (Fig. 6, D–F). Whereas sun and shade samples, having slightly different Chl a/b (Table II), varied in both Soret and Qy regions of the absorption spectra (shade-sun), the difference between shade and shadeL, for which the Lx → L exchange in L2 and V1 represented the only distinction (Table II; Fig. 2), indicated a small effect in the Soret region with hardly any difference in the Qy region (Fig. 6D). The fluorescence yields of these native LHCII samples varied only marginally (<5%; Fig. 6E), suggesting a minor role of Lx → L exchange (in shadeL) or substitution of these pigments with V (in sun) for energy transfer efficiency within isolated LHCII complexes. Furthermore, we measured comparable fluorescence yields in LHCII trimers of I. sapindoides and Arabidopsis (Fig. 6E), despite their different pigment-binding affinities and the absence of Lx in the latter, which also supports the notion that Lx, L, and V are equivalent in terms of energy transfer efficiency within isolated LHCII. Removal of xanthophylls from V1 by the β-DM treatment (Fig. 2) did not significantly alter fluorescence yield in trimeric LHCIIs (Fig. 6E, inset), confirming the previous demonstration that the xanthophylls in V1 are not involved in light harvesting (Caffarri et al., 2001). Hence, the Lx → L exchange in L2 seems to modify the environment of some Chls to affect Chl a → Chl a as well as xanthophyll → Chl a energy transfer efficiency within Lhcb5 (Figs. 4 and 5), but not within Lhcb1 and LHCII (Fig. 6).
Figure 6.
Spectral properties of the recombinant Lhcb1 (A–C) reconstituted with N + Lx (solid line), N + L (dashed line), or N + L + Lx (dash-dotted line) and the native trimeric LHCIIs obtained with 0.6% α-DM (D–F) from shade (solid line), shadeL (dashed line), and sun (dash-dotted line) leaves of I. sapindoides and Arabidopsis (dotted line). A and D, Room-temperature absorption spectra. Magnified difference spectra (×3) are also shown for the difference between the samples reconstituted with N + L + Lx and N + Lx (NLLx − NLx, solid bold line) and with N + L and N + L + Lx (NL − NLLx, dashed bold line) for the recombinant Lhcb1 and between shade and shadeL (shade − shadeL, solid bold line) and between shade and sun (shade − sun, dashed bold line) for the native trimeric LHCIIs. Details of the spectral region between 450 and 500 nm can be seen in the insets. All spectra were normalized to Chl content. B and E, Comparison of fluorescence yield at room temperature. The error bar at the emission peak indicates sd (n = 3). Fluorescence emission was recorded with an excitation wavelength of 625 nm. For the native LHCIIs, data from the samples obtained by solubilization with 1.0% β-DM (having the V1 site empty) are also shown in the inset. C and F, Room-temperature fluorescence excitation spectra. Fluorescence was detected at 682 nm and spectra were normalized to the maximum. Pigment composition of the recombinant Lhcb1 samples is given in Table IV and that of the native trimeric LHCIIs in Table II. Xanthophyll composition of the native trimeric LHCIIs of I. sapindoides isolated with α- or β-DM is illustrated in Figure 2.
DISCUSSION
Lx → L Exchange Occurs in V1 and L2 Sites
It is widely accepted that V molecules in the peripheral site V1 of trimeric LHCIIs represent the major substrate pool for rapid deepoxidation in the V cycle (Caffarri et al., 2001). Upon deepoxidation to Z, these molecules are thought to be redistributed within the thylakoid membranes (Verhoeven et al., 1999) to replace not only the pigments in V1, but also those in the internal L2 sites of different Lhcs (Bassi et al., 1999; Morosinotto et al., 2003). Indeed, occurrence of such V → Z exchange in internal binding sites has been demonstrated by using recombinant Lhcs of both PSII and PSI (Jahns et al., 2001; Morosinotto et al., 2002a; Wehner et al., 2004, 2006). Analogously, V1 has been suggested to be a possible binding site for Lx (Matsubara et al., 2005) based on the similar distribution patterns of Lx and V among different pigment-protein complexes (Matsubara et al., 2003, 2005) and their parallel deepoxidation kinetics during light exposure (Bungard et al., 1999; Matsubara et al., 2001, 2005; García-Plazaola et al., 2002, 2003). In the case of I. sapindoides, retention of high levels of photoconverted L in sun leaves has been attributed in part to the slowly reversible binding (“lock in”) of L in the L2 site (Matsubara et al., 2005).
In this study, we examined the localization of Lx and photoconverted L in the antenna complexes to identify the xanthophyll-binding sites for the Lx → L exchange. Our data in Figure 2 clearly show that Lx is the dominant xanthophyll species in V1 and L2 of LHCIIs in shade leaves. The short light treatment resulted in the replacement of Lx with L in both V1 and L2, whereas long-term sun acclimation led to accumulation of L in L2 and, to a lesser extent, in V1. These short- and long-term responses of Lx and L were similar in monomeric and trimeric Lhcs (Table II) as well as PSI-LHCI (Table III). Thus, we conclude that the Lx → L exchange occurs in the same binding sites as the V → Z exchange, namely, V1 and L2 sites of the antenna complexes of PSII and PSI, regulated presumably by the same mechanism. It is worth noting that a major difference between I. sapindoides and previously analyzed plants is the extent of xanthophyll exchange in LHCII. In fact, the activity of Lx → L exchange in Inga LHCII is very high (Table II; Fig. 2) compared with the activity of V → Z exchange reported in LHCII of other plants (Färber et al., 1997; Verhoeven et al., 1999; Caffarri et al., 2001; Jahns et al., 2001). Currently, it is not possible to attribute this enhanced Lx → L exchange in LHCII of I. sapindoides to different protein structure and/or different affinity of Lx → L exchange with respect to V → Z exchange. Additional work is needed to elucidate this point.
What could be the causes of the retention of high L, but not Z and A, after short-term light exposure (Table I)? Slower postillumination recovery of Lx compared with V seems to be a common feature of the Lx-accumulating plants studied thus far (Matsubara et al., 2001, 2005; García-Plazaola et al., 2002; Snyder et al., 2005), which raised the question as to whether the conversions between Lx and L function as a true cycle (Matsubara et al., 2005; Snyder et al., 2005). One possible explanation for the delayed Lx recovery is slower release of locked-in L from L2, resulting in slower epoxidation of L than Z (Matsubara et al., 2005). Although such apoprotein steric hindrance could explain dark retention of L in L2, the finding of L in the peripheral V1 site in shadeL (Fig. 2) and in the free pigment fraction (Supplemental Table S1; Matsubara et al., 2003, 2005) demands an additional or alternative explanation. For instance, lower catalytic activity of the epoxidase enzyme for L compared with Z may underlie the delayed recovery of Lx. Epoxidation of L to Lx has been regarded as the key step for the occurrence of the Lx cycle (Matsubara et al., 2003), but the enzyme responsible for this reaction has not been identified yet. The most likely candidate is ZE, which catalyzes epoxidation reactions in the β-ring of the deepoxidized β,β-xanthophylls Z and A in the V cycle. So far, however, there has been only one study in which the activity of ZE was tested for L (Bouvier et al., 1996). Although the experiment by Bouvier et al. (1996) using ZE from a non-Lx plant (Capsicum annuum) indicated little ZE activity for L, the affinity of this enzyme to L may have been increased in Lx plants, presumably by mutation following gene duplication (Matsubara et al., 2003), to bring about substantial accumulation of Lx in shade. Identification and characterization of putative ZE enzymes from Lx plants could verify this hypothesis.
Physiological Role of the Lx Cycle
The operation of the V cycle has been associated with the regulation of thermal energy dissipation in the PSII Lhcs (Demmig-Adams and Adams, 1992a; Horton et al., 1996, 2005; Gilmore, 2001; Morosinotto et al., 2003; Holt et al., 2004; Dall'Osto et al., 2005). Based on the similar chemical structures of the pigments, as well as the parallel deepoxidation kinetics of the two xanthophyll cycles, an analogy has been drawn with the V cycle when speculating about the functions of the Lx cycle. Hence, a possible role of the Lx cycle in energy dissipation has been suggested (Matsubara et al., 2001, 2003; García-Plazaola et al., 2002, 2003) and the effect of photoconverted L on energy dissipation has been examined (García-Plazaola et al., 2003; Matsubara et al., 2005). Whereas the involvement of L in energy dissipation, direct or indirect, has been demonstrated in mutant and transgenic plants with altered L levels (Niyogi et al., 1997; Pogson et al., 1998; Pogson and Rissler, 2000; Lokstein et al., 2002), unequivocal evidence of energy dissipation by photoconverted L in the Lx cycle has not been presented yet due mainly to the difficulty in separating the effects of the two xanthophyll cycles operating in parallel.
On the other hand, pronounced accumulation of Lx in shade and its quick conversion to L upon short illumination or substitution by L and V during long-term acclimation to high irradiance (Tables I and II; Fig. 2) may imply a unique function for Lx under shade conditions. Consistent with this notion, we found a high fluorescence yield in Lhcb5 reconstituted with Lx (Fig. 4C). As the absorbed light energy can either be reemitted as fluorescence or dissipated as heat in isolated antenna complexes, the high fluorescence yield of Lhcb5 with Lx indicates that less energy was lost via thermal dissipation. It should be noted that this high fluorescence yield was measured in the sample despite the presence of a small amount of A (Table IV), which may have exerted an opposite, dissipative effect (Gilmore et al., 1998; Wentworth et al., 2000). Hence, the fluorescence yield of the recombinant Lhcb5 should have been even higher if it had been reconstituted with 100% Lx. According to the previous observation that fluorescence yields vary little between the Lhcb5 samples reconstituted with L alone, V alone, or a mixture of L, N, and V (Frank et al., 2001), the fluorescence yield we found in Lhcb5 with Lx would exceed not only those of the samples with L and L + Lx, but also with V and L + N + V, suggesting a peculiar effect of Lx (i.e. increasing light-harvesting efficiency) in Lhcb5.
Whereas L is most likely the major xanthophyll species in L1 of LHCII in I. sapindoides (Fig. 2), as is the case in many other species, the occurrence of Lx in the L1 site cannot be ruled out. The small pool of Lx found in the internal binding sites of trimeric LHCIIs from shadeL and sun (Fig. 2, B and C) could represent such Lx molecules bound in L1, analogous to the nonconvertible pool of V in the V cycle (Jahns et al., 2001). Furthermore, the Lx-binding affinity seems to differ among Lhcs, as is exemplified by the higher Lx:L ratio of Lhcb5 with L + Lx compared with Lhcb1 with N + L + Lx (Table IV). Thus, we propose that Lx could occupy both L1 and L2 (and also V1 for trimeric LHCII) in some of the antenna complexes and the light-induced Lx → L exchange in L2 may modify excitation energy transfer (Fig. 4). In this scenario, Lx slowly accumulates under prolonged shade conditions to increase light-harvesting efficiency, but is quickly converted into L by VDE upon light exposure, which results in lower energy transfer efficiency (Fig. 4C) and probably also higher photoprotective efficiency (Matsubara et al., 2005). Although Z formation in the V cycle is needed for strong energy dissipation (Demmig-Adams and Adams, 1992a; Gilmore, 2001; Morosinotto et al., 2003; Holt et al., 2004, 2005; Horton et al., 2005), for mitigation of photooxidative damage (Havaux and Niyogi, 1999; Havaux et al., 2004), and thus the pool size of the V cycle increases in sun leaves (Table I), Lx may play an important role in acclimation to shade environments. In addition to large antenna size conferring an increased absorption cross section upon PSII (Anderson, 1986; Anderson et al., 1988), substantial accumulation of Lx may facilitate efficient excitation energy transfer within the extended antenna complexes under deeply shaded conditions in which light severely limits photosynthesis.
There are a few points in this hypothesis that need to be addressed in the future. First, the effect of Lx may not be confined within the intrasubunit energy transfer analyzed in this study. Although our data in Figure 6 showed that Lx binding does not significantly alter energy transfer within isolated Lhcb1 or trimeric LHCII, Lx → L exchange in the peripheral V1 site (Fig. 2) may modify the macroorganization of antenna complexes, similar to the proposed role of V → Z exchange in V1 in the allosteric model of energy dissipation (Horton et al., 1991, 2005). In this context, it is also worth mentioning that PSII-LHCII supercomplexes show higher stability in I. sapindoides than in Arabidopsis (Fig. 1), although its relevance to Lx is not clear. Effects of the Lx cycle on the protein macroorganization are yet to be investigated in more detail. Second, photoprotective functions of the Lx cycle need to be examined. It has been suggested that the locked-in L molecules in the internal binding sites may contribute to quenching of Chl triplets (Matsubara et al., 2005). Engagement of L in energy dissipation (Niyogi et al., 1997; Pogson et al., 1998; Pogson and Rissler, 2000; Lokstein et al., 2002) also awaits demonstration in Lx plants. Notably, we found that binding of Lx or L did not significantly alter the susceptibility of the recombinant Lhcb5 and Lhcb1 to photobleaching (Supplemental Fig. S2). Future experiments could clarify photoprotective properties of these β,ɛ-xanthophylls.
α- and β-Car in Photoacclimation
The comparison between sun and shade leaves points to a photoacclimatory shift between β,ɛ-carotenoids and β,β-carotenoids (Table I). Accumulation of α-Car in addition to β-Car has been reported in leaves of shade-tolerant species or shade-grown plants (Thayer and Björkman, 1990; Demmig-Adams and Adams, 1992b; Siefermann-Harms, 1994; Demmig-Adams, 1998). The levels of these two carotenes change in tropical tree species during acclimation to high light intensities, with α-Car decreasing and β-Car increasing by sun exposure (Tables I and III; Krause et al., 2001, 2004). In I. sapindoides, shade leaves were characterized by higher α/β-Car and a larger pool of the Lx-cycle pigments, whereas sun leaves had lower α/β-Car and a larger pool of the V-cycle pigments, suggesting general up-regulation of the carotenoid biosynthesis in the β,ɛ-branch in shade and the β,β-branch in sun. Although accumulation of Lx does not always accompany accumulation of α-Car and vice versa (e.g. Matsubara et al., 2003), the occurrence of Lx and α-Car under shade environments and the replacement of these β,ɛ-carotenoids by the β,β-carotenoids under sun-exposed environments strongly support the notion that the carotenoids function as allosteric modulators of photosystem activity (Formaggio et al., 2001; Morosinotto et al., 2003; Horton et al., 2005).
The contribution of α-Car to better light harvesting, similar to the role of Lx in shade acclimation proposed above, has been suggested previously (Krause et al., 2001). On the other hand, β-Car is considered an important photoprotectant, scavenging reactive oxygen species (Telfer, 2002) and mediating PSII cyclic electron transport via cytochrome b559 (Hanley et al., 1999; Telfer, 2002). Whereas the high-light-sensitive phenotype of Arabidopsis lut5 plants (Kim and DellaPenna, 2006), accumulating α-Car at the expense of β,β-carotenoids, is consistent with the functions of β-Car and/or the V-cycle pigments under light stress, no obvious phenotype indicative of an increased light-harvesting efficiency under low light has been observed in lut5 plants (S. Matsubara, G. Giuliano, R. Bassi, unpublished data). In this study, the difficulty in obtaining pure PSII core complexes from thylakoids of I. sapindoides did not allow us to study functions of α- and β-Car in PSII. Although the band 5b samples (enriched in PSII core complexes) from shade and shadeL exhibited higher absorption and fluorescence excitation in the spectral region >450 nm compared with sun (Supplemental Fig. S1), these spectroscopic features are probably attributable to the amounts of antenna proteins still attached to the PSII core complexes (Table III) rather than the levels of α- and β-Car. Isolation of Lhc-free PSII core complexes from plants accumulating α-Car, such as Inga or lut5, could facilitate elucidation of the role of α-Car in the PSII core.
Function of Red-Shifted Chl Forms in PSI-LHCI
A striking difference in photoacclimation of PSII and PSI is the capacity to modify the antenna size. In contrast to the marked down-sizing of the PSII antennae in sun leaves, PSI exhibited no noticeable alteration in the antenna size under sun and shade environments (Table III). The situation is contradictory for Arabidopsis in which both unchanged (Ballottari et al., 2007) and increasing (Bailey et al., 2001) PSI antenna size have been reported under strong illumination. The apparent inflexibility of the PSI antenna size in I. sapindoides could be explained by stable association of the PSI-LHCI holocomplex, embracing gap and linker pigments at the interface of the pigment-protein complexes (Ben Shem et al., 2003; Ballottari et al., 2004; Klimmek et al., 2005; Morosinotto et al., 2005a). The biochemical data presented here do not provide any information about the involvement of gap carotenoids (L, V, and β-Car in the case of Arabidopsis; Ballottari et al., 2004; Morosinotto et al., 2005a) in the light-dependent carotenoid variations in PSI-LHCI of I. sapindoides. However, the observed changes in the carotenoid composition, no matter where these pigments were, did not substantially affect the spectroscopic characteristics of PSI-LHCI (Fig. 3A).
An important discovery for PSI-LHCI was the conspicuous spectral feature of the red-most Chl forms. The major band in the low-temperature fluorescence emission spectra of PSI-LHCI, which is ascribed to low-energy Chls in LHCI, was strongly red-shifted in I. sapindoides compared with the corresponding band of Arabidopsis (Fig. 3C). To our knowledge, these red Chl forms of I. sapindoides have the lowest energy level so far documented for higher plants. Given the presumed role of Lx in light harvesting, it is tempting to associate these peculiar red Chl forms with PSI light harvesting under shade environments. In fact, it has been suggested that red Chl forms may significantly contribute to PSI light absorption under shade environments in which light is strongly enriched in the red spectral region (Rivadossi et al., 1999). However, PSI-LHCI from I. sapindoides did not show higher absorption of red light compared with Arabidopsis at room temperature or at low temperature (Fig. 3B). The observed difference in fluorescence emission spectra is therefore very likely due to a larger bandwidth of the red band rather than a shift of its absorption maximum. In any case, because there is little difference in the absorption spectra, it is unlikely that these pronounced red Chl forms in PSI lead to a large increase in red-light absorption under physiological conditions.
Rather, it is worth mentioning that the presence of these red Chl forms was accompanied by diminished efficiency of excitation energy transfer from the antenna pigments to the PSI core in both sun and shade leaves (Fig. 3A), suggesting that the relative importance of carotenoids as light-harvesting pigments may be lower in PSI of I. sapindoides compared to Arabidopsis. Instead, these carotenoids could serve as effective quenchers of triplet-state red Chl forms, another important function of carotenoids, as has been recently shown in LHCI of Arabidopsis (Carbonera et al., 2005; Croce et al., 2007). In the work by Carbonera et al. (2005), it was demonstrated in Lhca4 that lack of red Chls leads to a reduced efficiency of triplet quenching by carotenoids. Hence, we hypothesize that the pronounced red Chl forms, which draw excitation energy to themselves and whose triplets can be effectively quenched by nearby carotenoids, may augment photoprotective efficiency of PSI-LHCI in I. sapindoides. It has been shown in Arabidopsis that the formation of red Chl forms involves excitonic interaction between two Chl a molecules A5 and B5 (Morosinotto et al., 2002b, 2005b), as well as some of the gap Chls and intersubunit interactions (Morosinotto et al., 2005a). The strong red Chl forms in I. sapindoides invite further exploration of their origin and physiological significance.
CONCLUSION
To operate linear electron transport efficiently under diverse light environments, higher plants have evolved multifaceted mechanisms to adjust the balance of light energy absorption between the two photosystems. During shade acclimation, I. sapindoides slowly accumulates Lx in Lhcs, which, along with an increase in the antenna size, enables efficient light harvesting by PSII. When shade leaves are suddenly exposed to strong light (e.g. by canopy gap formation), Lx → L exchange takes place in L2 and V1 sites of Lhcs in parallel with V → Z exchange that induces strong dissipation. The rapid, but slowly reversible, Lx → L conversion may represent one of the earliest steps in long-term sun acclimation, converting efficient light-harvesting centers to efficient photoprotective centers. During the later steps of sun acclimation, the V-cycle pool size increases in the antenna complexes and the relative abundance of carotenes shifts from α- to β-Car in the core complexes. These dynamic changes in the carotenoid composition may reflect the roles of these pigments in modulating the photosystem activities. Furthermore, strong red Chl forms in PSI-LHCI may enhance photoprotection via triplet quenching by carotenoids, which could play an important role, especially under shade environments enriched in >700 nm. Together, Lx accumulation in Lhcs and pronounced red Chl forms in PSI-LHCI may confer an advantage upon I. sapindoides for seedling survival in deep shade of the forest floor, as well as for shade acclimation and maintenance of mature leaves inside a dense canopy or after canopy gap closure.
MATERIALS AND METHODS
Plant Material
Sun and shade leaves of Inga sapindoides Willd were collected from outer and inner canopy branches of a tree growing in the Humid Tropics Biome at Eden Project (Cornwall, UK). For light treatment of shade leaves, some inner canopy branches were cut, the stems were immediately put in water, and the adaxial surface of the leaves was illuminated for 30 min with a halogen lamp that gave a light intensity of approximately 200 μmol m−2 s−1 (measured on leaves). These light-exposed shade leaves (shadeL) were then wrapped with moist tissues and kept in a plastic bag in the dark for approximately 24 h before freezing in liquid N2. Nontreated sun and shade leaves were also subjected to the same dark adaptation of 24 h before freezing. The measurements of the maximal PSII efficiency (Fv/Fm), calculated as (Fm − F0)/Fm, where Fm and F0 are the maximal and minimal fluorescence intensity in a dark-adapted state (van Kooten and Snel, 1990), were performed by using a PAM-2100 (Walz) at the end of the dark treatment.
For some experiments, thylakoids from Arabidopsis (Arabidopsis thaliana) L. Heynh Columbia ecotype grown under a controlled condition (100 μmol m−2 s−1, 19°C, 8-h light/16-h dark) were also used for comparison.
Thylakoid Purification and Isolation of Native Pigment-Protein Complexes
Thylakoid membranes were isolated from leaves and solubilized as described in Caffarri et al. (2001). Thylakoid samples containing 0.5 mg Chl/mL were solubilized with 0.6% α-DM and separated by Suc gradient ultracentrifugation. Two Suc gradient fractions (bands 5 and 6), containing PSII core and PSI-LHCI, respectively, were further purified by another solubilization with 0.6% α-DM (band 5) or 0.8% β-DM (band 6) followed by a second Suc gradient ultracentrifugation. For identification of xanthophyll species in the V1 site, trimeric LHCIIs (band 3) were also isolated with 1.0% β-DM.
In Vitro Reconstitution of Lhcb1 and Lhcb5 Monomeric Complexes
Lhcb1 from Hordeum vulgare and Lhcb5 from Arabidopsis were expressed in the SG13009 strain of Escherichia coli and isolated following a protocol described previously (Nagai and Thøgersen, 1987). In vitro reconstitution of the recombinant proteins with purified pigments was performed according to Croce et al. (2002). Pure Chl a and Chl b were purchased from Sigma-Aldrich, whereas Lx was obtained from CaroteNature. L and N were purified from spinach (Spinacia oleracea) thylakoids by using a HPLC. Lhcb1 (420 μg apoprotein) was reconstituted with 240 μg Chl (Chl a/b = 2.3), 25 μg N, and 65 μg L, Lx, or L:Lx (1:1). Lhcb5 (420 μg apoprotein) was reconstituted with 240 μg Chl (Chl a/b = 3.0) and 60 μg L, Lx, or L:Lx (1:1).
Electrophoresis and Immunoblotting Analyses
Nondenaturing Deriphat-PAGE was performed according to the method developed by Peter and Thornber (1991) with the following modifications: The stacking gel had 3.5% (w/v) acrylamide (38:1 acrylamide/bis-acrylamide) and the resolving gel had an acrylamide concentration gradient from 4.5% to 11.5% (w/v) stabilized by a glycerol gradient from 8% to 16% (w/v). Both gels contained 12 mm Tris and 48 mm Gly (pH 8.5). For the nondenaturing gel, thylakoid samples (0.5 mg Chl/mL) were solubilized with 0.6% α-DM, vortexed for 1 min, kept on ice for 10 min, and centrifuged at 13,000 rpm for 15 min to remove unsolubilized material. Samples containing 30 μg Chl were then loaded in each lane of the gel.
SDS-PAGE analysis was preformed as described in Ballottari et al. (2004). After SDS-PAGE, polypeptides were transferred to a nitrocellulose membrane (Sartorius) using a blot system from Bio-Rad. The polypeptides were then identified with specific antibodies.
Pigment Analyses
Pigments were extracted with 80% acetone and assayed by the HPLC method developed by Gilmore and Yamamoto (1991). The data from the HPLC analysis were then verified by fitting the absorption spectra of the acetone extracts with the spectra of individual pure pigments (Croce et al., 2002). Absorption spectra were recorded as described below.
Spectroscopic Analyses
Spectroscopic analyses were performed for the isolated native complexes and the recombinant proteins in 10 mm HEPES (pH 7.5), 0.2 m Suc, and 0.06% α-DM (or β-DM). Room temperature absorption spectra were recorded by using a spectrophotometer SLM-Aminco DK2000 (Aminco). Fluorescence excitation and emission spectra were measured at room temperature and/or low temperature with a Jasco FP-777 spectrofluorimeter (Jasco). Circular dichroism spectra were obtained at 10°C with a Jasco 600 spectropolarimeter.
Deconvolution and Fitting of Spectra
Absorption and fluorescence excitation spectra of reconstituted Lhcb5 and Lhcb1 samples were fitted with the spectra of individual pigments in the protein matrix (Croce et al., 2000). As multiple solutions are possible for such a deconvolution analysis, two constraints were employed for the fitting based on the biochemical data: (1) Pigment-binding ratios (Chl a/b and Chl/xanthophylls) were fixed to the values determined by the biochemical analysis and (2) the carotenoid-binding stoichiometry was kept constant. For example, binding in the internal xanthophyll-binding sites L1 and L2 of Lhcb5 gives rise to two different spectroscopic forms of L (or Lx) for the samples reconstituted with L (or Lx) as the single xanthophyll species (Croce et al., 2000). Thus, only the deconvolution solutions with two xanthophyll spectral forms with 1:1 ratio were considered for the sample reconstituted with L or Lx alone. For the sample reconstituted with L + Lx, up to four spectral forms of xanthophylls (two L forms found in the sample with L and two Lx forms found in the sample with Lx) were allowed for the deconvolution.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Spectral characteristics of the PSII-enriched fractions (band 5b).
Supplemental Figure S2. Photobleaching kinetics of the recombinant Lhcb5 and Lhcb1 reconstituted with Lx or L.
Supplemental Table S1. Carotenoid composition of free-pigment fractions (band 1).
Supplementary Material
Acknowledgments
We thank Pavan Umate for preparing the green gel for Figure 1D. S.M. and C.B.O. are grateful to Tony Kendle, Donald Murray, and Dina Gallick for their support for the experiment in the Humid Tropics Biome at Eden Project (Cornwall, UK).
This work was supported in part by grants from the National Ministry for University and Research (grant no. FIRB RBLA0345SF_002) and the Trento Provincial Government (grant no. SAMBAx2 to R.B. and T.M.).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Anderson JM (1986) Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol 37 93–136 [Google Scholar]
- Anderson JM, Chow WS, Goodchild DJ (1988) Thylakoid membrane organization in sun/shade acclimation. Aust J Plant Physiol 15 11–26 [Google Scholar]
- Aspinall-O'Dea M, Wentworth M, Pascal A, Robert B, Ruban A, Horton P (2002) In vitro reconstitution of the activated zeaxanthin state associated with energy dissipation in plants. Proc Natl Acad Sci USA 99 16331–16335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P (2001) Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213 794–801 [DOI] [PubMed] [Google Scholar]
- Ballottari M, Dall'Osto L, Morosinotto T, Bassi R (2007) Contrasting behaviour of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282 8947–8958 [DOI] [PubMed] [Google Scholar]
- Ballottari M, Govoni C, Caffarri S, Morosinotto T (2004) Stoichiometry of LHCI antenna polypeptides and characterization of gap and linker pigments in higher plants photosystem I. Eur J Biochem 271 4659–4665 [DOI] [PubMed] [Google Scholar]
- Bassi R (1986) Studies on the leaf of Trapa natants: polymorphism of chloroplasts and microbodies. Cytobios 45 109–121 [Google Scholar]
- Bassi R, Croce R, Cugini D, Sandonà D (1999) Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc Natl Acad Sci USA 96 10056–10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi R, Pineau B, Dainese P, Marquardt J (1993) Carotenoid binding proteins of photosystem II. Eur J Biochem 212 297–303 [DOI] [PubMed] [Google Scholar]
- Ben Shem A, Frolow F, Nelson N (2003) Crystal structure of plant photosystem I. Nature 426 630–635 [DOI] [PubMed] [Google Scholar]
- Björkman O (1981) Responses to different quantum flux densities. In OL Lange, PS Nobel, CB Osmond, H Ziegler, eds, Encyclopedia of Plant Physiology, Vol. 12A. Physiological Plant Ecology I, Responses to the Physical Environment. Springer-Verlag, Berlin, pp 57–107
- Bouvier F, D'Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B (1996) Xanthophyll biosynthesis: cloning, expression, functional reconstitution, and regulation of β-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J Biol Chem 271 28861–28867 [DOI] [PubMed] [Google Scholar]
- Bungard RA, Ruban RV, Hibberd JM, Press MC, Horton P, Scholes JD (1999) Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc Natl Acad Sci USA 96 1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffarri S, Croce R, Breton J, Bassi R (2001) The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J Biol Chem 276 35924–35933 [DOI] [PubMed] [Google Scholar]
- Carbonera D, Agostini G, Morosinotto T, Bassi R (2005) Quenching of chlorophyll triplet states by carotenoids in reconstituted Lhca4 subunit of peripheral light-harvesting complex of photosystem I. Biochemistry 44 8337–8346 [DOI] [PubMed] [Google Scholar]
- Chow WS, Aro E-M (2005) Photoinactivation and mechanisms of recovery. In TJ Wydrzynski, K Satoh, eds, Photosystem II. The Light-Driven Water:Plastoquinone Oxidoreductase. Springer, Dordrecht, The Netherlands, pp 627–648
- Croce R, Canino G, Ros F, Bassi R (2002) Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry 41 7334–7343 [DOI] [PubMed] [Google Scholar]
- Croce R, Cinque G, Holzwarth AR, Bassi R (2000) The Soret absorption properties of carotenoids and chlorophylls in antenna complexes of higher plants. Photosynth Res 64 221–231 [DOI] [PubMed] [Google Scholar]
- Croce R, Mozzo M, Morosinotto T, Romeo A, Hienerwadel R, Bassi R (2007) Singlet and triplet state transitions of carotenoids in the antenna complexes (Lhca) of higher plants photosystem I. Biochemistry 46 3846–3855 [DOI] [PubMed] [Google Scholar]
- Croce R, Weiss S, Bassi R (1999) Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J Biol Chem 274 29613–29623 [DOI] [PubMed] [Google Scholar]
- Dainese P, Bassi R (1991) Subunit stoichiometry of the chloroplast photosystem II antenna system and aggregation state of the component chlorophyll a/b binding proteins. J Biol Chem 266 8136–8142 [PubMed] [Google Scholar]
- Dall'Osto L, Caffarri S, Bassi R (2005) A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39 474–482 [Google Scholar]
- Demmig-Adams B, Adams WWIII (1992. a) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43 599–626 [Google Scholar]
- Demmig-Adams B, Adams WWIII (1992. b) Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ 15 411–419 [Google Scholar]
- Färber A, Young AJ, Ruban AV, Horton P, Jahns P (1997) Dynamics of xanthophyll cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants. Plant Physiol 115 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formaggio E, Cinque G, Bassi R (2001) Functional architecture of the major light-harvesting complex from higher plants. J Mol Biol 314 1157–1166 [DOI] [PubMed] [Google Scholar]
- Frank HA, Das SK, Bautista JA, Bruce D, Vasil'ev S, Crimi M, Croce R, Bassi R (2001) Photochemical behavior of xanthophylls in the recombinant photosystem II antenna complex CP26. Biochemistry 40 1220–1225 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Hernández A, Errasti E, Becerril JM (2002) Occurrence and operation of the lutein epoxide cycle in Quercus species. Funct Plant Biol 29 1075–1080 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Hernández A, Olano JM, Becerril JM (2003) The operation of the lutein epoxide cycle correlates with energy dissipation. Funct Plant Biol 30 319–324 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Hormaetxe K, Hernández A, Olano JM, Becerril JM (2004) The lutein epoxide cycle in vegetative buds of woody plants. Funct Plant Biol 31 815–823 [DOI] [PubMed] [Google Scholar]
- Gilmore AM (2001) Xanthophyll cycle-dependent nonphotochemical quenching in photosystem II: mechanistic insights gained from Arabidopsis thaliana L. mutants that lack violaxanthin deepoxidase activity and/or lutein. Photosynth Res 67 89–101 [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Shinkarev VP, Hazlett TL, Govindjee (1998) Quantitative analysis of the effects of intrathylakoid pH and xanthophyll cycle pigments on chlorophyll a fluorescence lifetime distributions and intensity in thylakoids. Biochemistry 37 13582–13593 [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY (1991) Resolution of lutein and zeaxanthin using a nonendcapped, lightly carbon-loaded C-18 high-performance liquid chromatographic column. J Chromatogr 543 137–145 [Google Scholar]
- Hanley J, Deligiannakis Y, Pascal A, Faller P, Rutherford AW (1999) Carotenoid oxidation in photosystem II. Biochemistry 38 8189–8195 [DOI] [PubMed] [Google Scholar]
- Havaux M, Dall'Osto L, Cuiné S, Giuliano G, Bassi R (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279 13878–13888 [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieber AD, Kawabata O, Yamamoto HY (2004) Significance of the lipid phase in the dynamics and functions of the xanthophyll cycle as revealed by PsbS overexpression in tobacco and in vitro de-epoxidation in monogalactosyldiacylglycerol micelles. Plant Cell Physiol 45 92–102 [DOI] [PubMed] [Google Scholar]
- Holt NE, Fleming GR, Niyogi KK (2004) Toward an understanding of the mechanism of nonphotochemical quenching in green plants. Biochemistry 43 8281–8289 [DOI] [PubMed] [Google Scholar]
- Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Nature 307 433–436 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Rees D, Noctor G, Pascal AA, Young A (1991) Control of the light harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll protein complex. FEBS Lett 292 1–4 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47 655–684 [DOI] [PubMed] [Google Scholar]
- Horton P, Wentworth M, Ruban A (2005) Control of the light harvesting function of chloroplast membranes; the LHCII-aggregation model for non-photochemical quenching. FEBS Lett 579 4201–4206 [DOI] [PubMed] [Google Scholar]
- Jahns P, Wehner A, Paulsen H, Hobe S (2001) De-epoxidation of violaxanthin after reconstitution into different carotenoid binding sites of light-harvesting complex II. J Biol Chem 276 22154–22159 [DOI] [PubMed] [Google Scholar]
- Kim J, DellaPenna D (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid β-ring hydroxylase CYP97A3. Proc Natl Acad Sci USA 103 3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimmek F, Ganeteg U, Ihalainen JA, van Roon H, Jensen PE, Scheller HV, Dekker JP, Jansson S (2005) Structure of the higher plant light harvesting complex I: in vivo characterization and structural interdependence of the lhca proteins. Biochemistry 44 3065–3073 [DOI] [PubMed] [Google Scholar]
- Krause GH, Grube E, Koroleva OY, Barth C, Winter K (2004) Do mature shade leaves of tropical tree seedlings acclimate to high sunlight and UV radiation? Funct Plant Biol 31 743–756 [DOI] [PubMed] [Google Scholar]
- Krause GH, Koroleva OY, Falling JW, Winter K (2001) Acclimation of tropical tree seedlings to excessive light in simulated tree-fall gaps. Plant Cell Environ 24 1345–1352 [Google Scholar]
- Kühlbrandt W, Wang DN, Fujiyoshi Y (1994) Atomic model of plant light-harvesting complex by electron crystallography. Nature 367 614–621 [DOI] [PubMed] [Google Scholar]
- Lee AI-C, Thornber JP (1995) Analysis of the pigment stoichiometry of pigment-protein complexes from barley (Hordeum vulgare). Plant Physiol 107 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403 391–395 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light harvesting complex at 2.72 Å′ resolution. Nature 428 287–292 [DOI] [PubMed] [Google Scholar]
- Lokstein H, Tian L, Polle JEW, DellaPenna D (2002) Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim Biophys Acta 1553 309–319 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Gilmore AM, Ball MC, Anderson JM, Osmond CB (2002) Sustained downregulation of photosystem II in mistletoes during winter depression of photosynthesis. Funct Plant Biol 29 1157–1169 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Gilmore AM, Osmond CB (2001) Diurnal and acclimatory responses of violaxanthin and lutein epoxide in the Australian mistletoe Amyema miquelii. Aust J Plant Physiol 28 793–800 [Google Scholar]
- Matsubara S, Morosinotto T, Bassi R, Christian A-L, Fischer-Schliebs E, Lüttge U, Orthen B, Franco AC, Scarano FR, Förster B, et al (2003) Occurrence of the lutein-epoxide cycle in mistletoes of the Loranthaceae and Viscaceae. Planta 217 868–879 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Naumann M, Martin R, Nichol C, Rascher U, Morosinotto T, Bassi R, Osmond B (2005) Slowly reversible de-epoxidation of lutein-epoxide in deep shade leaves of a tropical tree legume may “lock-in” lutein-based photoprotection during acclimation to strong light. J Exp Bot 56 461–468 [DOI] [PubMed] [Google Scholar]
- Morosinotto T, Ballottari M, Klimmek F, Jansson S, Bassi R (2005. a) The association of the antenna system to photosystem I in higher plants. J Biol Chem 280 31050–31058 [DOI] [PubMed] [Google Scholar]
- Morosinotto T, Baronio R, Bassi R (2002. a) Dynamics of chromophore binding to lhc proteins in vivo and in vitro during operation of the xanthophyll cycle. J Biol Chem 277 36913–36920 [DOI] [PubMed] [Google Scholar]
- Morosinotto T, Caffarri S, Dall'Osto L, Bassi R (2003) Mechanistic aspects of the xanthophyll dynamics in higher plant leaves. Physiol Plant 119 347–354 [Google Scholar]
- Morosinotto T, Castelletti S, Breton J, Bassi R, Croce R (2002. b) Mutation analysis of lhca1 antenna complex. J Biol Chem 277 36253–36261 [DOI] [PubMed] [Google Scholar]
- Morosinotto T, Mozzo M, Bassi R, Croce R (2005. b) Pigment-pigment interactions in lhca4 antenna complex of higher plants photosystem I. J Biol Chem 280 20612–20619 [DOI] [PubMed] [Google Scholar]
- Moya I, Silvestri M, Vallon O, Cinque G, Bassi R (2001) Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. Biochemistry 40 12552–12561 [DOI] [PubMed] [Google Scholar]
- Nagai K, Thøgersen HC (1987) Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol 153 461–481 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50 333–359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB, Anderson JM, Ball MC, Egerton JJG (1999) Compromising efficiency: the molecular ecology of light resource utilisation in terrestrial plants. In MC Press, JC Scholes, M Baker, eds, Advances in Physiological Plant Ecology. Blackwell, Oxford, pp 1–24
- Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W, Ruban AV (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436 134–137 [DOI] [PubMed] [Google Scholar]
- Peter GF, Thornber JP (1991) Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J Biol Chem 266 16745–16754 [PubMed] [Google Scholar]
- Pogson BJ, Niyogi KK, Björkman O, DellaPenna D (1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci USA 95 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson BJ, Rissler HM (2000) Genetic manipulation of carotenoid biosynthesis and photoprotection. Philos Trans R Soc Lond B Biol Sci 355 1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadossi A, Zucchelli G, Garlaschi FM, Jennings RC (1999) The importance of PSI chlorophyll red forms in light-harvesting by leaves. Photosynth Res 60 209–215 [Google Scholar]
- Ruban AV, Lee PJ, Wentworth M, Young AJ, Horton P (1999) Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J Biol Chem 274 10458–10465 [DOI] [PubMed] [Google Scholar]
- Schurr U, Walter A, Rascher U (2006) Functional dynamics of plant growth and photosynthesis—from steady-state to dynamics—from homogeneity to heterogeneity. Plant Cell Environ 29 340–352 [DOI] [PubMed] [Google Scholar]
- Siefermann-Harms D (1994) Light and temperature control of season-dependent changes in the α- and β-carotene content of spruce needles. J Plant Physiol 143 488–494 [Google Scholar]
- Snyder AM, Clark BM, Bungard RA (2005) Light-dependent conversion of carotenoids in the parasitic angiosperm Cuscuta reflexa L. Plant Cell Environ 28 1326–1333 [Google Scholar]
- Telfer A (2002) What is β-carotene doing in the photosystem II reaction centre? Philos Trans R Soc Lond B Biol Sci 357 1431–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima I, Sakaguchi S, Hara N (1986) Intra-leaf and intracellular gradients in chloroplast ultrastructure of dorsiventral leaves illuminated from the adaxial side during their development. Plant Cell Physiol 27 1023–1031 [Google Scholar]
- Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23 331–343 [DOI] [PubMed] [Google Scholar]
- Thayer SS, Björkman O (1992) Carotenoid distribution and de-epoxidation in thylakoid pigment-protein complexes from cotton leaves and bundle-sheath cells of maize. Photosynth Res 33 213–225 [DOI] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25 147–150 [DOI] [PubMed] [Google Scholar]
- Verhoeven AS, Adams WWIII, Demmig-Adams B, Croce R, Bassi R (1999) Xanthophyll cycle pigment localization and dynamics during exposure to low temperatures and light stress in Vinca major. Plant Physiol 120 727–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann TC, Evans JR (2002) Profiles of light absorption and chlorophyll within spinach leaves from chlorophyll fluorescence. Plant Cell Environ 25 1313–1323 [Google Scholar]
- Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56 435–447 [DOI] [PubMed] [Google Scholar]
- Wehner A, Grasses T, Jahns P (2006) De-epoxidation of violaxanthin in the minor antenna proteins of photosystem II, Lhcb4, Lhcb5, and Lhcb6. J Biol Chem 281 21924–21933 [DOI] [PubMed] [Google Scholar]
- Wehner A, Storf S, Jahns P, Schmid HR (2004) De-epoxidation of violaxanthin in light-harvesting complex I proteins. J Biol Chem 279 26823–26829 [DOI] [PubMed] [Google Scholar]
- Wentworth M, Ruban AV, Horton P (2000) Chlorophyll fluorescence quenching in isolated light harvesting complexes induced by zeaxanthin. FEBS Lett 471 71–74 [DOI] [PubMed] [Google Scholar]
- Yamamoto HY (2006) Functional roles of the major chloroplast lipids in the violaxanthin cycle. Planta 224 719–724 [DOI] [PubMed] [Google Scholar]
- Yamamoto HY, Higashi RM (1978) Violaxanthin de-epoxidase: lipid composition and substrate specificity. Arch Biochem Biophys 190 514–522 [DOI] [PubMed] [Google Scholar]
- Young AJ (1993) Occurrence and distribution of carotenoids in photosynthetic systems. In AJ Young, G Britton, eds, Carotenoids in Photosynthesis. Chapman and Hall, London, pp 16–71
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.