Abstract
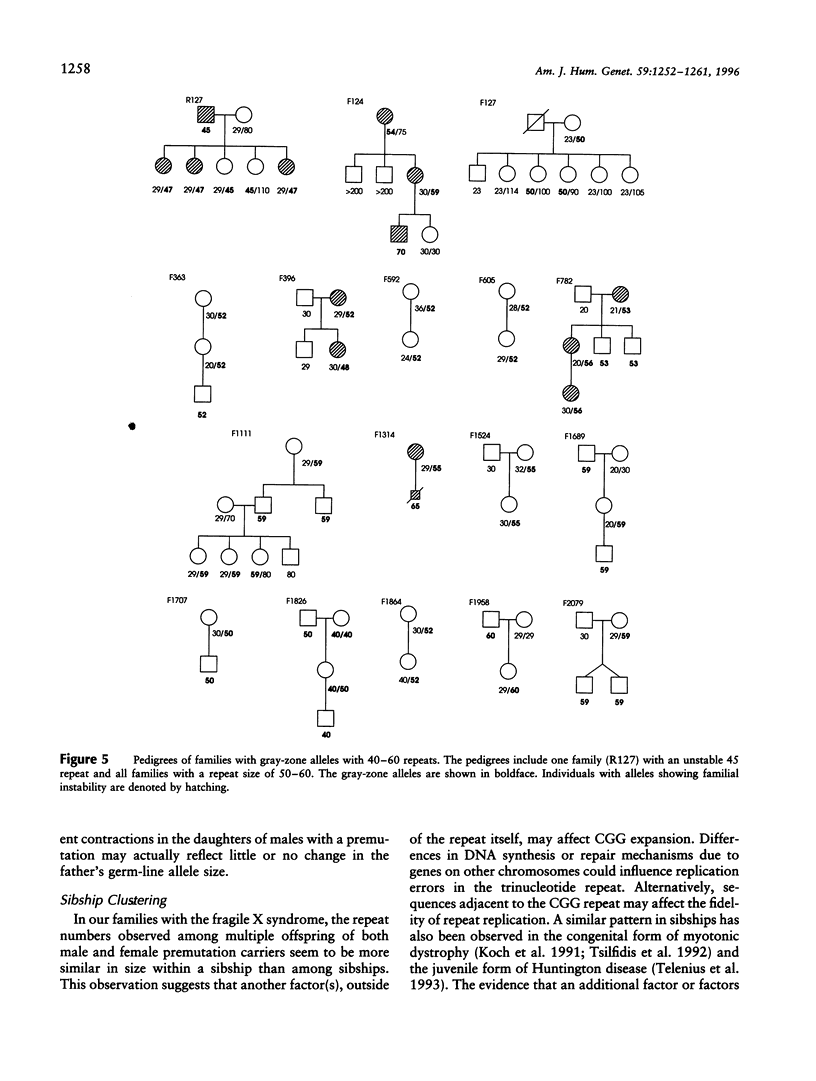
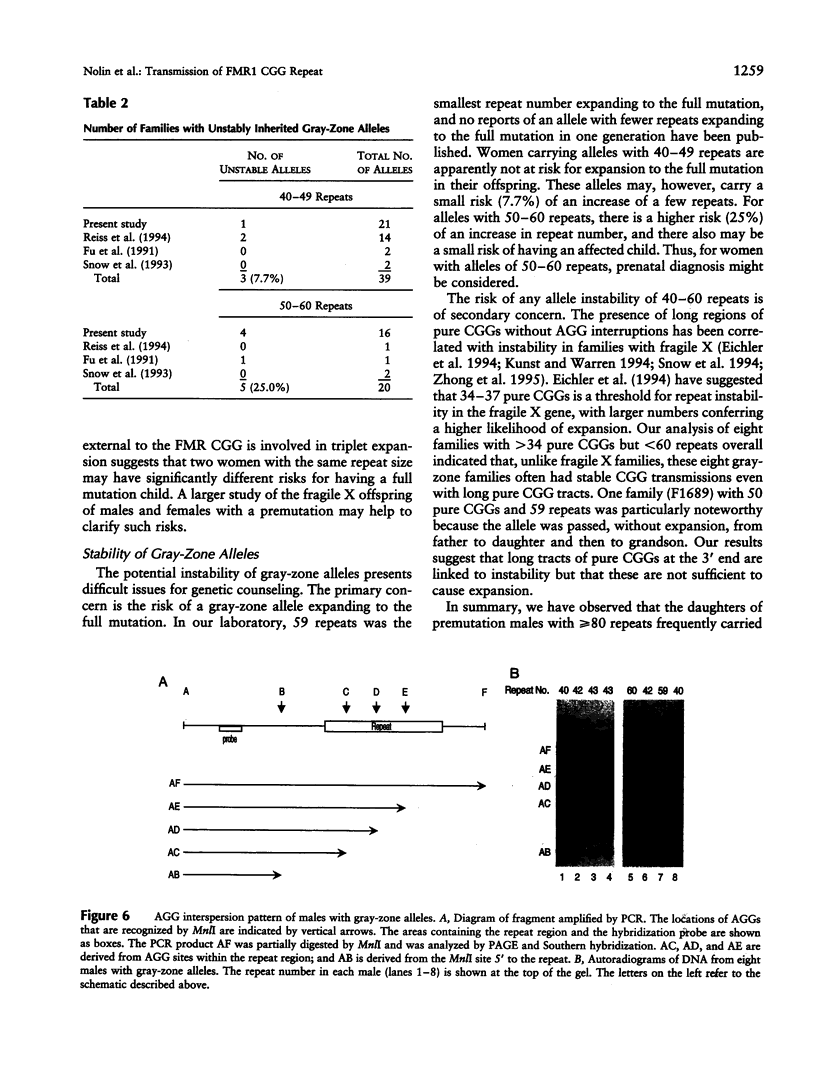
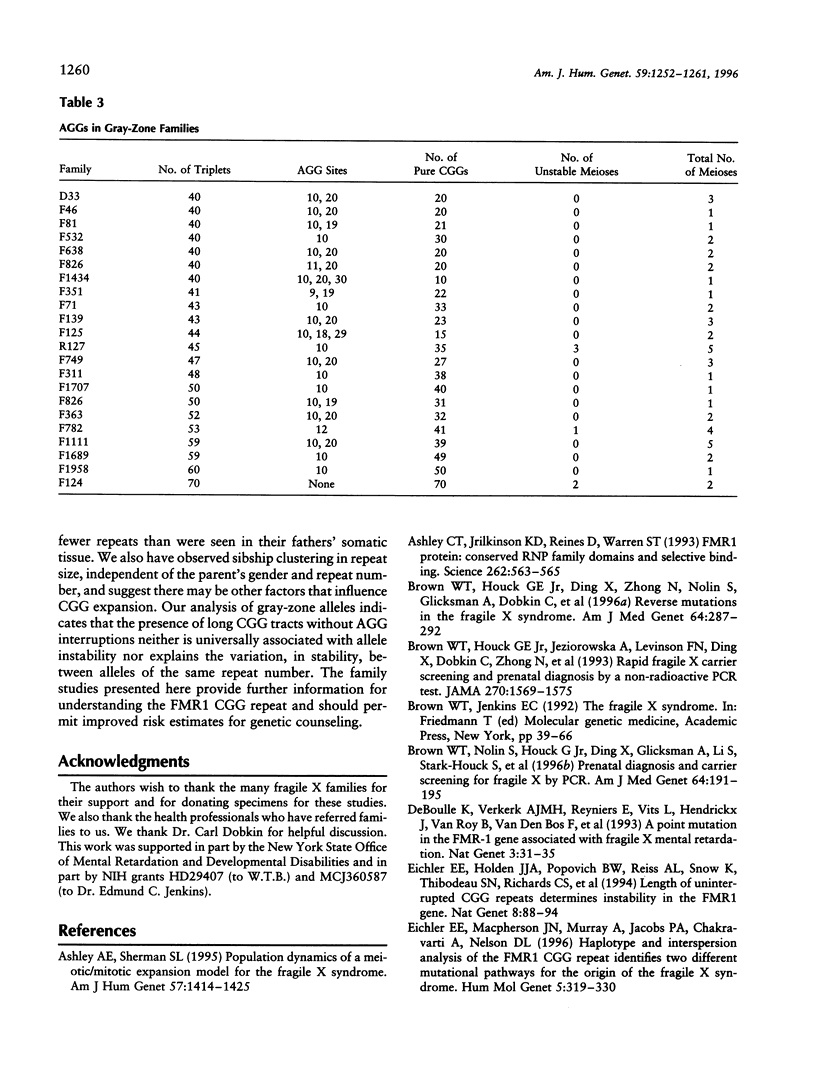
To better define the nature of FMR1 CGG-repeat expansions, changes in allele sizes for 191 families with fragile X and for 33 families with gray-zone repeats (40-60) were analyzed. Expansion of the fragile X chromosome to the full mutation was seen in 13.4% of offspring from premutation mothers with 56-59 repeats, 20.6% of those with 60-69 repeats, 57.8% of those with 70-79 repeats, 72.9% of those with 80-89 repeats, and 97.3% of those with 90-199 repeats. For premutation fathers, the majority (62%) of their daughters had a larger repeat number, while a few had either a smaller (22%) or the same (16%) repeat number, compared with their fathers' sizes. However, daughters with a smaller repeat number were observed only if their fathers had > or = 80 repeats. Fifteen (39.5%) of 38 such daughters carried a smaller repeat than did their fathers. We observed that a similar repeat number was inherited more often than expected by chance, among the members of a sibship segregating fragile X. This familial clustering, observed in the offspring of both males and females with a premutation, implies there may be an additional factor, independent of parental repeat size, that influences CGG-repeat instability. Instability in gray-zone allele transmissions was observed in 25% of alleles with 50-60 CGGs but in <8% of those with 40-49 CGGs. Examination of gray-zone allele organization revealed that long tracts of pure CGGs (>34) are not always unstably transmitted. These results raise new questions regarding the familial factors that may determine transmission expansions.
Full text
PDF

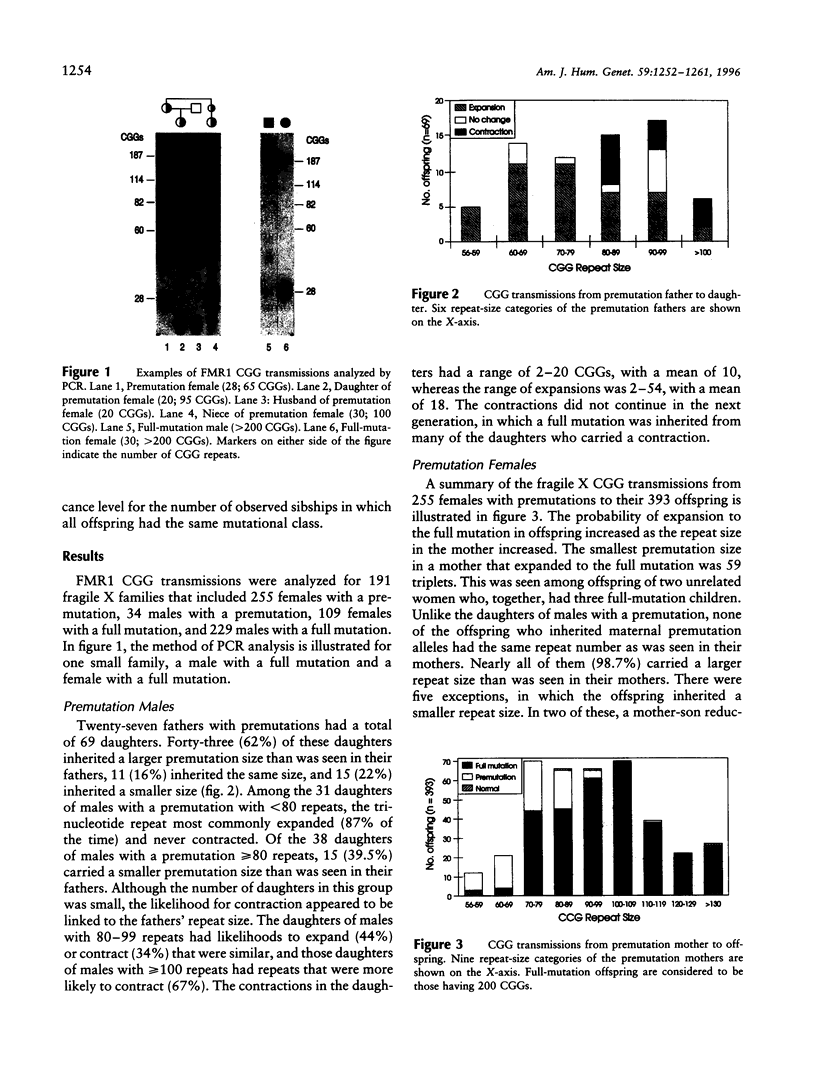
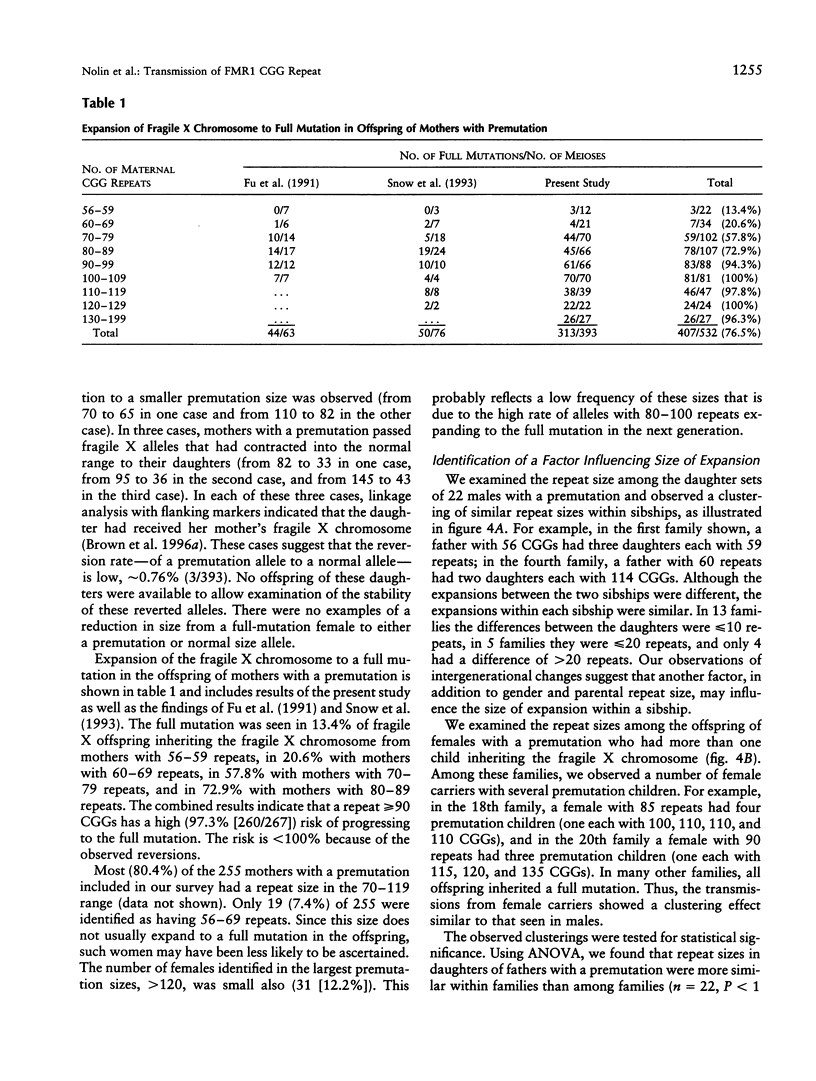
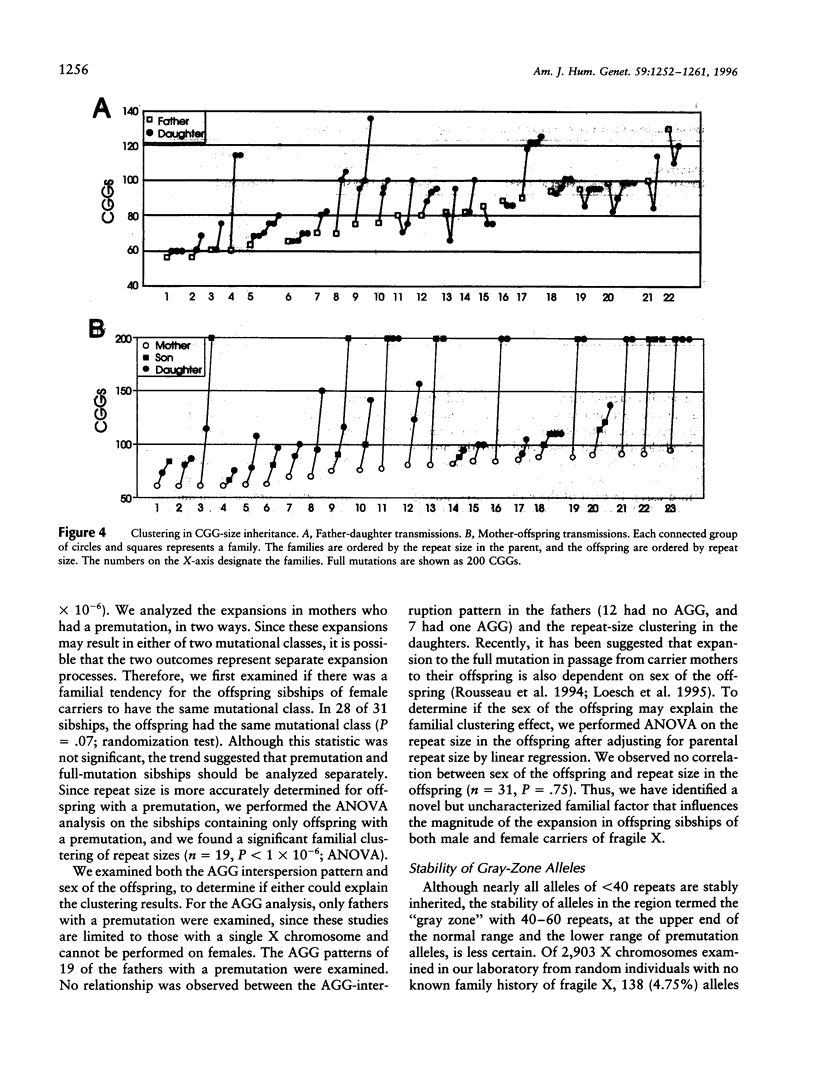


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley A. E., Sherman S. L. Population dynamics of a meiotic/mitotic expansion model for the fragile X syndrome. Am J Hum Genet. 1995 Dec;57(6):1414–1425. [PMC free article] [PubMed] [Google Scholar]
- Ashley C. T., Jr, Wilkinson K. D., Reines D., Warren S. T. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993 Oct 22;262(5133):563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Brown W. T., Houck G. E., Jr, Ding X., Zhong N., Nolin S., Glicksman A., Dobkin C., Jenkins E. C. Reverse mutations in the fragile X syndrome. Am J Med Genet. 1996 Aug 9;64(2):287–292. doi: 10.1002/(SICI)1096-8628(19960809)64:2<287::AID-AJMG11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Brown W. T., Houck G. E., Jr, Jeziorowska A., Levinson F. N., Ding X., Dobkin C., Zhong N., Henderson J., Brooks S. S., Jenkins E. C. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 1993 Oct 6;270(13):1569–1575. [PubMed] [Google Scholar]
- Brown W. T., Nolin S., Houck G., Jr, Ding X., Glicksman A., Li S. Y., Stark-Houck S., Brophy P., Duncan C., Dobkin C. Prenatal diagnosis and carrier screening for fragile X by PCR. Am J Med Genet. 1996 Jul 12;64(1):191–195. doi: 10.1002/(SICI)1096-8628(19960712)64:1<191::AID-AJMG34>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- De Boulle K., Verkerk A. J., Reyniers E., Vits L., Hendrickx J., Van Roy B., Van den Bos F., de Graaff E., Oostra B. A., Willems P. J. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993 Jan;3(1):31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Holden J. J., Popovich B. W., Reiss A. L., Snow K., Thibodeau S. N., Richards C. S., Ward P. A., Nelson D. L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994 Sep;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Macpherson J. N., Murray A., Jacobs P. A., Chakravarti A., Nelson D. L. Haplotype and interspersion analysis of the FMR1 CGG repeat identifies two different mutational pathways for the origin of the fragile X syndrome. Hum Mol Genet. 1996 Mar;5(3):319–330. doi: 10.1093/hmg/5.3.319. [DOI] [PubMed] [Google Scholar]
- Fisch G. S., Snow K., Thibodeau S. N., Chalifaux M., Holden J. J., Nelson D. L., Howard-Peebles P. N., Maddalena A. The fragile X premutation in carriers and its effect on mutation size in offspring. Am J Hum Genet. 1995 May;56(5):1147–1155. [PMC free article] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gedeon A. K., Baker E., Robinson H., Partington M. W., Gross B., Manca A., Korn B., Poustka A., Yu S., Sutherland G. R. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1992 Aug;1(5):341–344. doi: 10.1038/ng0892-341. [DOI] [PubMed] [Google Scholar]
- Heitz D., Devys D., Imbert G., Kretz C., Mandel J. L. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant of the transition to full mutation. J Med Genet. 1992 Nov;29(11):794–801. doi: 10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M. C., Grewal P. K., Davies K. E. Precursor arrays for triplet repeat expansion at the fragile X locus. Hum Mol Genet. 1994 Sep;3(9):1553–1560. doi: 10.1093/hmg/3.9.1553. [DOI] [PubMed] [Google Scholar]
- Hirst M., Grewal P., Flannery A., Slatter R., Maher E., Barton D., Fryns J. P., Davies K. Two new cases of FMR1 deletion associated with mental impairment. Am J Hum Genet. 1995 Jan;56(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Koch M. C., Grimm T., Harley H. G., Harper P. S. Genetic risks for children of women with myotonic dystrophy. Am J Hum Genet. 1991 Jun;48(6):1084–1091. [PMC free article] [PubMed] [Google Scholar]
- Kunst C. B., Warren S. T. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994 Jun 17;77(6):853–861. doi: 10.1016/0092-8674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Huggins R., Petrovic V., Slater H. Expansion of the CGG repeat in fragile X in the FMR1 gene depends on the sex of the offspring. Am J Hum Genet. 1995 Dec;57(6):1408–1413. [PMC free article] [PubMed] [Google Scholar]
- Lugenbeel K. A., Peier A. M., Carson N. L., Chudley A. E., Nelson D. L. Intragenic loss of function mutations demonstrate the primary role of FMR1 in fragile X syndrome. Nat Genet. 1995 Aug;10(4):483–485. doi: 10.1038/ng0895-483. [DOI] [PubMed] [Google Scholar]
- Macpherson J. N., Curtis G., Crolla J. A., Dennis N., Migeon B., Grewal P. K., Hirst M. C., Davies K. E., Jacobs P. A. Unusual (CGG)n expansion and recombination in a family with fragile X and DiGeorge syndrome. J Med Genet. 1995 Mar;32(3):236–239. doi: 10.1136/jmg.32.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer H., de Graaff E., Merckx D. M., Jongbloed R. J., de Die-Smulders C. E., Engelen J. J., Fryns J. P., Curfs P. M., Oostra B. A. A deletion of 1.6 kb proximal to the CGG repeat of the FMR1 gene causes the clinical phenotype of the fragile X syndrome. Hum Mol Genet. 1994 Apr;3(4):615–620. doi: 10.1093/hmg/3.4.615. [DOI] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Reiss A. L., Kazazian H. H., Jr, Krebs C. M., McAughan A., Boehm C. D., Abrams M. T., Nelson D. L. Frequency and stability of the fragile X premutation. Hum Mol Genet. 1994 Mar;3(3):393–398. doi: 10.1093/hmg/3.3.393. [DOI] [PubMed] [Google Scholar]
- Reyniers E., Vits L., De Boulle K., Van Roy B., Van Velzen D., de Graaff E., Verkerk A. J., Jorens H. Z., Darby J. K., Oostra B. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993 Jun;4(2):143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Sutherland G. R. Heritable unstable DNA sequences. Nat Genet. 1992 Apr;1(1):7–9. doi: 10.1038/ng0492-7. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Jacobs P. A., Morton N. E., Froster-Iskenius U., Howard-Peebles P. N., Nielsen K. B., Partington M. W., Sutherland G. R., Turner G., Watson M. Further segregation analysis of the fragile X syndrome with special reference to transmitting males. Hum Genet. 1985;69(4):289–299. doi: 10.1007/BF00291644. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Morton N. E., Jacobs P. A., Turner G. The marker (X) syndrome: a cytogenetic and genetic analysis. Ann Hum Genet. 1984 Jan;48(Pt 1):21–37. doi: 10.1111/j.1469-1809.1984.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Snow K., Doud L. K., Hagerman R., Pergolizzi R. G., Erster S. H., Thibodeau S. N. Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. Am J Hum Genet. 1993 Dec;53(6):1217–1228. [PMC free article] [PubMed] [Google Scholar]
- Snow K., Tester D. J., Kruckeberg K. E., Schaid D. J., Thibodeau S. N. Sequence analysis of the fragile X trinucleotide repeat: implications for the origin of the fragile X mutation. Hum Mol Genet. 1994 Sep;3(9):1543–1551. doi: 10.1093/hmg/3.9.1543. [DOI] [PubMed] [Google Scholar]
- Telenius H., Kremer H. P., Theilmann J., Andrew S. E., Almqvist E., Anvret M., Greenberg C., Greenberg J., Lucotte G., Squitieri F. Molecular analysis of juvenile Huntington disease: the major influence on (CAG)n repeat length is the sex of the affected parent. Hum Mol Genet. 1993 Oct;2(10):1535–1540. doi: 10.1093/hmg/2.10.1535. [DOI] [PubMed] [Google Scholar]
- Tsilfidis C., MacKenzie A. E., Mettler G., Barceló J., Korneluk R. G. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat Genet. 1992 Jun;1(3):192–195. doi: 10.1038/ng0692-192. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Väisänen M. L., Kähkönen M., Leisti J. Diagnosis of fragile X syndrome by direct mutation analysis. Hum Genet. 1994 Feb;93(2):143–147. doi: 10.1007/BF00210599. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Kotzot D., Hirst M. C., Manca A., Korn B., Schmidt A., Barbi G., Rott H. D., Poustka A., Davies K. E. A microdeletion of less than 250 kb, including the proximal part of the FMR-I gene and the fragile-X site, in a male with the clinical phenotype of fragile-X syndrome. Am J Hum Genet. 1992 Aug;51(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- Yu S., Mulley J., Loesch D., Turner G., Donnelly A., Gedeon A., Hillen D., Kremer E., Lynch M., Pritchard M. Fragile-X syndrome: unique genetics of the heritable unstable element. Am J Hum Genet. 1992 May;50(5):968–980. [PMC free article] [PubMed] [Google Scholar]
- Zhong N., Yang W., Dobkin C., Brown W. T. Fragile X gene instability: anchoring AGGs and linked microsatellites. Am J Hum Genet. 1995 Aug;57(2):351–361. [PMC free article] [PubMed] [Google Scholar]