Abstract
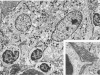
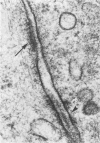
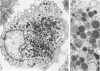
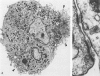
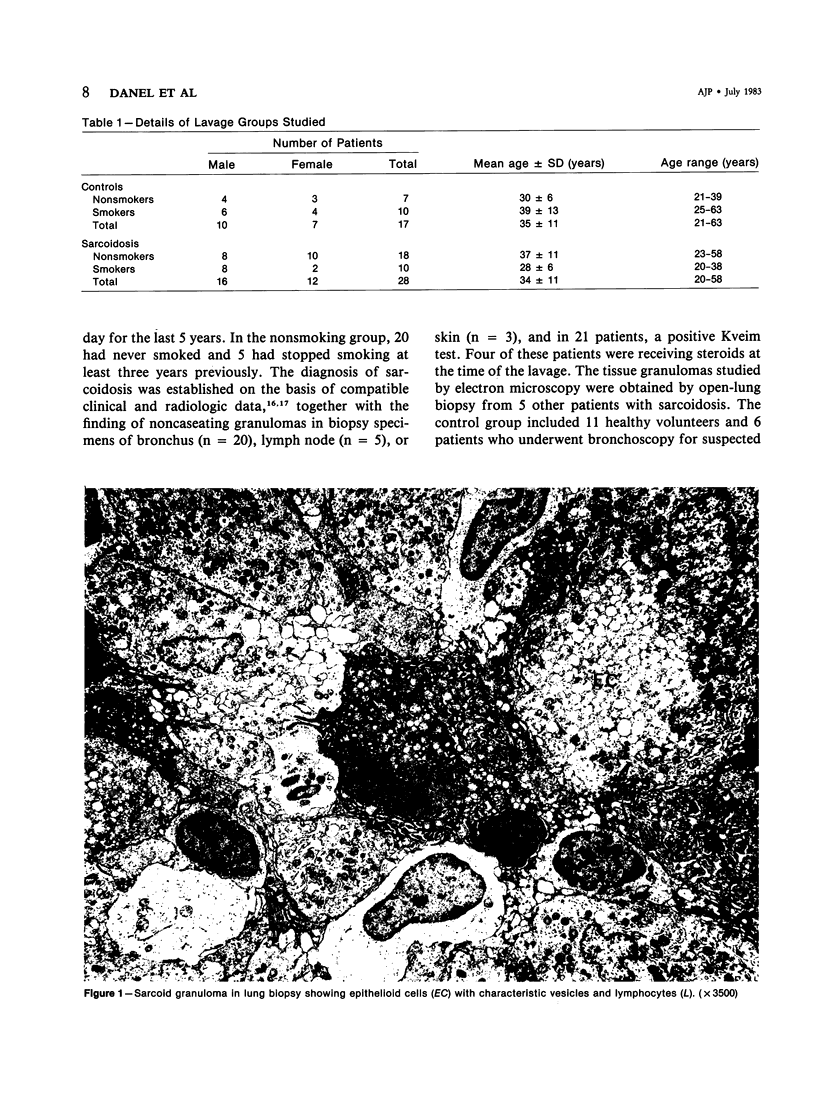
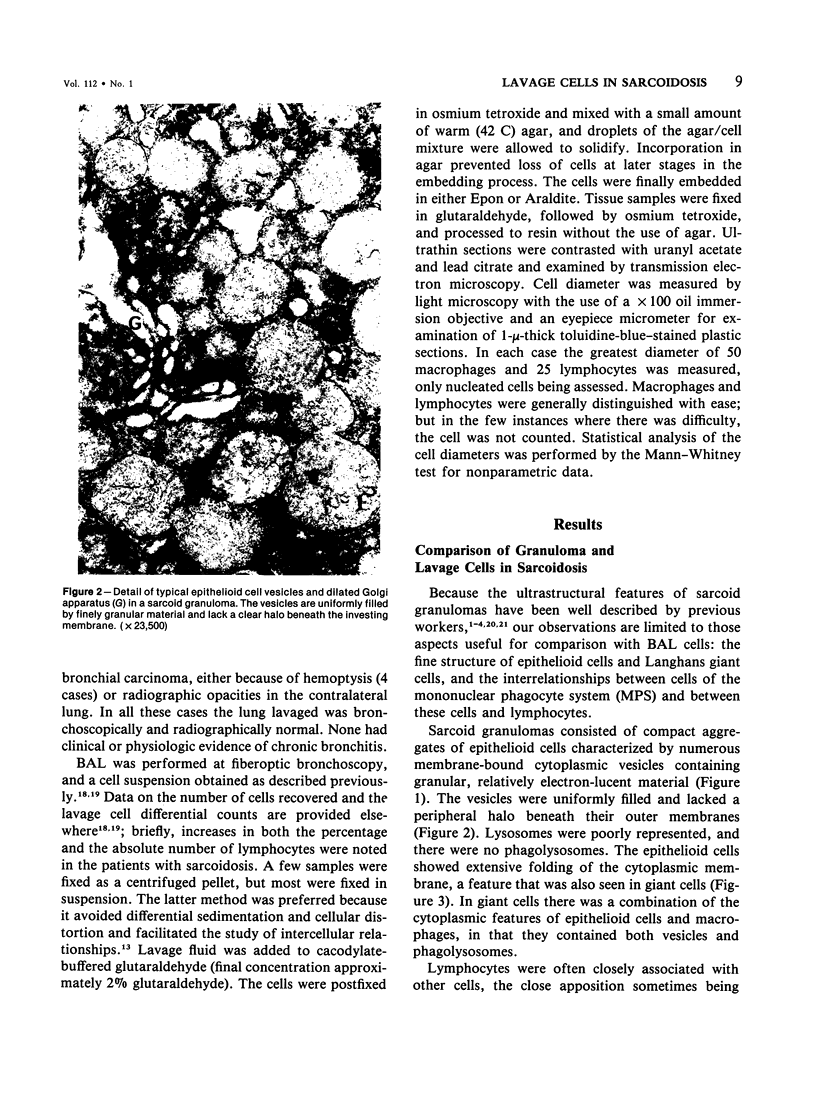
The authors undertook this study to determine whether there were any morphologic changes in bronchoalveolar lavage lymphocytes and macrophages in sarcoidosis and, in particular, to determine whether changes described previously in the mononuclear phagocytes of sarcoid granulomas were also evident in such cells obtained by lavage. Lavage cells from 28 sarcoidosis patients were studied by transmission electron microscopy and compared with lavage cells from 17 control subjects and with lung tissue granulomas from 5 sarcoidosis patients. Interactions between mononuclear phagocytes, especially subplasmalemmal linear densities, and between these cells and lymphocytes were observed in both the tissue granulomas and lavage specimens from sarcoidosis patients. Subplasmalemmal linear densities were never observed in control lavage specimens. Fully developed epitheloid cells were not identified in lavage specimens, but differences were nevertheless found between the lavage cells from sarcoidosis patients and control subjects: in particular, alveolar macrophages in sarcoidosis were larger and showed better developed pseudopodia, more marked polarity, less nuclear heterochromatin, and lysosomes that were larger and more numerous but less electron-dense than normal. Lymphocytes were also enlarged and contained more lysosomes. It is concluded that although there are only a few similarities between the cells of the granuloma and those obtained by bronchoalveolar lavage in sarcoidosis, there are noticeable differences between the lavage cells of sarcoidosis patients and control subjects. In sarcoidosis, a variable proportion (10-70%) of the lavage cells show morphologic features of "activation."
Full text
PDF
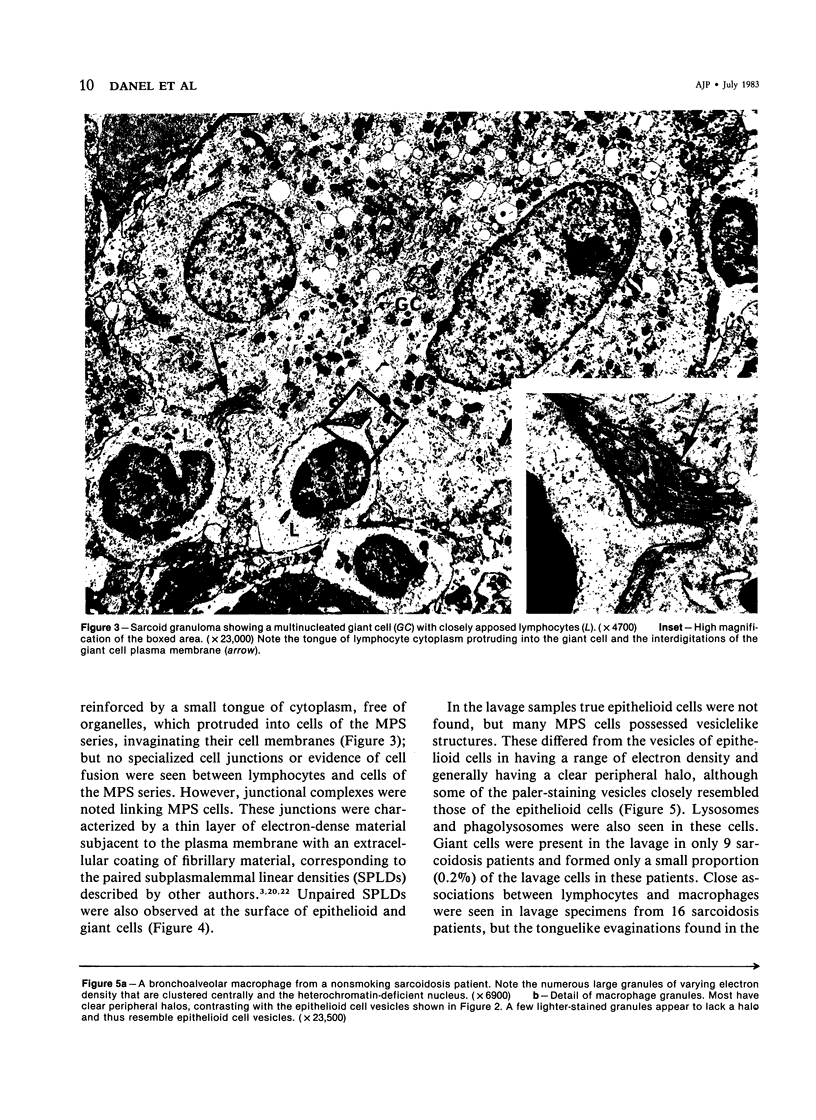
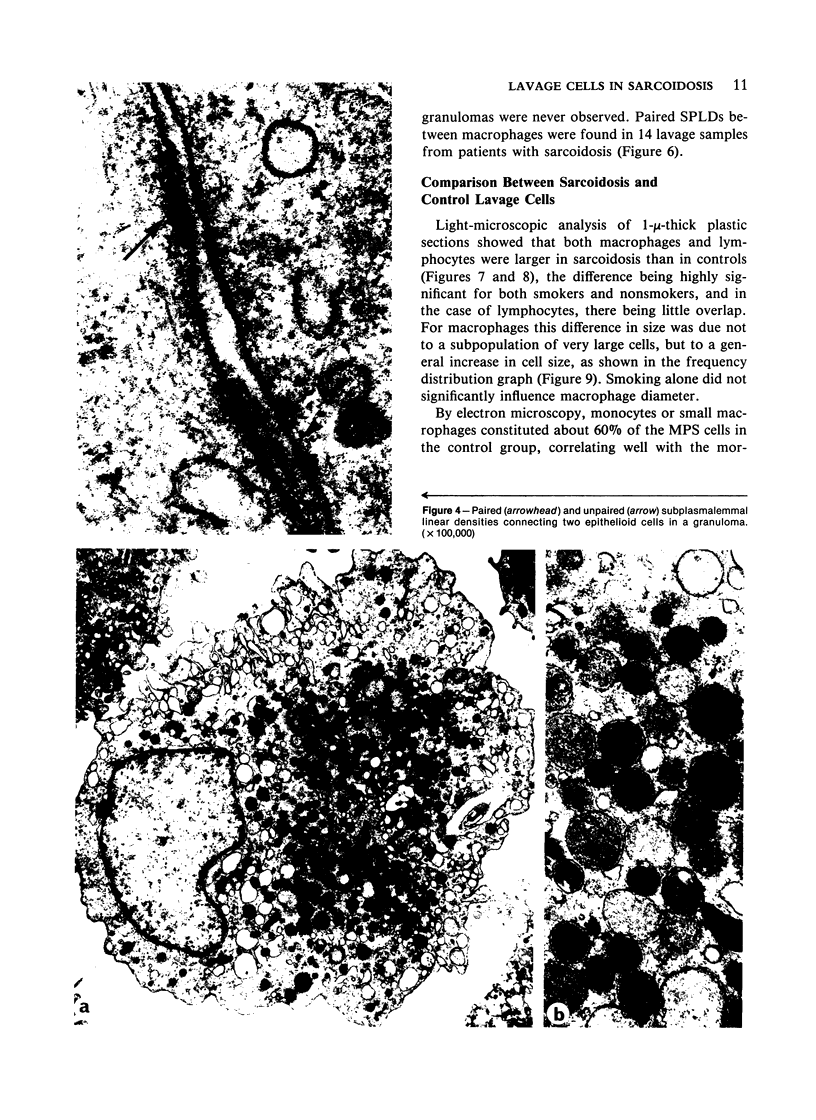
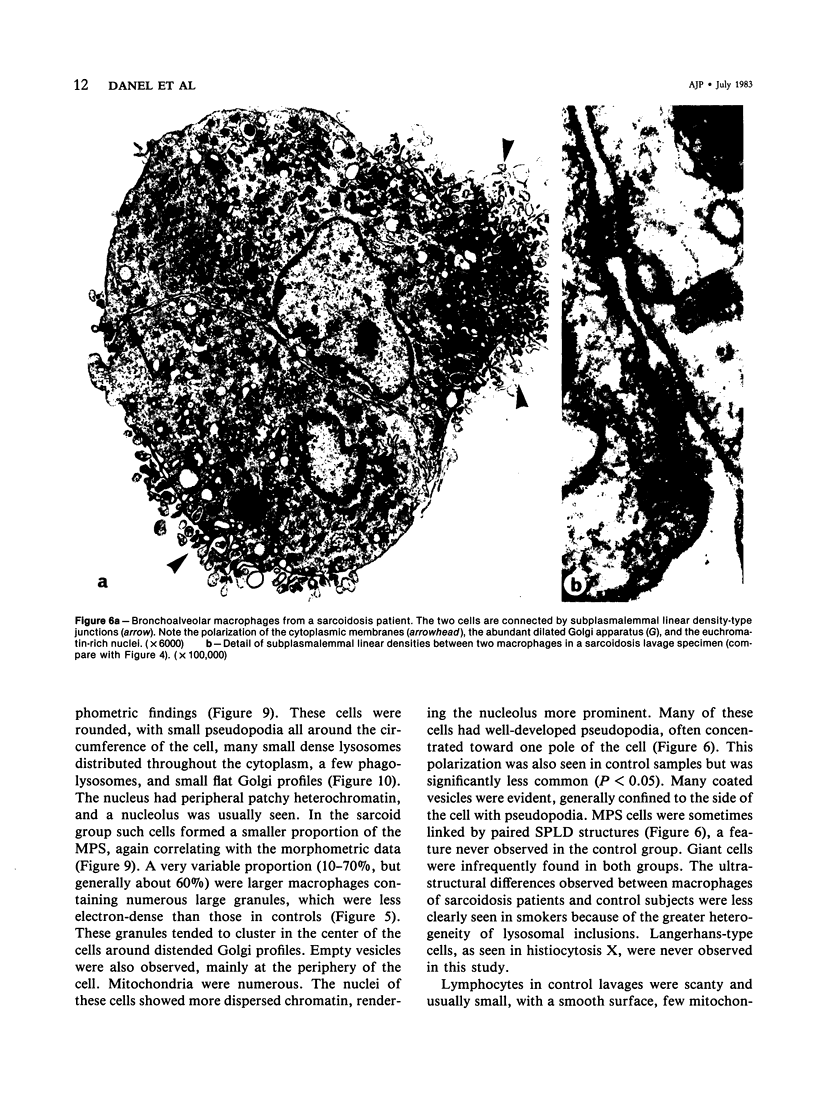
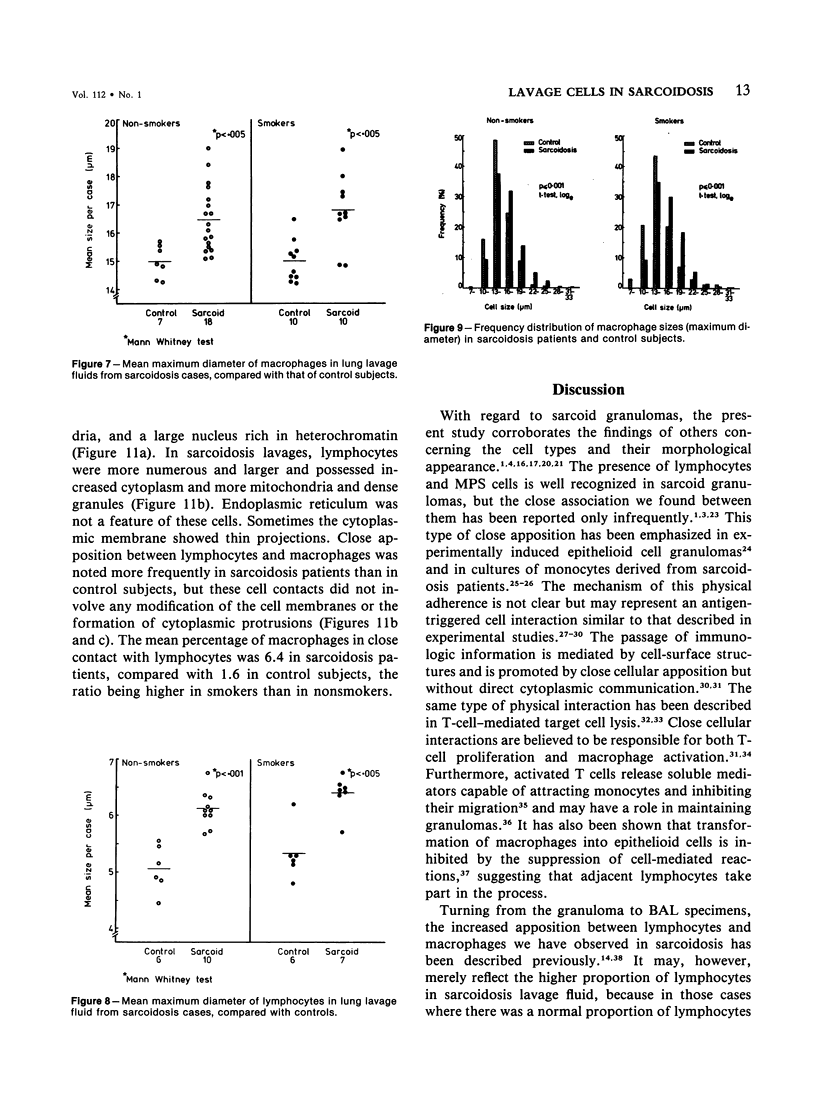



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG). Am J Pathol. 1974 Jul;76(1):17–48. [PMC free article] [PubMed] [Google Scholar]
- Basset F., Soler P., Jaurand M. C., Bignon J. Ultrastructural examination of broncho-alveolar lavage for diagnosis of pulmonary histiocytosis X: Preliminary report on 4 cases. Thorax. 1977 Jun;32(3):303–306. doi: 10.1136/thx.32.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P. Morphogenesis in blood lymphocytes stimulated with phytohaemagglutinin (PHA). A light and electron microscopic study. Acta Pathol Microbiol Scand Suppl. 1971;223(Suppl):1–70. [PubMed] [Google Scholar]
- Brody A. R., Craighead J. E. Cytoplasmic inclusions in pulmonary macrophages of cigarette smokers. Lab Invest. 1975 Feb;32(2):125–132. [PubMed] [Google Scholar]
- Caputo R., Gianotti F. Junctions between histiocytes: role of coated vesicles. J Ultrastruct Res. 1979 Sep;68(3):256–264. doi: 10.1016/s0022-5320(79)90158-8. [DOI] [PubMed] [Google Scholar]
- Clarke J. A., Salsbury A. J., Willoughby D. A. Some scanning electron-microscope observations on stimulated lymphocytes. J Pathol. 1971 Jun;104(2):115–118. doi: 10.1002/path.1711040205. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Rossman M. D. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980 Mar;92(3):406–416. doi: 10.7326/0003-4819-92-3-406. [DOI] [PubMed] [Google Scholar]
- Douglas S. D. Electron microscopic and functional aspects of human lymphocyte response to mitogens. Transplant Rev. 1972;11:39–59. doi: 10.1111/j.1600-065x.1972.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Schmidt M. E. Mononuclear phagocytes in sarcoidosis and granulomatous diseases. Mt Sinai J Med. 1977 Nov-Dec;44(6):761–766. [PubMed] [Google Scholar]
- Elias P. M., Epstein W. L. Ultrastructural observations on experimentally induced foreign-body and organized epithelioid-cell granulomas in man. Am J Pathol. 1968 Jun;52(6):1207–1223. [PMC free article] [PubMed] [Google Scholar]
- Gee J. B., Bodel P. T., Zorn S. K., Hinman L. M., Stevens C. A., Matthay R. A. Sarcoidosis and mononuclear phagocytes. Lung. 1978;155(3):243–253. doi: 10.1007/BF02730696. [DOI] [PubMed] [Google Scholar]
- Hanifin J. M., Cline M. J. Human monocytes and macrophages. Interaction with antigen and lymphocytes. J Cell Biol. 1970 Jul;46(1):97–105. doi: 10.1083/jcb.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. O., Swenson E. W., Johnson J. E., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam P. L., Turton C. W., Heard B., Lukoszek A., Collins J. V., Salsbury A. J., Turner-Warwick M. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980 Jan;35(1):9–18. doi: 10.1136/thx.35.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. J., Beaman B. L., Williams M. C., Yeager H., Jr The ultrastructure of bronchial macrophages and lymphocytes in sarcoidosis. Hum Pathol. 1979 Mar;10(2):155–163. doi: 10.1016/s0046-8177(79)80005-2. [DOI] [PubMed] [Google Scholar]
- Hedfors E., Holm G., Pettersson D. Activation of human peripheral blood lymphocytes by concanavalin A dependence of monocytes. Clin Exp Immunol. 1975 Nov;22(2):223–229. [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., White J. G., Repine J. E. Influence of cationic local anesthetics on the metabolism and ultrastructure of human alveolar macrophages. J Lab Clin Med. 1979 May;93(5):857–866. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Kawanami O., Ferrans V. J., Young R. C., Jr, Roberts W. C., Crystal R. G. Characterization of the inflammatory and immune effector cells in the lung parenchyma of patients with interstitial lung disease. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):407–412. doi: 10.1164/arrd.1981.123.4.407. [DOI] [PubMed] [Google Scholar]
- James D. G., Neville E., Siltzbach L. E. A worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321–334. doi: 10.1111/j.1749-6632.1976.tb47043.x. [DOI] [PubMed] [Google Scholar]
- James E. M., Williams W. J. Fine structure and histochemistry of epithelioid cells in sarcoidosis. Thorax. 1974 Jan;29(1):115–120. doi: 10.1136/thx.29.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. B., Lund S. Effect of sympathetic stimulation on the blood brain barrier dysfunction induced by amphetamine and by epileptic seizures. Acta Physiol Scand. 1978 Nov;104(3):281–286. doi: 10.1111/j.1748-1716.1978.tb06280.x. [DOI] [PubMed] [Google Scholar]
- Judd P. A., Finnegan P., Curran R. C. Pulmonary sarcoidosis: A clinico-pathological study. J Pathol. 1975 Apr;115(4):191–198. doi: 10.1002/path.1711150402. [DOI] [PubMed] [Google Scholar]
- Kataria Y. P., LoBuglio A. F., Bromberg P. A. Sarcoid lymphocytes: spontaneous transformation and release of macrophage migration inhibition activity. Am Rev Respir Dis. 1976 Mar;113(3):315–323. doi: 10.1164/arrd.1976.113.3.315. [DOI] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Crystal R. G. Subplasmalemmal linear densities in cells of the mononuclear phagocyte system in lung. Am J Pathol. 1980 Jul;100(1):131–150. [PMC free article] [PubMed] [Google Scholar]
- Kurti V., Mankiewicz E. In vitro study of macrophages from patients with sarcoidosis. Can Med Assoc J. 1972 Sep 23;107(6):509–515. [PMC free article] [PubMed] [Google Scholar]
- Lenzini L., Heather C. J., Rottoli L., Rottoli P. Studies on bronchoalveolar cells in human diseases. II. General morphology and ultrastructure of pulmonary macrophages and small mononuclear cells in sarcoidosis. Respiration. 1980;40(2):81–93. doi: 10.1159/000194256. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975 Jan 1;141(1):138–154. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann P. E., Cohen A. B., Finley T. N., Ladman A. J. Alveolar macrophages. Structural and functional differences between nonsmokers and smokers of marijuana and tobacco. Lab Invest. 1971 Aug;25(2):111–120. [PubMed] [Google Scholar]
- McCombs C., Michalski J. P., Talal N. Cellular interactions in lymphocyte proliferation: effect of syngeneic and xenogeneic macrophages. Cell Immunol. 1976 May;23(2):283–296. doi: 10.1016/0008-8749(76)90194-5. [DOI] [PubMed] [Google Scholar]
- NIH conference. Pulmonary sarcoidosis: a disease characterized and perpetuated by activated lung T-lymphocytes. Ann Intern Med. 1981 Jan;94(1):73–94. doi: 10.7326/0003-4819-94-1-73. [DOI] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Poulsen P. B. Cytological events in allo-stimulated lymphocytes triggered by exposure to stimulatory alloantigens. I. Changes in cell size, the mitochondrial areal density, and numerical density of the endoplasmic reticulum and the Golgi apparatus. Acta Pathol Microbiol Scand C. 1979 Apr;87C(2):131–140. [PubMed] [Google Scholar]
- Pratt S. A., Smith M. H., Ladman A. J., Finley T. N. The ultrastructure of alveolar macrophages from human cigarette smokers and nonsmokers. Lab Invest. 1971 May;24(5):331–338. [PubMed] [Google Scholar]
- Quan S. G., Golde D. W. Surface morphology of the human alveolar macrophage. Exp Cell Res. 1977 Oct 1;109(1):71–77. doi: 10.1016/0014-4827(77)90045-3. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Cell shape changes induced by cationic anesthetics. J Exp Med. 1976 Feb 1;143(2):290–304. doi: 10.1084/jem.143.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y. The importance of lymphocytes in pulmonary health and disease. Lung. 1978;155(3):225–242. doi: 10.1007/BF02730695. [DOI] [PubMed] [Google Scholar]
- Rosen Y., Vuletin J. C., Pertschuk L. P., Silverstein E. Sarcoidosis: from the pathologist's vantage point. Pathol Annu. 1979;14(Pt 1):405–439. [PubMed] [Google Scholar]
- Rossman M. D., Dauber J. H., Daniele R. P. Identification of activated T cells in sarcoidosis. Am Rev Respir Dis. 1978 Apr;117(4):713–720. doi: 10.1164/arrd.1978.117.4.713. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Glauert A. M. The mechanism of T-cell mediated cytotoxicity. VI. T-cell projections and their role in target cell killing. Immunology. 1979 Jan;36(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Soler P., Basset F. Morphology and distribution of the cells of a sarcoid granuloma: ultrastructural study of serial sections. Ann N Y Acad Sci. 1976;278:147–160. doi: 10.1111/j.1749-6632.1976.tb47026.x. [DOI] [PubMed] [Google Scholar]
- Soler P., Bernaudin J. F., Basset F., Basset G. Epithelioid cell and sarcoid-like granuloma. A review of human and experimental material. Mt Sinai J Med. 1977 Nov-Dec;44(6):767–771. [PubMed] [Google Scholar]
- Spector W. G. Immunologic components of granuloma formation. Epithelioid cells, giant cells, and sarcoidosis. Ann N Y Acad Sci. 1976;278:3–6. doi: 10.1111/j.1749-6632.1976.tb47010.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K., Madden S. C., Zeldis L. J. Fine structural alterations of interphase nuclei of lymphocytes stimulated to grwoth activity in vitro. J Cell Biol. 1968 Dec;39(3):630–660. doi: 10.1083/jcb.39.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L. Immunologic and nonimmunologic activation of macrophages. J Invest Dermatol. 1980 May;74(5):301–306. doi: 10.1111/1523-1747.ep12543509. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Warren K. S. A functional classification of granulomatous inflammation. Ann N Y Acad Sci. 1976;278:7–18. doi: 10.1111/j.1749-6632.1976.tb47011.x. [DOI] [PubMed] [Google Scholar]
- Yeager H., Jr, Williams M. C., Beekman J. F., Bayly T. C., Beaman B. L. Sarcoidosis: analysis of cells obtained by bronchial lavage. Am Rev Respir Dis. 1977 Nov;116(5):951–954. doi: 10.1164/arrd.1977.116.5.951. [DOI] [PubMed] [Google Scholar]