Abstract
Transient receptor potential (TRP) channels mediate responses in a large variety of signaling mechanisms. Most studies on mammalian TRP channels rely on heterologous expression, but their relevance to in vivo tissues is not entirely clear. In contrast, Drosophila TRP and TRP-like (TRPL) channels allow direct analyses of in vivo function. In Drosophila photoreceptors, activation of TRP and TRPL is mediated via the phosphoinositide cascade, with both Ca2+ and diacylglycerol (DAG) essential for generating the light response. In tissue culture cells, TRPL channels are constitutively active, and lipid second messengers greatly facilitate this activity. Inhibition of phospholipase C (PLC) completely blocks lipid activation of TRPL, suggesting that lipid activation is mediated via PLC. In vivo studies in mutant Drosophila also reveal an acute requirement for lipid-producing enzyme, which may regulate PLC activity. Thus, PLC and its downstream second messengers, Ca2+ and DAG, constitute critical mediators of TRP/TRPL gating in vivo.
Keywords: Ca2+ signaling, inositol lipid signaling, INAD, phospholipase C (PLC), phototransduction
INTRODUCTION
Channel proteins are of prime importance for the survival and function of virtually every cell. Ca2+-permeable channels are particularly important because Ca2+ is not only a charge carrier but also one of the most important second messengers. There are three main classes of Ca2+-permeable channels: voltage gated, ligand gated, and TRP. TRP channels constitute a large and diverse family of proteins that are expressed in many tissues and cell types. The name TRP is derived from a spontaneously occurring Drosophila mutant lacking TRP that responded to a continuous light with a transient receptor potential [hence, TRP (2)]. The Drosophila TRP (3) was later used to isolate the first mammalian TRP homologs (4, 5). The TRP superfamily is conserved throughout evolution from nematodes to humans (1). TRP channels mediate responses to light, nerve growth factors, pheromones, olfaction, taste, mechanical changes, temperature, pH, osmolarity, vasorelaxation of blood vessels, and metabolic stress. Furthermore, mutations in members of the TRP family are responsible for several diseases (for reviews, see 6–11).
TRP channels are classified into seven related subfamilies designated TRPC (canonical or classical), TRPM (melastatin), TRPN (NOMPC), TRPV (vanilloid receptor), TRPA (ANKTM1), TRPP (polycystin), and TRPML (mucolipin) (for reviews, see 6–8, 10, 12). All these TRP subfamilies are represented in the Drosophila genome (13), and three of them were initially discovered in Drosophila. The pioneering research and basic concepts on the properties and function of the channels in two of them (i.e., TRP and NOMPC) were established in Drosophila. The three subfamilies are TRPC, which includes the founding member of the TRP superfamily (TRP); TRPA, which includes the temperature detectors painless (14), pyrexia (15), and ANKTM1 (12, 16); and the TRPN subfamily, in which the no mechanical potential C (NOMPC) is the first member (17). NOMPC currently includes only one vertebrate homolog that was found in Zebra fish (18).
A great deal is known today about members of the mammalian TRP channels. However, the exact physiological function and gating mechanisms of most channels are still elusive. Furthermore, a discrepancy exists in the mechanism of channel activation and channel properties between the native and the heterologously expressed channels. Therefore, the Drosophila TRPC channels, which are robustly expressed in the photoreceptor cells, still constitute one of the most useful model systems for studying TRP channels because the physiological function is well defined and understood. It has been well established that the function of the Drosophila TRP channels is to generate the light-induced current (LIC) that produces the photoreceptor potential and the sensation of light. In most mammalian systems that use TRP channels, the primary physiological role of the channels is not entirely clear. Even in the few systems in which the physiological roles of the channels have been determined [e.g., pain mechanism, taste pheromone and temperature detection (19–26)], the gating mechanism of the channels is still unclear. The robust properties of the response to light, which dictate the use of literally a million copies of TRP channels and a similar number of their activating molecules in a single cell, have turned out to be very useful for studying the mechanism underlying TRP channel activation. This may prove useful in directing future studies on mammalian TRPs.
The present review compares the properties of Drosophila light-sensitive channels in the native photoreceptor cells to those deduced by studies on the same channels expressed in heterologous systems. Because the physiological function of the native photoreceptor channels is known, this comparison may help in evaluating the vast literature on heterologously expressed mammalian TRP channels, which has been the major way to study these channels. A property common to both Drosophila and mammalian TRPs is also covered in this review. This property is manifested by signal-induced translocation of specific TRP channels, which has important implications for cellular functions.
DROSOPHILA PHOTOTRANSDUCTION USES LARGE QUANTITIES OF HIGHLY ORGANIZED SIGNALING MOLECULES
In the history of biological research, specialized cells and tissues have proved to be crucial for the discovery of fundamental principles and mechanisms. Illuminating examples are the insightful and pioneering studies in which the squid giant axon and the torpedo electroplax were used (27). Drosophila photoreceptor cells are formed as highly specialized cells designed to maximize the sensitivity to light; they can detect single photons (28) and respond at high speed, producing the photoreceptor potential in response to intense lights within 10 msec (29). These remarkable capabilities dictate concentrating large quantities of signaling molecules, including the TRP channels, in a small volume. Indeed, Drosophila photoreceptors are highly polarized cells composed of two well-defined compartments: a signaling compartment (termed rhabdomere) and the cell body. The rhabdomere contains ~30,000 tightly packed actin-rich microvilli that harbor the signaling proteins required to generate the photoreceptor potential upon illumination. Each microvillus contains ~1000 rhodopsin molecules and ~100 TRP molecules leading to a total of ~30,000,000 and 3,000,000 rhodopsin and TRP channel subunits, respectively, in a photoreceptor cell (30) (Figure 1).
Figure 1.

The Drosophila rhabdomere and submicrovillar cisternae (SMC) are shown in this electron micrograph. The electron-dense region shows the tightly packed microvilli. Each microvillus, which contains ~1000 rhodopsin and ~100 TRP molecules, is connected by a narrow neck to the cell body. A system of minute sac-like cisternae is located near the base of the microvilli (arrow). These represent the putative inositol 1,4,5-trisphosphate (InsP3)-sensitive Ca2+ stores that might play a crucial role in phototransduction. A part of the nucleus (N) with a double membrane is also shown. (Modified from Reference 38 with permission from Current Opinion in Neurobiology.)
The signaling membrane is composed of the microvilli and the nearby extension of smooth endoplasmic reticulum termed submicrovillar cisternae (SMC, Figure 1). It has been well established that flies in general and Drosophila photoreceptors in particular use the phosphoinositide cascade for vision (31, 32) (Figure 2). Upon absorption of light, the major rhodopsin [encoded by the ninaE (neither inactivation nor after-potential E) gene (33, 34)] is converted into the active metarhodopsin state, which activates a heterotrimeric G protein (dGq). This leads to activation of phospholipase C [PLCβ4, encoded by the norpA (no-receptor-potential A) gene (31)] and subsequent opening of two classes of light-sensitive channels, TRP and TRP-like [encoded by the trp and trpl genes (3, 35)] (for reviews see 7, 30, 36–38). Genetic elimination of these channels completely abolished the robust response to light, indicating that these proteins make up the light-sensitive channels (39, 40). A third channel subunit, designated TRPγ, was also found in the eye, but its function is still unclear (41). Deactivation of the channels and light adaptation are regulated in part by the eye-specific protein kinase C [PKC, encoded by the inaC gene (42)].
Figure 2.
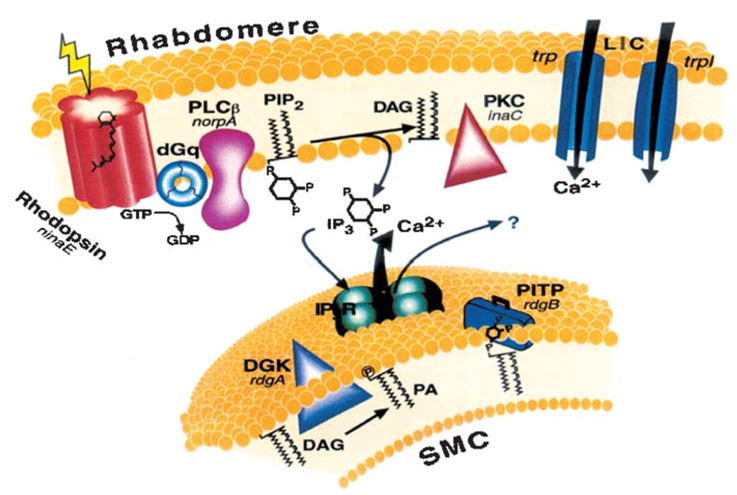
The phosphoinositide cascade of vision. Cloned genes (for all mutants that are available) are shown in italics, next to their corresponding proteins. Upon absorption of light, rhodopsin (ninaE gene) is converted to the active metarhodopsin state, which activates a heterotrimeric G protein (dGq). This leads to activation of phospholipase C (PLCβ, norpA gene) and subsequent opening of two classes of light-sensitive channels encoded at least in part by trp and trpl genes. PLC catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into the soluble second messenger InsP3 and the membrane-bound diacylglycerol (DAG). DAG is recycled to PIP2 by the phosphatidyl inositol (PI) cycle shown in an extension of the smooth endoplasmic reticulum called submicrovillar cisternae (SMC, shown on the bottom). DAG is converted to phosphatidic acid (PA) via DAG kinase (DGK, rdgA gene). After conversion to PI, PI is presumed to be transported back to the microvillar membrane by the PI transfer protein (PITP, encoded by the rdgB gene). The InsP3 receptor (InsP3R) is an internal Ca2+ channel that opens and releases Ca2+ upon binding of InsP3. (Modified from Reference 7 with permission from Physiological Reviews.)
PLC catalyzes the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) into the soluble second messenger inositol-1,4,5-trisphosphate (InsP3) and the membrane-bound diacylglycerol (DAG). DAG is recycled back to PIP2 by the phosphatidyl inositol (PI) cycle in the SMC (43). DAG is converted to phosphatidic acid (PA) via DAG kinase [encoded by the rdgA (retinal degeneration A) gene (44)] and into CDP-DAG via CD synthetase [encoded by the cds gene (45)]. After conversion, PI is presumed to be transported back to the microvillar membrane by the PI transfer protein [PITP, encoded by the rdgB gene (46)]. Both RDGA and RDGB have been immunolocalized to the SMC (47, 48) (Figure 2).
The SMC at the base of the rhabdomere has been proposed by analogy to other insects to represent Ca2+ stores endowed with InsP3 receptor (InsP3R), which is an internal Ca2+ channel that opens and releases Ca2+ upon binding of InsP3. The roles of InsP3 and the InsP3R in Drosophila phototransduction are still unclear (9).
The spatial localization of the signaling molecules in the microvilli is not random but highly organized by a signaling web composed of the multi-PDZ scaffold protein INAD (inactivation-no-after-potential D protein), which is anchored to the microvillar membrane via the TRP channel (49, 50). Genetic elimination of INAD resulted in mislocalization and degradation of the signaling molecules and strong suppression of the response to light (51).
Organization in Signaling Complexes
The power of the molecular genetics of Drosophila, which has been applied in an unbiased manner by forward genetics to screen for visual mutants, led to the discovery of novel proteins. The scaffold protein INAD was discovered in a screen for mutants with abnormal prolonged depolarizing after-potential (PDA, 52). In this screen, conducted by Pak (53), a mutant designated inaD was isolated. The inaD gene was cloned and sequenced by Shieh & Zhu (54). Pioneering studies by Huber and colleagues (55) have shown that INAD binds not only TRP but also PLC (NORPA) and eye-specific PKC (INAC). Investigators further found that the INAD scaffold protein consists of five ~90 amino acid protein interaction motifs called PDZ (PSD95, DLG, ZO1) domains (51). These domains are recognized as protein modules that bind to a diversity of signaling, cell adhesion, and cytoskeletal proteins (56–58) by specific binding to target sequences typically, though not always, in the final three residues of the carboxy-terminal. TRPL appears not to be a member of the complex because, unlike INAC, NORPA, or TRP, it remains strictly localized to the microvilli in the inaD1 null mutant (51) and it translocates without the signaling complex outside the rhabdomere upon illumination (59). However, coimmunoprecipitation studies by Montell and colleagues (60) have shown that TRP and TRPL interact directly when heterologously expressed in 293T cells as well as in Drosophila head extracts. Montell and colleagues have also shown that, in addition to PLC, PKC, and TRP, other signaling molecules such as TRPL, calmodulin (CaM), rhodopsin (61), and NINAC (62) bind to the INAD signaling complex. Recently, Minke and colleagues (62a) have found that the sole member of the ERM (ezrin/radixin/moesin) family in Drosophila, Dmoesin (63), binds to the TRP and TRPL channels in the dark. ERM proteins constitute a bridge between the actin cytoskeleton and the plasma membrane by binding to membrane proteins (64) [including mammalian TRP channels (65)]. Therefore, the association of proteins, which coimmunoprecipitate with the channels but do not bind directly to the PDZ domains of INAD (66), might be indirect via the actin cytoskeleton and Dmoesin in a dynamic process.
Additional evidence that TRPL functions as a channel in a native system without the need for INAD came from studies on neuropeptide-stimulated fluid transport in Drosophila Malpighian (renal) tubule (67). Interestingly, all photoreceptor channel proteins TRP, TRPL, and TRPγ are expressed in the Malpighian tubule. However, mutant analysis showed that only the TRPL channels are functional in neuropeptide-stimulated fluid transport and Ca2+ signaling in this system (67). Strikingly, INAD is not expressed in the Malpighian tubule, indicating that functional TRPL channels do not need INAD for function both in heterologous expression systems (68) and in a native system of the Malpighian tubule (67). This study further suggests that TRP does not form a functional channel without INAD.
Biochemical studies in Calliphora have revealed that both INAD and TRP are targets for phosphorylation by the nearby eye PKC (INAC, 69). Investigators have established an important aspect of the supramolecular complex formation by showing that TRP plays a major role in localizing the entire INAD multimolecular complex. INAD is correctly localized to the rhabdomeres in inaC mutants (where eye PKC is missing) and in norpA mutants (where PLC is missing), but is severely mislocalized in null trp mutants (70), thus indicating that TRP (but not PLC or PKC) is essential for localization of the signaling complex to the rhabdomere. The study of the above mutants was also used to show that TRP and INAD do not depend on each other to be targeted to the rhabdomeres, and thus that INAD-TRP interaction is not required for targeting but rather for anchoring the signaling complex (70). Additional experiments show that INAD has other functions in addition to anchoring the signaling complex (51). One important function is to preassemble the proteins of the signaling complex (49). Another important function, at least in the case of PLC, is to prevent degradation of the unbound signaling protein by a still unknown mechanism (49). The prevention of PLC degradation is important for keeping an accurate stochiometry among the signaling proteins (71). An accurate stochiometry between Gαq and PLC is particularly important because PLC functions as a GTPase activating protein. Accordingly, the inactivation of Gαq by its target, PLC, has proven essential for the high temporal and intensity resolution of the response to light (71).
The organization of the Drosophila photoreceptor’s signaling proteins in a specific cellular compartment in the form of a multimolecular signaling complex seems to be a common mechanism. Recent studies in mammalian cells showed that the adaptor protein, termed Homer, facilitates a physical association between TRPC1 and the InsP3R that is required for the TRPC1 channel to respond to signals. The TRPC1-Homer-InsP3R complex is dynamic, and its disassembly parallels TRPC1 activation (72). In other studies, the mammalian TRPC3 has been found in a specific microdomain called caveoli. TRPC3 constitutes a part of a multimolecular signaling complex containing Ezrin and key Ca2+ signaling proteins, including PLCβ1 and Gαq/11, which are involved in Ca2+-mediated regulation of TRPC3 channel activity and cytoskeletal reorganization (73). Another study reveals that PLCγ1 binds to and regulates the TRPC3 channel, although this interaction requires a partial pleckstrin homology (PH) domain of PLCγ1 and TRPC3 (74). Another study has shown that the ERM adaptator Na/H exchanger regulatory factor (NHERF, or EBP50) via its first PDZ domain associates with PLCβ, TRPC4, and TRPC5, and regulates channel activity and subcellular localization (65, 75, 76). Another scaffold protein, termed spinophillin, regulates Ca2+ signaling (77), but interaction with TRPC channels was not demonstrated for this protein. These data strongly suggest that Dmoesin-TRP and TRP-INAD interactions found in Drosophila are evolutionarily conserved mechanisms with important functional roles.
FUNCTIONAL TRPL CHANNELS WITH NATIVE PROPERTIES ARE READILY EXPRESSED IN HETEROLOGOUS SYSTEMS
TRP Channels with Native Properties Failed to be Expressed in Heterologous Systems
Both the Drosophila TRP and TRPL channels have been expressed in several heterologous systems. However, there is a marked difference in the outcome of these heterologous expressions between the TRP and TRPL channels. Although there is wide agreement that the expression of TRPL channels resulted in a functional TRPL channel in the host cells (68), this result does not occur in the TRP channel. The few reports on TRP expression seem to indicate that the expressed channels lead either to activation of endogenous host cell channels (see discussion in Reference 78) or to novel currents grossly different from those obtained in the native system; single-channel activity has not been published (60, 78, 79). These results strongly suggest that the measured currents did not arise from activation of Drosophila TRP channels.
Heterologous Expression of TRPL Channels
TRPL channels were expressed in several expression systems (60, 78, 80–84). The main functional characteristics of the expressed channels are, in general, similar to those of the native Drosophila TRPL channels. However, although the native TRPL channels are closed in the dark and open upon illumination, the expressed channels are constitutively active. Owing to the strong outward rectification of the TRPL-mediated current (see Figure 3C), it is not always obvious that these channels are already in their active state at physiological resting potential (i.e., ~−50 mV). However, the active state of the channels is readily revealed when the holding voltage is stepped to positive values (i.e., +150 mV), unlike the closed state of the native channel in the dark (see Figure 3C, right). The open probability of the expressed channels can be modulated (enhanced or suppressed) pharmacologically (see Figure 3B), and this fact may give the impression that these modulations mimic the opening and closing of the channels under physiological condition. However, one has to bear in mind that these are already active channels and these modulations may have limited value in understanding the physiological gating mechanism.
Figure 3.
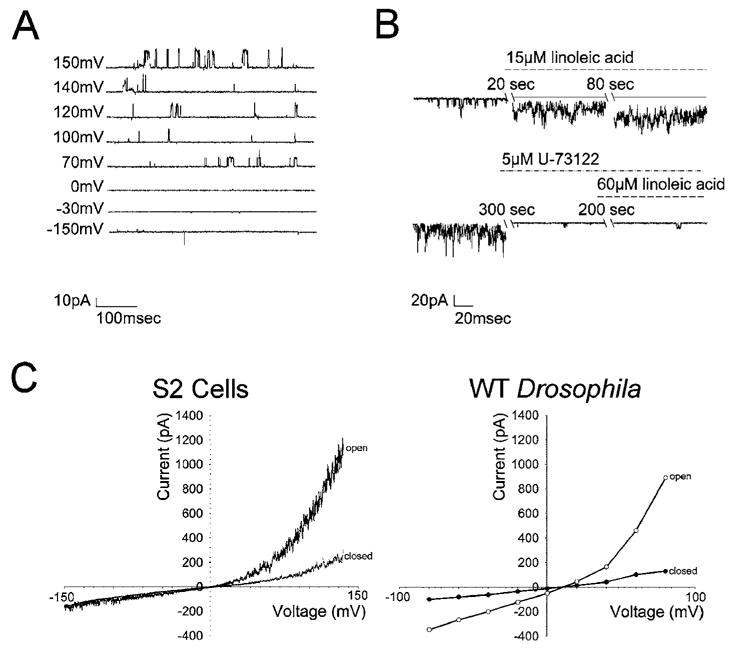
Linoleic acid affects TRPL channels via PLC. Recordings were made 24 h after induction of TRPL expression in S2 cells stably expressing trpl. (A) Traces of single-channel activity in cell-attached patch clamp recordings at various voltages as indicated at the left of each trace. (B) Single-channel recordings using cell-attached patch in physiological solution containing 0.5 mM EGTA (no Ca2+ added) (top). Application of 15 μM linoleic acid induced robust activation of single channels (bottom). The experiment shown in the top trace was repeated, except that application of the PLC inhibitor U-73122 (5 μM) resulted in a complete block of the excitatory effect of linoleic acid. (C) Current-voltage relationship measured during whole-cell recordings from S2 cell expressing the TRPL channel (left). The upper curve (open) was recorded during spontaneous activity of the TRPL channels, and the lower curve (closed ) was recorded after an increase in cellular Ca2+ inactivated the open channels. Current-voltage relationship measured during whole-cell recordings from wild-type (WT) Drosophila photoreceptor (right). The lower curve (closed ) was recorded in the dark, and the upper curve (open) was recorded after application of metabolic inhibitors.
Hardie and colleagues (81) showed that the native TRPL channel and the heterologously expressed TRPL channel in the S2 expression system have the same single-channel conductance, ion selectivity, inhibition by Mg2+, power spectra of channel noise, and current-voltage relationship. Both the expressed and the native channels seem to require PLC for activation. Channel activation of the native channels by light requires PLC, as evidenced by a virtually complete and reversible block of channel activation by light in a temperature-sensitive PLC mutant (53, 85). Similarly, the constitutive activity of the expressed TRPL channels was greatly enhanced by activation of endogenous PLC or by external application of exogenous PLCs (68). Accordingly, coexpression of the TRPL channel along with either the muscarinic or the histamine (H1) receptors led to an increased channel activity upon application of carbachol (81), histamine, thrombin, and U46619 (80, 86–88). Consistent with this finding, activation of PLC via the G protein by application of GTPγS also activated the TRPL channels, and inhibition of the G protein pathway by GDPβS suppressed TRPL activity (87).
Both Native and Heterologously Expressed TRPL Channels Reveal Antagonistic Effects of Ca2+ and Variable Dependence on the Intracellular Ca2+ Stores
THE ROLE OF Ca2+ IN ACTIVATION OF TRP AND TRPL CHANNELS IN THE NATIVE SYSTEM
Ca2+ is known both to facilitate (positive feedback effect) and to inactivate (negative feedback effect) the Drosophila response to light (89–91). Interestingly, there is a difference in the effect of Ca2+ between the TRP and the TRPL channels: Facilitation of the LIC is mediated via the TRP channels but not via the TRPL channels (90). Moreover, the Ca2+ influx via the TRP channels during the light response inactivates the TRPL channels. Therefore, the LIC in response to intense light is mediated almost exclusively via the TRP channels, and the TRPL channels are inactivated, making the LIC of normal Drosophila and that of trpl null mutant very similar (39, but see 92). The complex interaction of Ca2+ with the TRP and TRPL channels should critically depend on the spatial geometry of the signaling compartment, which is composed of tightly packed ~60-nm-wide and ~1-μm-long microvilli (Figure 1). The addition of only a few Ca2+ ions to a microvillus dramatically raises its Ca2+ concentration to the μM range. The local Ca2+ concentration in the microvilli depends on a delicate balance between a large influx via the channels and a very efficient and highly localized extrusion via the Na+-Ca2+ exchanger (93, 94). This highly specialized structure should be taken into account when the role of Ca2+ is compared in Drosophila photoreceptors and in other cells and tissues that use TRP channels. This specialized structure is especially relevant when comparing the effect of Ca2+ on TRP channels in the native tissue and in cultured cells (95).
The role of Ca2+ in the generation of the LIC has been a controversial issue (96, 97). There is strong evidence that the presence of Ca2+ is necessary for the generation of the LIC. Accordingly, during a critical developmental time window, at the last pupa stages, Drosophila photoreceptors are incapable of responding to light. Strikingly, application of Ca2+ via the whole-cell recording pipette to blind pupa photoreceptors produces a transient response to light similar to that of the trp mutant (98). Consistent with this finding, prolonged (~30 min) Ca2+ deprivation in the isolated ommatidia preparation (99, 100) and much shorter deprivation (<3 min) in intact flies (101) initially eliminates the steady state response to continuous light of wild-type flies, making the LIC similar to that of the trp mutant. The trp phenotype in Ca2+ buffered conditions is followed by total, but reversible, abolition of the LIC (99, 102). Investigators have suggested that these effects arise from illumination-dependent depletion of PIP2 in the rhabdomere via a still unknown mechanism affecting PLC (102). Additional evidence showing that Ca2+ is necessary for the generation of the LIC came from experiments in which internal dialysis of the cell interior with a 10-mM-fast Ca2+ buffer, BAPTA, combined with zero external Ca2+ (1 mM EGTA) abolished the response to intense lights in a reversible manner (Figure 4). Dialysis of the cell interior with the slower Ca2+ buffer, EGTA, under the same conditions did not abolish the LIC (101), indicating that fast chelating of Ca2+ is required to abolish the LIC. This finding suggests that the Ca2+-mediated effect on excitation is very fast. Because the Ca2+ affinity is similar for both BAPTA and EGTA, the above result further suggests that the source of Ca2+ for light excitation in the above experiments is from the cell interior (101). Experiments in which caged Ca2+ was photoreleased failed to excite the photoreceptors when applied to blind mutants without rhodopsin (ninaE ) or PLC (norpA). However, in wild-type flies, photorelease of caged Ca2+ strongly facilitated the LIC when applied at the rising phase of the response to light (103).
Figure 4.

Ca2+ is required for light- and DNP (2,4-dinitrophenol)-induced activation of the TRP and TRPL channels. (A) Whole-cell recordings in which the recording pipette contained 10 mM BAPTA but no ATP or NAD. Also, no Ca2+ but 1 mM EGTA was added to the external solution. Application of orange light ~1 min after the beginning of the recordings elicited no response. Replacing the external solution by Ringer’s solution containing 10 mM Ca2+ (second arrow; after application of DNP to the external medium, first arrow) had no effect in the dark in this particular cell, although the subsequent application of the orange light induced a large LIC. (B, top) The paradigm of (A) was repeated in another retina. The LIC was not abolished but became very small and relatively slow. Application of DNP (arrow) had no significant effect in the dark. Subsequent replacement of the bath solution with Ringer’s solution containing 10 mM Ca2+ (arrow) elicited after a delay a robust, initially oscillating inward current with kinetics similar to that of the LIC under similar conditions (see C). (B, bottom) The paradigm of (B, top) was repeated in another cell, except that application of 10 mM Ca2+ ( first arrow) was not preceded by application of DNP. In this case application of 10 mM Ca2+ did not induce any fast inward current in the dark; however, when DNP was applied (second arrow) a fast oscillating inward current was induced in the dark. (C) The paradigm of (B, top) was repeated, except that 7.5 mM BAPTA was included in the pipette. The inset shows the LIC and the oscillating response to application of Ca2+ in a fast time scale. (D) A histogram comparing the averaged peak amplitude of the LIC recorded from cells when 7.5 mM BAPTA (7.5 mM) or 10 mM BAPTA (10 mM) was added to the solution of the recording pipette. (From Reference 101, with permission from Cell Calcium.)
Taken together, the present data strongly suggest that Ca2+ is necessary but not sufficient for excitation and that a combined action of Ca2+ and an additional factor, operating upstream to PLC, are required for excitation.
THE EFFECTS OF Ca2+ ON HETEROLOGOUSLY EXPRESSED TRPL CHANNELS
Heterologous expression of TRPL channels reported by several groups revealed opposite effects of Ca2+: The TRPL response to histamine in Sf9 cells coexpressing the histamine receptor and TRPL was examined by Schultz and colleagues (104). In the range of 1–10 μM Ca2+ there was a marked inhibition in the response of the TRPL channels to histamine, which increased with Ca2+ concentration. However, the kinetics of TRPL activation by histamine showed the opposite effect: The response to histamine at low Ca2+ concentrations had a relatively long latency, whereas at high Ca2+ concentrations the activation by histamine had a short latency. These results suggest that Ca2+ has a dual effect on the TRPL channel, reminiscent of the effects Ca2+ has on the LIC in Drosophila. In Drosophila photoreceptors, Ca2+ suppressed the amplitude but sped up the kinetics of the LIC in a mechanism known as light adaptation. At zero external Ca2+ the kinetics of the LIC slows down because Ca2+ is also required for excitation.
Schilling and colleagues (68) have shown that after inhibition of the ER Ca2+ pump by thapsigargin, a high Ca2+ concentration (10 mM) has only an inhibitory effect on TRPL channels expressed at high levels in Sf9 cells. However, at low expression levels of TRPL channels, there is an initial facilitation of channel activity by a very high Ca2+ concentration (50 mM) followed by inhibition, suggesting a dual role for Ca2+ on the activity of TRPL channels.
S2 cells co-expressing the muscarinic receptor and the TRPL channels behave differently. In these experiments Hardie & Raghu (105) found that application of carbachol, which releases Ca2+ from internal stores, facilitates TRPL channel activity. However, internal perfusion with BAPTA (10 mM) reduced but did not abolish the response to carbachol, leading to the conclusion that Ca2+ is not the primary activator of the channels.
Flockerzi and colleagues (106) found that heterologously expressed TRPL channels in CHO cells do not show any channel activity at low cellular Ca2+ levels below 50 nM. However, the expressed TRPL channels showed a marked increase in channel activation by application of Ca2+ up to 3 μM. Interestingly, no inhibitory effect of Ca2+ was observed even at higher concentrations. In another study, researchers reported that similar results were obtained when TRPL channels were expressed in COS cells (80). In addition, they showed that 10 mM BAPTA blocked the constitutive opening of the TRPL channels in COS cells. An additional study found, in the same experimental system, that a rise in cellular Ca2+ is required for activation of TRPL channels (84).
The apparently conflicting reports on the effects of Ca2+ on heterologously expressed TRPL channels may arise from overexpression of the channels in different expression systems (see below).
ACTIVATION OF THE TRPL CHANNELS BY MANIPULATION OF Ca2+ STORES
Investigators have clearly demonstrated that depletion of the InsP3-sensitive Ca2+ stores by thapsigargin or by application of InsP3 in cells deprived of Ca2+ by Ca2+ buffers activates several types of mammalian TRPC channels (107–109). In contrast, no activation of the light-sensitive channels during store depletion by thapsigargin was observed in Drosophila (96, 97).
There is wide agreement that modulation of the Ca2+ stores by thapsigargin affects the TRPL channels in a large variety of expression systems. TRPL channels expressed in Sf9, S2, CHO, and 293T cells increased their activity in response to store depletion by thapsigargin (60, 84, 110, 111). The only exception is the Xenopus oocytes expression system (87). The detailed studies of TRPL channel activation by thapsigargin gave different results in the different expression systems: Yagodin and colleagues (111) measured the level of TRPL activation after a 10 min incubation with 1 μM thapsigargin in the presence of 1 mM EGTA in the S2 expression system. This protocol resulted in opening of TRPL channels, suggesting that Ca2+ store depletion but not Ca2+ per se is required for channel activation. In contrast to this study, TRPL channels expressed in Sf9 cells responded to thapsigargin only when the extracellular medium contained Ca2+ (110). Application of thapsigargin in a divalent free medium did not lead to channel activation, whereas application of Ca2+ to the external medium resulted in activation of the channels. Researchers therefore concluded that the TRPL channels are not store-operated channels but rather channels that are activated by Ca2+ release from internal stores following receptor activation.
In CHO cells, store depletion by thapsigargin resulted in a minor activation of the TRPL channels but blocked the effect of exogenous application of InsP3, which strongly activated the channels (84). Researchers concluded that the release of Ca2+ from internal stores is required for TRPL activation. InsP3 was also shown to activate the TRPL channels in several expression systems. Xenopus oocytes expressing TRPL channels revealed an elevation in TRPL channel activity after application of InsP3. Researchers suggested that TRPL opening resulted from direct binding of InsP3 to the channels (88). In contrast, 10 mM BAPTA applied to CHO cells expressing TRPL channels completely blocked the effect of InsP3 on TRPL openings. These experiments are consistent with the previous experiments, suggesting that release of Ca2+ from internal stores is required to open the TRPL channels. In contrast, Sf9 and S2 expression systems show no (or negligible) response of the TRPL channels to InsP3 (110, 111).
Taken together, the results of the above studies clearly show that heterologously expressed TRPL channels respond differently to similar experimental protocols when expressed in different expression systems or when expressed at different levels in the same expression system. Importantly, the response of the TRPL channels in the native photoreceptor cells with similar experimental protocols is markedly different. Toward reconciliation of the conflicting results described above, we suggest that the TRPL channels expressed in heterologous systems are already in their activated state, and thus the various pharmacological manipulations only modulate the activity of already activated channels. Because the TRPL channels are expressed without additional exogenous proteins (except for the receptor), the channels obviously require participation of endogenous signaling proteins for their activation and regulation (e.g., Gq protein, PLC, CaM, protein kinases, and phosphatases). Therefore, the pharmacological modulations of the channels’ activity most likely affected both the channels and their activator/modulator proteins of the host cells. For example, PLCβ1 was facilitated by Ca2+ in vitro (112). In contrast, the Drosophila PLC (most homologous to PLCβ4) has a bell-shaped response to Ca2+ in which low Ca2+ facilitates and high Ca2+ inhibits the PLC (113). Accordingly, high levels of Ca2+ are expected to facilitate TRPL channels in a specific expression system containing PLCβ1 but to inhibit TRPL channels in an expression system containing PLCβ4. Similarly, binding of CaM either activates or inhibits TRPL activity; therefore, the levels of CaM in the host cell can determine the facilitation/inhibition levels of channel activity by Ca2+.
CALMODULIN INTERACTIONS WITH TRPL
The TRPL channel was first discovered by Kelly and colleagues (35) as a Drosophila gene that encodes a CaM-binding protein. These authors were also the first to show that the amino acid sequences of TRP and TRPL are typical for channel proteins (35). Two putative CaM-binding sites, designated CBS1 and CBS2, have been identified on the C-terminal of TRPL (35). In vitro experiments on CBS1 revealed that it binds to CaM in a Ca2+-dependent manner at Ca2+ concentrations of 500 nM, which are significantly higher than the resting level (114). At this concentration, all four binding domains of CaM bind Ca2+. Although CBS2 was initially reported to be Ca2+ independent, it was later found in one study to bind CaM in a Ca2+-dependent manner (IC50 of 0.1 μM Ca2+ and 3.3 μM Ca2+ for CBS1 and CBS2, respectively) (114). The CaM IC50 for binding to CBS1 and CBS2 is very low (up to 200 nM), while CBS1 has a higher affinity to CaM than CBS2 (114). Because CaM concentrations in Drosophila photoreceptors are very high, reaching 500 μM (115), the only factor affecting CaM-binding to CBS1 and CBS2 is the cellular Ca2+ concentration (106).
Binding of CaM to CBS1 is regulated by phosphorylation. Accordingly, CBS1 has two phosphorylation sites: a PKC site and a PKA site. Phosphorylation at the PKC site showed no effect on the CaM-binding capability, whereas phosphorylation at the PKA site greatly reduced the CaM ability to bind to the channel. The phosphorylation by PKC, though, affects the phosphorylation by PKA and reduces it by 70% (114).
Zuker and colleagues (40) produced a Drosophila mutant lacking the CBS1 domain and an additional mutant lacking the CBS2 domain of TRPL. When placed on a trpl;trp null background, both mutants showed slow light responses relative to mutants on a similar double-null background that express the normal TRPL channel. The mutant lacking CBS1 showed slower responses than the mutant lacking CBS2. These results suggest that CaM-binding to TRPL exerts a negative feedback effect that speeds up the light response arising from activation of the TRPL channels (40).
The effect of CaM on the TRPL channels was tested in several expression systems. In contrast to the in vivo system, in heterologous expression studies both positive and negative effects of CaM on TRPL activity have been reported. Accordingly, TRPL channels expressed in Xenopus oocytes inactivated when CaM was blocked by CaM inhibitors. Injection of 1 μM CaM increased TRPL channel activity, while a higher concentration of 3 μM blocked TRPL activity altogether (87). Expression of a TRPL channel lacking CBS1 blocked the activating effect of CaM, whereas expression of a TRPL channel lacking CBS2 had no effect on CaM modulations. CaM inhibitors also affected the TRPL channel lacking CBS2, but had no effect on TRPL lacking CBS1 (88). In contrast to the above findings, TRPL channels expressed in Sf9 cells show a dramatic inhibitory effect of Ca2+ on channel activity. This effect is not mediated via CaM, however, as addition of CaM or CaM inhibitors (such as calmidazolium) had no effect on the TRPL activity (104).
In conclusion, CaM shows different effects on the TRPL channel in different expression systems. Because Ca2+ and CaM are tightly linked, it is difficult to know whether CaM is involved in TRPL channel activation or only in its inhibition. Furthermore, it is unclear if these two processes act solely on the TRPL channel or affect other stages of the signal transduction cascade.
Mammalian TRPs also have CaM-binding domains (116). Accordingly, TRPV1 channel expressed in Xenopus oocytes shows inhibition of the channel activity by Ca2+ with an IC50 of 60 μM. This effect is mediated by CaM, as application of Ca2+-CaM, but not of Ca2+ or CaM separately, inhibits the channel. In this system, no activating effects of either Ca2+-CaM or Ca2+ were observed. An additional study on TRPV6 revealed a Ca2+-dependent binding of CaM at a unique site of TRPV6, which facilitated channel inactivation. Interestingly, facilitation of channel inactivation was counteracted by PKC-mediated phosphorylation of the CaM-binding site (117). In contrast to TRPL and TRPV6, TRPV1 binds CaM in a Ca2+-independent manner at a site located at the N-terminal of the channel (116). In agreement with the inhibitory effects of Ca2+-CaM, Zhu and colleagues (118, 118a) have demonstrated that TRPC3 and TRPC4 are inhibited by Ca2+-CaM but not by Ca2+ or CaM alone. Interestingly, InsP3R binds to TRPC3 and TRPC4 at the same site as CaM (designated CIRB, CaM/InsP3 receptor-binding) in a competitive manner. CIRB is conserved in all TRPC channels, including the Drosophila TRP channel. However, the Drosophila InsP3R has several modifications in the CIRB-binding domain that prevent TRP binding, suggesting that InsP3R does not bind to TRP or TRPL in Drosophila phototransduction (118).
ACTIVATION OF THE TRP AND TRPL CHANNELS IN THE PHOTORECEPTOR CELL IN THE DARK
To interpret the experimental results obtained from heterologously expressed TRPL channels, it is useful to compare these results to those obtained from studies on the TRPL channels of the native photoreceptor cells. Unfortunately, the signaling membranes of the photoreceptor cells (the rhabdomeres) where the light-sensitive channels reside are not accessible to the patch pipette under conditions that preserve the response to light. Therefore, the characteristics of the channels under physiological conditions have been derived indirectly from studies using whole-cell patch clamp recordings from isolated ommatidia, where the patch pipette was attached to the cell body and did not reveal single-channel activity. The use of shot noise analysis to derive single-channel properties during the light response under physiological conditions is problematic because current fluctuations during light are dominated by the relatively slow fluctuations of the summed responses to single photons (quantum pumps, 119, 120). To overcome these difficulties, Hardie & Minke (120) derived the TRP and TRPL channel properties indirectly from cells that undergo uncontrolled spontaneous activation of the TRP and TRPL channels in the dark during whole-cell recordings (see Figure 3C and Reference 120).
It is important to realize that in the isolated ommatidia preparation, which has been the major preparation for studying the properties of the TRP and TRPL channels in the native system, the channels are readily and spontaneously activated in the dark. This dark activation is strongly facilitated by prior illumination. The spontaneous channel opening is irreversible and accompanied by a marked shift of the positive reversal potential to ~0 voltage. The bump noise and the response to light are also lost during this process (120). Detailed studies on the mechanism underlying spontaneous activation of the channels revealed that it arises from metabolic exhaustion. Unlike the in vivo intact preparation in which the metabolites for ATP production in the photoreceptor cells come from the pigment (glia) cells (121), in the isolated ommatidia preparation used for whole-cell recordings the photoreceptor cells are stripped from their pigment cells. The lack of pigment cells limits ATP production, especially under illumination, which consumes large quantities of ATP. Application of ATP and its precursor, NAD, into the cells via the patch pipette is essential to prevent spontaneous activation of the channels, while intense light is still followed by spontaneous channel activation even in the presence of ATP and NAD in the pipette.
A study aiming to elucidate the mechanism underlying the spontaneous activation of the channels in the dark was carried out by Minke and colleagues (122). In this study they found that spontaneous openings of the channels can be obtained not only in the isolated ommatidia preparation but also in vivo in the intact fly by inducing anoxia with N2. In the intact preparation, in contrast to the isolated ommatidia, channel opening in the dark by anoxia is completely reversible upon removal of anoxia. This study showed that anoxia rapidly and reversibly depolarizes the photoreceptors and induces Ca2+ influx into these cells in the dark. It further showed that openings of the light-sensitive channels, which mediate these effects, could also be obtained by mitochondrial uncouplers or by depletion of ATP in photoreceptor cells, whereas the effects of illumination and all forms of metabolic stress were additive. Effects similar to those found in wild-type flies were also found in mutants with strong defects in rhodopsin, Gq protein, or PLC. Genetic elimination of both TRP and TRPL channels prevented the effects of anoxia, mitochondrial uncouplers, and depletion of ATP, thus demonstrating that the TRP and TRPL channels are sensitive targets of metabolic stress. These results suggest that a constitutive ATP-dependent process is required to keep these channels closed in the dark, a requirement that would make them sensitive to metabolic stress (122).
In a follow-up study, Minke and colleagues (101) further showed that ATP depletion or inhibition of PKC activated the TRP channels, whereas photorelease of caged ATP or application of phorbol ester antagonized channel openings in the dark. Furthermore, Mg2+-dependent stable phosphorylation events by ATPγS or protein phosphatase inhibition by calyculin A abolished activation of the TRP and TRPL channels either by light or by metabolic inhibition. Although a high reduction of cellular Ca2+ with 10 mM BAPTA abolished channel activation by light or metabolic inhibition, subsequent application of Ca2+ to the extracellular medium (in the presence of 10 mM BAPTA in the pipette) combined with ATP depletion induced a robust dark current that was reminiscent of light responses (Figure 4). These results suggest that the combined action of Ca2+and phosphorylation-dephosphorylation reactions activates the TRP and TRPL channels in the dark (101).
A more specific mechanism for activation of the TRP and TRPL channels by metabolic inhibition in the dark has been proposed by Hardie and colleagues (123). According to this mechanism, activation of the channels by metabolic inhibition is primarily because DAG kinase failed to phosphorylate DAG owing to ATP depletion. Accumulation of DAG owing to intrinsic spontaneous activity (leak) of PLC, either during metabolic inhibition or in a mutant lacking DAG kinase (the rdgA mutant), activates the TRP and TRPL channels, which remain constitutively open and do not respond to light (124). The constitutive activity of the Ca2+-permeable TRP and TRPL channels presumably leads to a toxic increase in cellular Ca2+ followed by photoreceptor degeneration in the rdgA mutant. This model suggests that DAG or its metabolites, polyunsaturated fatty acids (PUFAs), are second messengers of excitation (125). In support of this model, genetic elimination of the TRP channels partially rescued the degeneration and the LIC of the rdgA mutant (126). In addition, the LIC of PLC mutants with minimal PLC activity and with a very small residual response to light was partially rescued in double mutant combinations that also eliminated the DAG kinase (123). Although DAG surrogates activate heterologously expressed TRPL channels (110), so far activating the TRP and TRPL channels in the photoreceptor cells by exogenous application of DAG or its surrogates has proved impossible. In contrast, application of PUFAs to both isolated ommatidia of Drosophila and heterologously expressed TRPL channels in S2 cells did activate the TRP/TRPL or TRPL channels, respectively (125). The kinetics of channel activation by PUFAs in Drosophila is, however, slower by 2–3 orders of magnitude relative to activation by light and has similar kinetics and magnitude to activation by metabolic inhibition.
In summary, activation of the TRP and TRPL channels by metabolic inhibition in the dark has been a useful tool to decouple the activity of the TRP and TRPL channels from the phototransduction machinery and to study their properties in isolation in the native system. Furthermore, these studies show that a combined action of cellular Ca2+ and DAG is required for channel activation. It is not clear, however, if activation of the channels by metabolic inhibition is equivalent to the mechanism underlying physiological activation of the channels by light.
PLC HAS AN ESSENTIAL ROLE IN ACTIVATION OF THE TRP AND TRPL CHANNELS IN PHOTORECEPTOR CELLS
PLC has long been recognized as an essential enzyme for the generation of the response to light because the response to supersaturating light intensity is virtually abolished in the nearly null PLC mutant norpA (29, 53). Activation of PLC results in three molecular events: breakdown of PIP2, production of DAG, and production of InsP3. A role for DAG in light excitation has been demonstrated by Hardie and colleagues (126).
The Role of InsP3
In Drosophila, the participation of InsP3 in light excitation has been seriously challenged by genetic elimination of the apparently single InsP3R, because InsP3R elimination had no effect on light excitation (127, 128). Because the expected role 2+ of InsP3 is to release Ca from internal stores via binding to the InsP3R, it is still possible that light-induced production of InsP3 leads, in a still unclear way, to a small, local Ca2+ elevation in the InsP3R-defective mutant. Such a small localized increase in Ca2+ combined with production of DAG can initiate the positive feedback effect of Ca2+ on excitation by opening a few TRP channels located at the base of the microvilli, leading to a normal light response. The critical future test for the role of InsP3 in light excitation is to examine the effects of manipulating the endogenous InsP3 levels upon its production by light. It is interesting to note that the InsP3R antagonist, 2-APB, reversibly blocks the LIC upstream of channel activation (129).
It should be noted that in several invertebrate species, a clear role for InsP3 in excitation via Ca2+ release from internal stores has been demonstrated (130–133). InsP3 has been shown to participate in excitation of mammalian TRPC channels where its binding to InsP3R induces TRP channel openings via direct coupling of TRPC3 to the InsP3R (134).
The Role of PIP2 Depletion
Although PIP2 has been implicated in regulation of various channels (135), including mammalian TRPV, TRPM (136, 137), and the heterologously expressed TRPL channel (110), there is strong evidence that depletion of PIP2 cannot account for activation of the TRP and TRPL channels in Drosophila in vivo. Although activity of TRPL channels expressed in Sf9 cells was suppressed by application of PIP2, suggesting that breakdown of PIP2 may open the channels (110), experiments in the fly point to the opposite conclusion. In Drosophila, PIP2 levels were measured in vivo in transgenic flies by the activity of targeted expression of Kir2.1 channels, acting as biosensors for PIP2 concentration (102). In these transgenic flies, depletion of PIP2 by light in conditions that block its regeneration inhibits activation of the TRP and TRPL channels. These experiments showed that in the trp mutant or in wild-type flies under Ca2+ deprivation, continuous light strongly reduced PIP2 levels in the rhabdomere and closed the TRP or TRPL channels in correlation with PIP2 depletion (102). Additional Ca2+-deprivation experiments in vivo in intact Drosophila strongly suggest that breakdown of PIP2 does not activate the TRP channels as well (see figure 7 in Reference 122).
In summary, several experiments have indicated that application of exogenous Ca2+ in the dark or in blind mutants failed to activate the TRP and TRPL channels (96, 103). However, application of Ca2+ to Ca2+-deprived cells when combined with metabolic inhibition excites the photoreceptors in the dark with magnitude and speed of the light response under similar conditions (Figure 4). These results together with the evidence obtained from studies of the rdgA mutant strongly suggest that a combined action of Ca2+ and DAG resulting from light activation of PLC mediates excitation.
PLC HAS AN EXCITATORY ROLE IN ACTIVATION OF HETEROLOGOUSLY EXPRESSED TRPL CHANNELS
TRPL channels expressed in various cell lines are spontaneously active. However, when the TRPL channel was coexpressed with G protein–coupled receptors, application of agonists greatly enhanced channel activity (81). These observations strongly suggest that TRPL is a promiscuous channel that uses the endogenous G protein and PLC of the host cell for its activation. A fact of significant importance was reported by Schilling and colleagues (110), who showed that the PLC inhibitor U-73122, but not its inactive analog, blocked the spontaneous TRPL activity in Sf9 cells as well as the enhanced channel activity by carbachol. M. Parnas and B. Minke (unpublished observations) have verified the striking blocking effect of U-73122 on TRPL channel activity in S2 cells (Figure 3). These results suggest that the spontaneous TRPL channel activity is mediated by spontaneous (leak) activity of PLC in the host cells. A support for the important role of PLC in activation of TRPL came from the experiments in which application of exogenous PLCβ2 enhanced TRPL channel activity (110).
Importantly, Schilling and colleagues (110) demonstrated that the large facilitation of TRPL channel activity by PUFAs, initially reported by Hardie and colleagues (125) in S2 cells, was also inhibited by the PLC inhibitor U-73122 in Sf9 cells expressing TRPL. This striking result was also verified by M. Parnas and B. Minke (unpublished observations) in S2 cells (Figure 3B). The conclusion from these experiments is that activation of the TRPL channels by PUFAs is mediated via PLC and does not result from direct activation of the channels. A strong support for this notion came from recent experiments of Broadie and colleagues (138) in a Drosophila mutant with defects in an integral plasma membrane protein with a sequence of a putative DAG lipase designated rolling blackout (rbo). Photoreceptors are enriched with the RBO DAG lipase protein. Temperature-sensitive (ts) rbo mutants show reversible elimination of phototransduction in a light-dependent manner within minutes, demonstrating acute requirement for the protein. Conditional ts rbo mutants show activity-dependent depletion of DAG and concomitant accumulation of PIP and PIP2 within minutes of induction at the restrictive temperature, strongly suggesting rapid downregulation of PLC activity. A strong indication that inhibition of phototransduction by the rbo mutation is not at the level of the TRP and TRPL channel activation came from induction of anoxia in the ts rbo mutants, which readily opened the channels when phototransduction was blocked. Additional support for lipid regulation of PLC activity came from a recent study by Mikoshiba and colleagues (139), who demonstrated a novel crosstalk between DAG and InsP3-mediated Ca2+ signaling through PLC activation by a DAG analog in tissue culture cells. Taken together, the above results suggest that PUFAs, the products of DAG lipase, regulate PLC activity presumably via its partial PH domain (74) and do not activate the channels directly.
The difficulty in applying this conclusion to fly photoreceptors arises from the finding of Hardie and colleagues (123) that PUFAs activate the TRP and TRPL channels in the native system of a nearly null PLC mutant of Drosophila regardless of application time. In addition, they showed in this study that unlike the time-independent PUFAs’ action, metabolic inhibitors induced openings of the channels in the PLC mutant only temporarily, during ~20 min of residual PLC activity following illumination. A reconciliation of the apparently conflicting data on PUFAs’ actions in vivo and in the tissue culture cells may come from many reports showing that PUFAs have multiple actions, including activation of PLC and inhibition of protein kinases, either directly (140) or by acting as metabolic inhibitors (141). Therefore, application of PUFAs to the PLC mutant is capable of inducing a residual PLC activation combined with metabolic inhibition, leading to channel activation via DAG accumulation. Accordingly, in the minimal system of the heterologously expressed TRPL channels, the specific targets of PUFAs’ metabolic inhibition are most likely missing, and only the effect on PLC activation is realized, whereas in the native system of the fly both effects of PUFAs are realized.
In summary, although all studies on Drosophila phototransduction indicate that both DAG and Ca2+ are required for excitation, there are different views regarding the specific mechanism that triggers channel opening. A summary of these views is given in Table 1.
TABLE 1.
The triggering mechanisms of TRP and TRPL channels
| Supporting evidence | Difficulties | |
|---|---|---|
| Excitation by lipids | Inhibition of DAG kinase enhances channel activity in vivo (123, 124, 126) | Application of DAG has no effect on channel excitation in vivo (S. Frechter and B. Minke, unpublished data) |
| The LIC of a PLC mutant with a very small residual response to light was partially rescued in the double mutant PLC/DAG kinase (123, 124) | Removal of a putative DAG lipase does not block channel opening (138) | |
| PUFAs open the channels both in vivo and in heterologous expression systems (124, 125) | The PUFAs’ effect in heterologous expression systems is blocked upon inhibition of PLC (110)
PUFAs’ activation kinetics in vivo is slower by 2–3 orders of magnitude relative to the activation by light (123, 125) |
|
| Ca2+ is necessary but not sufficient for excitation | 10 mM BAPTA but not 10 mM EGTA blocks channel activation by light and metabolic inhibition. In Ca2+ deprived cells application of Ca2+ combined with the metabolic inhibition mimics the response to light in the dark (kinetics and amplitude) (101) | Photorelease of caged Ca2+ in blind mutants does not open the channels (103) |
| Embryonic Drosophila photoreceptors are incapable of responding to light unless first provided with Ca2+ via the whole-cell pipette (98) | Genetic elimination of the single known InsP3R in Drosophila has no effect on excitation (128) | |
| Application of the InsP3R antagonist 2APB reversibly blocks the light response (129) | 2APB is known to have nonspecific effects (129) |
LIGHT-REGULATED TRANSLOCATION OF THE TRPL CHANNEL
To achieve a very high gain, which is required to produce the single photon responses to dim lights, the photoreceptor cell maximizes the current produced by a single photon using both the TRP channels and a relatively high level of TRPL channels. To prevent saturation of the light response upon an increase in ambient light, the Ca2+ flow via the TRP channel shuts off the TRPL channels and also induces its translocation out of the signaling membranes (59, 91, 99). This fascinating and novel mechanism to fine-tune visual responses is also used by other cellular signaling mechanisms such as fine-tuning of growth cone pathfinding via the action of a growth factor on TRPC5 activation (142).
The Amount of TRPL but not of TRP, PLC, PKC, or INAD Present in the Rhabdomeres is Regulated by Light
A relatively high level of TRPL, observed in photoreceptor membranes of dark-raised flies, rapidly decreased when the flies were transferred to light (Figure 5). Furthermore, the amount of rhabdomeral TRPL of flies that were kept in light for 16 h was close to the detection limit of the Western blot analysis but increased to a high level within 1 h after the transfer of the flies to darkness (Figure 5). The amount of rhabdomeral TRP and also that of other components of the INAD signaling complex, PLCβ, ePKC, and INAD, was unaffected by light (Figures 5 and 6). This finding indicates that the changes in the rhabdomeral TRPL level is specific to this ion channel subunit and are not, for example, a consequence of a massive light-dependent photoreceptor membrane shedding. Radioimmune assays and Western blot analysis revealed that the reduction of TRPL levels in the rhabdomers results from translocation of TRPL rather than from its degradation and de novo synthesis (59).
Figure 5.
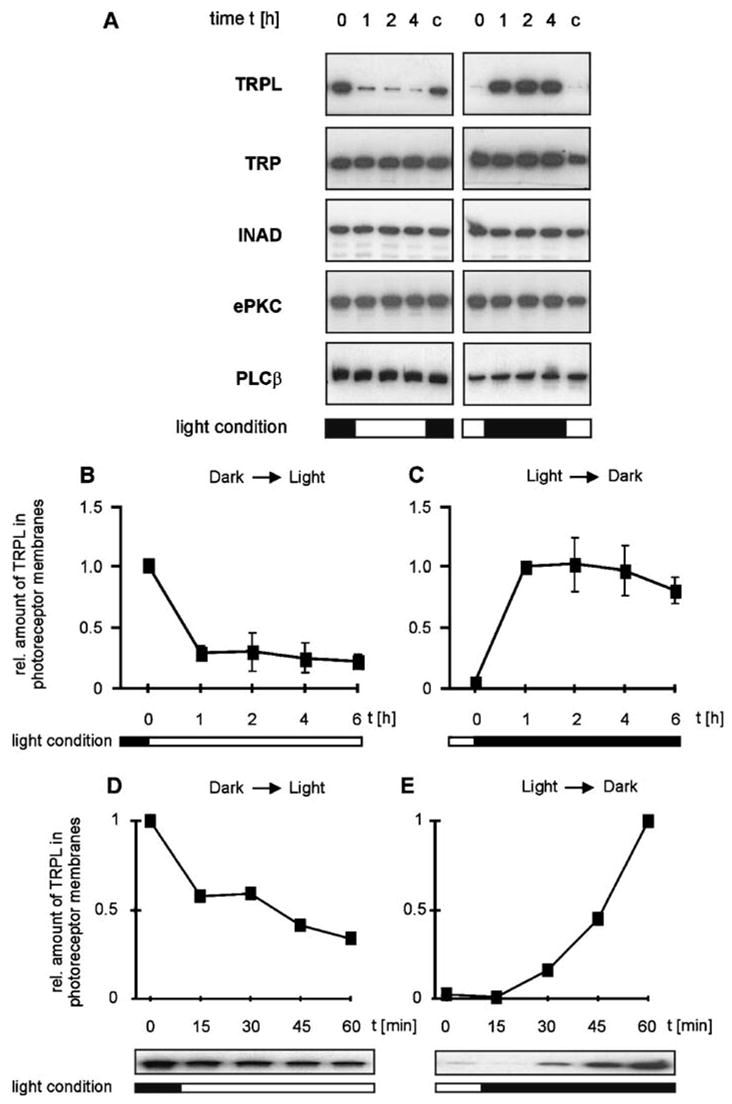
The amount of rhabdomeral TRPL but not TRP undergoes rapid light-induced changes. (A) Western blot analysis of phototransduction proteins present in the photoreceptor membrane of flies kept under different light conditions. Flies (Calliphora, mutant chalky) were raised in darkness or light for 16 h (t = 0 h) and were then switched from darkness to light (left panels) or vice versa (right panels). Photoreceptor membranes were isolated from dissected fly eyes at t = 0 and 1, 2, and 4 h (t = 1–4 h) after the change of light condition. A control group of flies (C) was kept under the original light condition during the experiment and was dissected at t = 5 h. (B–E) Time courses of the light-dependent increase and decrease of the TRPL level in rhabdomeral photoreceptor membranes. Flies were treated as described in (A). The time courses shown in (B) and (C) comprise 6 h after the change of light condition. Time courses revealing the changes occurring within the first hour after light change are shown in (D) and (E). (From Reference 59 with permission from Neuron.)
Figure 6.

Light-dependent translocation of TRPL molecules in the photoreceptor cells of Drosophila compound eyes. Cross sections through wild-type Drosophila (A–F) eyes of light- and dark-raised flies were double labeled with rhodamin-coupled wheat germ agglutinin, which specifically labels rhabdomeral photoreceptor membranes (red fluorescence) and antibodies against TRPL, TRP, and INAD, as indicated. The overlay of both markers appears yellow. Scale bar in (A), 10 μm. (From Reference 59 with permission from Neuron.)
The Mechanism that May Underlie TRPL Translocation
Direct visualization of intracellular movements of TRPL in photoreceptors upon illumination was obtained by immunolabeling of cross sections through Calliphora and Drosophila eyes. This study revealed that unlike TRPL, TRP and INAD are confined to the rhabdomeres, independent of whether the flies are kept in darkness or in light prior to sectioning (Figure 5). Antibodies directed against TRPL specifically labeled the rhabdomere area of cross-sectioned eyes obtained from dark-raised flies (Figure 6). In the eye sections of light-raised flies the TRPL-specific immunofluorescence was distributed over the cell body of the photoreceptor cell and was not detected in the rhabdomeres (Figure 6) in line with the Western blot analysis (59).
Because the translocation of TRPL depends on illumination, the question arises whether the response to light through activation of the TRP and TRPL channels is the trigger for TRPL movement to the cell body. Using immunocytochemistry, TRPL translocation from the rhabdomere to the cell body was tested in the nearly null PLC mutant, norpAP24 (31), the null mutant of TRP, trpP343 (40), and the null INAD mutant, inaD1. Young inaD flies contain rhabdomeral TRP, which is most likely detached from the signaling complex and therefore degrades with age and is largely missing in older inaD flies (51). Light-induced translocation of TRPL to the cell body was observed in young inaD flies but did not occur in the trp null mutant or in old inaD flies. In norpAP24 mutants, translocation of TRPL tagged with green fluorescent protein (TRPL-eGFP) was blocked. These findings reveal that when TRP is missing, as in the trp mutant and in old inaD flies, TRPL internalization is not observed (59). Therefore, the presence of TRP seems to be required for TRPL internalization. If the lack of PLC blocks TRPL translocation as found in vivo, then the block of TRPL translocation, by the absence of either TRP or PLC, suggests that light-induced Ca2+ influx is the trigger for TRPL translocation. This is because lack of PLC completely blocks Ca2+ influx via the light-activated channels, whereas genetic elimination of TRP eliminates the main route of Ca2+ entry into the photoreceptor cell (100, 143).
Translocation of TRPL Affects the Characteristics of the Light-Induced Current
The LIC of wild-type flies is composed of two independent components arising from activation of the TRP and TRPL channels (91). Patch clamp whole-cell recordings in isolated ommatidia of Drosophila were used to examined two properties of the LIC that discriminate between the contribution of the TRP and TRPL channels to the LIC: the block by La3+ and the reversal potential, which is ~15 mV and ~0 mV for the TRP and TRPL channels, respectively (91).
Application of La3+ in micromolar concentration is known specifically to block the TRP but not the TRPL channels (100, 144, 145). In wild-type Drosophila, application of La3+ specifically blocks the TRP channels leaving a residual response, which is mediated by the TRPL channels and is indistinguishable from the response measured in trp mutant photoreceptors. In wild-type flies kept in light (light-raised), application of La3+ largely reduced the peak amplitude of the LIC in response to intense light and modified the waveform of the response to prolonged light displaying the typical trp phenotype. In dark-raised wild-type flies, application of La3+ under identical experimental conditions had a much smaller effect on the peak amplitude of the LIC in response to similar light intensity. The weak trp phenotype in dark-raised flies indicates a reduced effect for La3+.
Quantitative analysis at dim lights shows that La3+ suppressed the LIC, suggesting a relative contribution of TRPL to the LIC of ~9% and 38%, in light-and dark-raised flies, respectively. Roughly similar conclusions were derived from measurements of the change in the reversal potential of light- and dark-raised flies, together suggesting that a significantly larger number of functional TRPL channels is present in dark-raised flies relative to light-raised flies (59).
Translocation of TRPL Induces Long-Term Adaptation
Measurements of the light sensitivity of dark- and light-raised flies in vivo revealed that wild-type flies kept in darkness are very sensitive to dim background lights and respond within a relatively wide dynamic range, having a relatively low sensitivity to small changes in stimulus intensity. Wild-type flies kept in light are different: (a) They are less sensitive to dim background lights, and (b) they have a smaller dynamic range, but (c) their photoreceptors are more sensitive to small changes in light intensity within their dynamic range. The fact that trpl mutants, when kept in either light or darkness, behave similarly to light-raised wild-type flies strongly suggests that translocation of TRPL underlies the fine-tuning of long-term adaptation (59).
Translocation of Mammalian TRP Channels
Recent studies on the mammalian TRPC5 channel revealed that growth factor stimulation initiated rapid (~2 min) incorporation into the plasma membrane of HEK-293 cells of expressed TRPC5 from vesicles held in reserve just under the plasma membrane. This incorporation was specific to TRPC5 and was not observed for TRPC1. Channel incorporation was measured by evanescent field of total internal reflection fluorescence microscopy and biotin surface labeling. Strikingly, TRPC5 incorporation into the plasma membrane resulted in a dramatic increase in the typical current produced by these channels, indicating insertion of functional channels. Epidermal growth factor–induced incorporation of functional TRPC5 channels requires phosphatidylinositol 3-kinase (PI3K), the Rho GTPase, Rac1, and phosphatidyl inositol 4-phosphate 5-kinase alpha (PIP5Kα). The role of ERM proteins, which are known to be regulated by Rho GTPase, was not investigated in the study. It is hypothesized that Rac1 mediates the rapid insertion of TRPC5 into the plasma membrane through stimulation of PIP5Kα and increased channel availability. This process also occurs in hippocampal neurons in primary culture, where it affects the process of growth cone extension, suggesting that growth factor–induced extension, which is known to be regulated by Ca2+ influx, is mediated by regulated insertion of the Ca2+-permeable TRPC5 channels (142).
Internalization of TRPC3, together with its signaling complex Gq/11, PLCβ1, and caveolin-1, was reported to occur following treatment with the protein phosphatase inhibitor caliculin A, which also induced reorganization of the actin cytoskeleton (73). This channel internalization led to a marked attenuation in divalent cation (Sr2+) entry induced by application of OAG, reminiscent of the reduction of TRPL-dependent currents in Drosophila upon translocation of TRPL. An additional study by Ambudkar and colleagues (146) revealed that carbachol-stimulation of epithelial cells resulted in translocation of TRPC3 to the plasma membrane in a mechanism that was inhibited by cleavage of the SNARE protein VAMP2 (vesicle-associated membrane protein 2). These data suggest that VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulation of Ca2+ influx (146). Another TRPC channel reported to be regulated by exocytosis is TRPC6. Stimulation of the M3 muscarinic receptor resulted in translocation of TRPC6 to the plasma membrane in a timescale that coincided with activation by Ca2+ influx (147).
Translocation of another mammalian TRP channel (TRPV2) was reported for Balb/c 3T3 cells. These cells expressed the TRPV2 channel, which is regulated by the insulin-like growth factor (IGF-1). Interestingly, stimulation of the cells by IGF-1 induced translocation of the channels from internal vesicles to the plasma membrane (148). No functional consequence of this translocation was tested, and similar experiments in heterologously expressed TRPV1 did not reveal any translocation (142).
In summary, translocation of TRP channels of both Drosophila and mammalian cells emerges as a novel and important regulatory mechanism with wide implications to a variety of signaling mechanisms.
CONCLUDING REMARKS
Channel proteins are capable of carrying relatively large currents, and, therefore, a relatively small number of channels is sufficient to produce large potential differences. Therefore, channel proteins are expressed in relatively small quantities in the native tissue. This rule applies to most TRP channels and makes in vivo studies of TRP channels difficult. In addition, activation and regulation of TRP channels seem to require multiple protein-protein interactions that are difficult to study in heterologous systems in which critical components are missing or exist in an abnormal stoichiometry. The Drosophila photoreceptor cell, which uses TRP channels and their activating proteins in huge quantities, combined with the great power of the Drosophila molecular genetics, is a unique signaling system. This system allows studies of TRP activation and gating mechanism in the native system of the eye.
Although a comparison of the single-channel properties of the native and expressed TRPL channels revealed many similarities, a profound difference between them is the constitutive activity of heterologously expressed TRPL channels, which is not observed in the native system in the dark. To account for this difference, investigators have suggested that the TRPγ subunit, which is missing in the heterologous system, is required to keep the channels closed in the photoreceptor cell (41). A different explanation based on substantial evidence (e.g., Figure 3) is that the endogenous PLC of the host cell (and not the TRPL channel itself) is constitutively active and therefore activates the expressed TRPL channel. A similar explanation may hold for mammalian TRP channels that also show constitutive activity in heterologous systems. In the photoreceptor cells, there seem to be powerful mechanisms that are missing in the heterologous system, preventing spontaneous activation of PLC or triggering slow inactivation of its products and the consequent generation of light response in the dark.
Unlike the promiscuous TRPL channel that can use endogenous or exogenous (110) PLCs for activation, the Drosophila TRP channel seems to be a tightly regulated channel that requires the native PLC (NORPA) and perhaps other TRP-interacting proteins of the INAD signaling complex to function in heterologous systems. Future experiments should resolve this possibility. Mammalian TRP channels may include both promiscuous and tightly regulated TRP channels, whereas only the promiscuous channels show constitutive activity when expressed in tissue culture cells.
Acknowledgments
We thank Drs. Johannes Oberwinkler, Shmuel Muallem, Kendal Broadie, Barbara Niemeyer, Veit Flockerzi, Shaya Lev, and Ben Katz for critical reading of the manuscript and Johannes Oberwinkler for stimulating discussions. The experimental part of this review was supported by grants from the National Institutes of Health (EY 03529), the Israel Science Foundation (ISF), the Minerva Foundation, the U.S.-Israel Binational Science Foundation (BSF), and the German Israeli Foundation (GIF).
LITERATURE CITED
- 1.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–66. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 2.Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 3.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–23. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 4.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–56. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–98. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- 6.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 7.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–72. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 8.Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39:585–88. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- 9.Hardie RC. Regulation of TRP channels via lipid second messengers. Annu Rev Physiol. 2003;65:735–59. doi: 10.1146/annurev.physiol.65.092101.142505. [DOI] [PubMed] [Google Scholar]
- 10.Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channel. Sci STKE. 2001;90:Re1. doi: 10.1126/stke.2001.90.re1. http://stke.sciencemag.org/cgi/content/full/OCsigtrans;2001/90/re1. [DOI] [PubMed] [Google Scholar]
- 11.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–59. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 12.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–39. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 13.Montell C. The TRP superfamily of cation channels. Sci STKE. 20052005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 14.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–73. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Lee Y, Lee J, Bang S, Hyun S, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–10. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 16.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–23. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 17.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–34. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 18.Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 21.Caterina MJ, Montell C. Take a TRP to beat the heat. Genes Dev. 2005;19:415–18. doi: 10.1101/gad.1294905. [DOI] [PubMed] [Google Scholar]
- 22.Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–62. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 23.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–51. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 24.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–15. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 25.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 26.Zufall F, Munger SD. From odor and pheromone transduction to the organization of the sense of smell. Trends Neurosci. 2001;24:191–93. doi: 10.1016/s0166-2236(00)01765-3. [DOI] [PubMed] [Google Scholar]
- 27.Stryer L, Berg J, Tymoczko J. Biochemistry. New York: W.H. Freeman; 2002. [Google Scholar]
- 28.Wu CF, Pak WL. Quantal basis of photoreceptor spectral sensitivity of Drosophila melanogaster. J Gen Physiol. 1975;66:149–68. doi: 10.1085/jgp.66.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minke B, Selinger Z. The inositol-lipid pathway is necessary for light excitation in fly photoreceptors. In: Corey D, Roper SD, editors. Sensory Transduction. Vol. 47. New York: Rockefeller Univ. Press; 1992. pp. 202–17. [PubMed] [Google Scholar]
- 30.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–93. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 31.Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–33. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 32.Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, et al. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci USA. 1987;84:6939–43. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–50. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 34.Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40:851–58. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- 35.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–42. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 36.Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–68. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- 37.Ranganathan R, Malicki DM, Zuker CS. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. doi: 10.1146/annurev.ne.18.030195.001435. [DOI] [PubMed] [Google Scholar]
- 38.Minke B, Selinger Z. The roles of trp and calcium in regulating photoreceptor function in Drosophila. Curr Opin Neurobiol. 1996;6:459–66. doi: 10.1016/s0959-4388(96)80050-x. [DOI] [PubMed] [Google Scholar]
- 39.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–59. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 40.Scott K, Sun Y, Beckingham K, Zuker CS. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell. 1997;91:375–83. doi: 10.1016/s0092-8674(00)80421-3. [DOI] [PubMed] [Google Scholar]
- 41.Xu XZ, Chien F, Butler A, Salkoff L, Montell C. TRPγ, a Drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron. 2000;26:647–57. doi: 10.1016/s0896-6273(00)81201-5. [DOI] [PubMed] [Google Scholar]
- 42.Smith DP, Ranganathan R, Hardy RW, Marx J, Tsuchida T, Zuker CS. Photoreceptor deactivation and retinal degeneration mediated by a photoreceptor-specific protein kinase C. Science. 1991;254:1478–84. doi: 10.1126/science.1962207. [DOI] [PubMed] [Google Scholar]
- 43.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 44.Masai I, Okazaki A, Hosoya T, Hotta Y. Drosophila retinal degeneration A gene encodes an eye-specific diacylglycerol kinase with cysteine-rich zinc-finger motifs and ankyrin repeats. Proc Natl Acad Sci USA. 1993;90:11157–61. doi: 10.1073/pnas.90.23.11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu L, Niemeyer B, Colley N, Socolich M, Zuker CS. Regulation of PLC-mediated signalling in vivo by CDP-diacylglycerol synthase. Nature. 1995;373:216–22. doi: 10.1038/373216a0. [DOI] [PubMed] [Google Scholar]
- 46.Vihtelic TS, Hyde DR, O’Tousa JE. Isolation and characterization of the Drosophila retinal degeneration B (rdgB) gene. Genetics. 1991;127:761–68. doi: 10.1093/genetics/127.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masai I, Suzuki E, Yoon CS, Kohyama A, Hotta Y. Immunolocalization of Drosophila eye-specific diacylgylcerol kinase, rdgA, which is essential for the maintenance of the photoreceptor. J Neurobiol. 1997;32:695–706. doi: 10.1002/(sici)1097-4695(19970620)32:7<695::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Vihtelic TS, Goebl M, Milligan S, O’Tousa JE, Hyde DR. Localization of Drosophila retinal degeneration B, a membrane-associated phosphatidylinositol transfer protein. J Cell Biol. 1993;122:1013–22. doi: 10.1083/jcb.122.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsunoda S, Sun Y, Suzuki E, Zuker C. Independent anchoring and assembly mechanisms of INAD signaling complexes in Drosophila photoreceptors. J Neurosci. 2001;21:150–58. doi: 10.1523/JNEUROSCI.21-01-00150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu XZ, Choudhury A, Li X, Montell C. Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins. J Cell Biol. 1998;142:545–55. doi: 10.1083/jcb.142.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, et al. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–49. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 52.Minke B. Photopigment-dependent adaptation in invertebrates—implications for vertebrates. In: Stieve H, editor. The Molecular Mechanism of Photoreception. Vol. 34. Berlin: Springer-Verlag; 1986. pp. 241–65. [Google Scholar]
- 53.Pak WL. Drosophila in vision research. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1995;36:2340–57. [PubMed] [Google Scholar]
- 54.Shieh BH, Zhu MY. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16:991–98. doi: 10.1016/s0896-6273(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 55.Huber A, Sander P, Gobert A, Bahner M, Hermann R, Paulsen R. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996;15:7036–45. [PMC free article] [PubMed] [Google Scholar]
- 56.Arnold DB, Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron. 1999;23:149–57. doi: 10.1016/s0896-6273(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 57.Dimitratos SD, Woods DF, Stathakis DG, Bryant PJ. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays. 1999;21:912–21. doi: 10.1002/(SICI)1521-1878(199911)21:11<912::AID-BIES3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 58.Schillace RV, Scott JD. Organization of kinases, phosphatases, and receptor signaling complexes. J Clin Invest. 1999;103:761–65. doi: 10.1172/JCI6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bahner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- 60.Xu XZS, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–64. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 61.Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- 62.Wes PD, Xu XZ, Li HS, Chien F, Doberstein SK, Montell C. Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat Neurosci. 1999;2:447–53. doi: 10.1038/8116. [DOI] [PubMed] [Google Scholar]
- 62a.Chorna-Ornan I, Tzarfaty V, Ankri-Eliahoo G, Joel-Almagor T, Meyer NE, et al. Light-regulated interaction of Dmoesin with TRP and TRPL channels is required for maintenance of photoreceptors. J Cell Biol. 2005;171:143–52. doi: 10.1083/jcb.200503014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat Cell Biol. 2002;4:782–89. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- 64.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–99. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 65.Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci. 2002;115:3497–508. doi: 10.1242/jcs.115.17.3497. [DOI] [PubMed] [Google Scholar]
- 66.Montell C. TRP trapped in fly signaling web. Curr Opin Neurobiol. 1998;8:389–97. doi: 10.1016/s0959-4388(98)80066-4. [DOI] [PubMed] [Google Scholar]
- 67.Macpherson MR, Pollock VP, Kean L, Southall TD, Giannakou ME, et al. Transient receptor potential-like channels are essential for calcium signaling and fluid transport in a Drosophila epithelium. Genetics. 2005;169:1541–52. doi: 10.1534/genetics.104.035139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Estacion M, Sinkins WG, Schilling WP. Stimulation of Drosophila TrpL by capacitative Ca2+ entry. Biochem J. 1999;341(Pt 1):41–49. [PMC free article] [PubMed] [Google Scholar]
- 69.Huber A, Sander P, Bahner M, Paulsen R. The TRP Ca2+ channel assembled in a signaling complex by the PDZ domain protein INAD is phosphorylated through the interaction with protein kinase C (ePKC) FEBS Lett. 1998;425:317–22. doi: 10.1016/s0014-5793(98)00248-8. [DOI] [PubMed] [Google Scholar]
- 70.Li HS, Montell C. TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J Cell Biol. 2000;150:1411–22. doi: 10.1083/jcb.150.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cook B, Bar-Yaacov M, Cohen-Ben-Ami H, Goldstein RE, Paroush Z, et al. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat Cell Biol. 2000;2:296–301. doi: 10.1038/35010571. [DOI] [PubMed] [Google Scholar]
- 72.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–89. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 73.Lockwich T, Singh BB, Liu X, Ambudkar IS. Stabilization of cortical actin induces internalization of transient receptor potential 3 (Trp3)-associated caveolar Ca2+ signaling complex and loss of Ca2+ influx without disruption of Trp3-inositol trisphosphate receptor association. J Biol Chem. 2001;276:42401–8. doi: 10.1074/jbc.M106956200. [DOI] [PubMed] [Google Scholar]
- 74.van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, et al. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- 75.Obukhov AG, Nowycky MC. TRPC5 activation kinetics are modulated by the scaffolding protein ezrin/radixin/moesin-binding phosphoprotein-50 (EBP50) J Cell Physiol. 2004;201:227–35. doi: 10.1002/jcp.20057. [DOI] [PubMed] [Google Scholar]
- 76.Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, et al. Association of mammalian Trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275:37559–64. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- 77.Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7:405–11. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 78.Gillo B, Chorna I, Cohen H, Cook B, Manistersky I, et al. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry. Proc Natl Acad Sci USA. 1996;93:14146–51. doi: 10.1073/pnas.93.24.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;267:C1501–5. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- 80.Hambrecht J, Zimmer S, Flockerzi V, Cavalie A. Single-channel currents through transientreceptor-potential-like (TRPL) channels. Pflugers Arch. 2000;440:418–26. doi: 10.1007/s004240000301. [DOI] [PubMed] [Google Scholar]
- 81.Hardie RC, Reuss H, Lansdell SJ, Millar NS. Functional equivalence of native light-sensitive channels in the Drosophila trp301 mutant and TRPL cation channels expressed in a stably transfected Drosophila cell line. Cell Calcium. 1997;21:431–40. doi: 10.1016/s0143-4160(97)90054-3. [DOI] [PubMed] [Google Scholar]
- 82.Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze DL, Schilling WP. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl ) protein of Drosophila. Biochem Biophys Res Commun. 1994;201:1050–56. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- 83.Kunze DL, Sinkins WG, Vaca L, Schilling WP. Properties of single Drosophila Trpl channels expressed in Sf9 insect cells. Am J Physiol. 1997;272:C27–34. doi: 10.1152/ajpcell.1997.272.1.C27. [DOI] [PubMed] [Google Scholar]
- 84.Zimmer S, Trost C, Wissenbach U, Philipp S, Freichel M, et al. Modulation of recombinant transient-receptor-potential-like (TRPL) channels by cytosolic Ca2+ Pflugers Arch. 2000;440:409–17. doi: 10.1007/s004240000292. [DOI] [PubMed] [Google Scholar]
- 85.Minke B, Selinger Z. Inositol lipid pathway in fly photoreceptors: excitation, calcium mobilization and retinal degeneration. In: Osborne NA, Chader GJ, editors. Progress in Retinal Research. Oxford: Pergamon; 1991. pp. 99–124. [Google Scholar]
- 86.Hu Y, Schilling WP. Receptor-mediated activation of recombinant Trpl expressed in Sf9 insect cells. Biochem J. 1995;305:605–11. doi: 10.1042/bj3050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lan L, Bawden MJ, Auld AM, Barritt GJ. Expression of Drosophila trpl cRNA in Xenopus laevis oocytes leads to the appearance of a Ca2+ channel activated by Ca2+ and calmodulin, and by guanosine 5′ [gamma-thio]triphosphate. Biochem J. 1996;316(Pt 3):793–803. doi: 10.1042/bj3160793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lan L, Brereton H, Barritt GJ. The role of calmodulin-binding sites in the regulation of the Drosophila TRPL cation channel expressed in Xenopus laevis oocytes by Ca2+, inositol 1,4,5-trisphosphate and GTP-binding proteins. Biochem J. 1998;330(Pt 3):1149–58. doi: 10.1042/bj3301149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hardie RC. Whole-cell recordings of the light induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc R Soc London Ser B. 1991;245:203–10. [Google Scholar]
- 90.Hardie RC, Minke B. Calcium-dependent inactivation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994;103:409–27. doi: 10.1085/jgp.103.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–59. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 92.Leung HT, Geng C, Pak WL. Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J Neurosci. 2000;20:6797–803. doi: 10.1523/JNEUROSCI.20-18-06797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oberwinkler J, Stavenga DG. Calcium imaging demonstrates colocalization of calcium influx and extrusion in fly photoreceptors. Proc Natl Acad Sci USA. 2000;97:8578–83. doi: 10.1073/pnas.97.15.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, Montell C. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron. 2005;45:367–78. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 95.Oberwinkler J. Calcium homeostasis in fly photoreceptor cells. Adv Exp Med Biol. 2002;514:539–83. doi: 10.1007/978-1-4615-0121-3_32. [DOI] [PubMed] [Google Scholar]
- 96.Hardie RC. Excitation of Drosophila photoreceptors by BAPTA and ionomycin: evidence for capacitative Ca2+ entry? Cell Calcium. 1996;20:315–27. doi: 10.1016/s0143-4160(96)90037-8. [DOI] [PubMed] [Google Scholar]
- 97.Ranganathan R, Bacskai BJ, Tsien RY, Zuker CS. Cytosolic calcium transients: spatial localization and role in Drosophila photoreceptor cell function. Neuron. 1994;13:837–48. doi: 10.1016/0896-6273(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 98.Hardie RC, Peretz A, Pollock JA, Minke B. Ca2+ limits the development of the light response in Drosophila photoreceptors. Proc R Soc London Ser B Biol Sci. 1993;252:223–29. doi: 10.1098/rspb.1993.0069. [DOI] [PubMed] [Google Scholar]
- 99.Cook B, Minke B. TRP and calcium stores in Drosophila phototransduction. Cell Calcium. 1999;25:161–71. doi: 10.1054/ceca.1998.0018. [DOI] [PubMed] [Google Scholar]
- 100.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–51. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 101.Agam K, Frechter S, Minke B. Activation of the Drosophila TRP and TRPL channels requires both Ca2+ and protein dephosphorylation. Cell Calcium. 2004;35:87–105. doi: 10.1016/j.ceca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 102.Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 2001;30:149–59. doi: 10.1016/s0896-6273(01)00269-0. [DOI] [PubMed] [Google Scholar]
- 103.Hardie RC. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J Neurosci. 1995;15:889–902. doi: 10.1523/JNEUROSCI.15-01-00889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Obukhov AG, Schultz G, Luckhoff A. Regulation of heterologously expressed transient receptor potential-like channels by calcium ions. Neuroscience. 1998;85:487–95. doi: 10.1016/s0306-4522(97)00616-7. [DOI] [PubMed] [Google Scholar]
- 105.Hardie RC, Raghu P. Activation of heterologously expressed Drosophila TRPL channels: Ca2+ is not required and InsP3 is not sufficient. Cell Calcium. 1998;24:153–63. doi: 10.1016/s0143-4160(98)90125-7. [DOI] [PubMed] [Google Scholar]
- 106.Trost C, Marquart A, Zimmer S, Philipp S, Cavalie A, Flockerzi V. Ca2+-dependent interaction of the trpl cation channel and calmodulin. FEBS Lett. 1999;451:257–63. doi: 10.1016/s0014-5793(99)00588-8. [DOI] [PubMed] [Google Scholar]
- 107.Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci USA. 1999;96:14669–71. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–72. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 109.Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–64. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 110.Estacion M, Sinkins WG, Schilling WP. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J Physiol. 2001;530:1–19. doi: 10.1111/j.1469-7793.2001.0001m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yagodin S, Hardie RC, Lansdell SJ, Millar NS, Mason WT, Sattelle DB. Thapsigargin and receptor-mediated activation of Drosophila TRPL channels stably expressed in a Drosophila S2 cell line. Cell Calcium. 1998;23:219–28. doi: 10.1016/s0143-4160(98)90120-8. [DOI] [PubMed] [Google Scholar]
- 112.Biddlecome GH, Berstein G, Ross EM. Regulation of phospholipase C-β1 by Gq and m1 muscarinic cholinergic receptor. Steady-state balance of receptor-mediated activation and GTPase-activating protein-promoted deactivation. J Biol Chem. 1996;271:7999–8007. doi: 10.1074/jbc.271.14.7999. [DOI] [PubMed] [Google Scholar]
- 113.Running Deer JL, Hurley JB, Yarfitz SL. G protein control of Drosophila photoreceptor phospholipase C. J Biol Chem. 1995;270:12623–28. doi: 10.1074/jbc.270.21.12623. [DOI] [PubMed] [Google Scholar]
- 114.Warr CG, Kelly LE. Identification and characterization of two distinct calmodulin-binding sites in the Trpl ion-channel protein of Drosophila melanogaster. Biochem J. 1996;314:497–503. doi: 10.1042/bj3140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Porter JA, Yu M, Doberstein SK, Pollard TD, Montell C. Dependence of calmodulin localization in the retina on the NINAC unconventional myosin. Science. 1993;262:1038–42. doi: 10.1126/science.8235618. [DOI] [PubMed] [Google Scholar]
- 116.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C. Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci USA. 2001;98:3600–5. doi: 10.1073/pnas.051511398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Z, Tang Y, Zhu MX. Increased inwardly rectifying potassium currents in HEK-293 cells expressing murine transient receptor potential 4. Biochem J. 2001;354:717–25. doi: 10.1042/0264-6021:3540717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118a.Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, et al. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci USA. 2001;98:3168–73. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barash S, Minke B. Is the receptor potential of fly photoreceptors a summation of single-photon responses? Comments Theor Biol. 1994;3:229–63. [Google Scholar]
- 120.Hardie RC, Minke B. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994;103:389–407. doi: 10.1085/jgp.103.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–85. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agam K, von Campenhausen M, Levy S, Ben-Ami HC, Cook B, et al. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci. 2000;20:5748–55. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hardie RC, Martin F, Chyb S, Raghu P. Rescue of light responses in the Drosophila “null” phospholipase C mutant, norpAP24, by diacylglycerol kinase mutant, rdgA, and by metabolic inhibition. J Biol Chem. 2003;278:18851–58. doi: 10.1074/jbc.M300310200. [DOI] [PubMed] [Google Scholar]
- 124.Hardie RC. TRP channels in Drosophila photoreceptors: the lipid connection. Cell Calcium. 2003;33:385–93. doi: 10.1016/s0143-4160(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 125.Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–59. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 126.Raghu P, Usher K, Jonas S, Chyb S, Polyanovsky A, Hardie RC. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron. 2000;26:169–79. doi: 10.1016/s0896-6273(00)81147-2. [DOI] [PubMed] [Google Scholar]
- 127.Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18:881–87. doi: 10.1016/s0896-6273(00)80328-1. [DOI] [PubMed] [Google Scholar]
- 128.Raghu P, Colley NJ, Webel R, James T, Hasan G, et al. Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene. Mol Cell Neurosci. 2000;15:429–45. doi: 10.1006/mcne.2000.0846. [DOI] [PubMed] [Google Scholar]
- 129.Chorna-Ornan I, Joel-Almagor T, Cohen-Ben-Ami H, Frechter S, Gillo B, et al. A common mechanism underlies vertebrate calcium signaling and Drosophila phototransduction. J Neurosci. 2001;21:2622–29. doi: 10.1523/JNEUROSCI.21-08-02622.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown JE, Rubin LJ, Ghalayini AJ, Tarver AP, Irvine RF, et al. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984;311:160–63. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- 131.del Pilar Gomez M, Nasi E. Membrane current induced by protein kinase C activators in rhabdomeric photoreceptors: implications for visual excitation. Proc Natl Acad Sci USA. 1998;93:11196–201. doi: 10.1523/JNEUROSCI.18-14-05253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fein A, Payne R, Corson DW, Berridge MJ, Irvine RF. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984;311:157–60. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- 133.Walz B, Liebherr H, Ukhanov K. Ca2+-dependent and Ca2+ release-dependent excitation in leech photoreceptors: evidence from a novel “inside-out” cell model. Cell Calcium. 2003;34:35–47. doi: 10.1016/s0143-4160(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 134.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, et al. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–82. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 135.Gomez MD, Nasi E. A direct signaling role for phosphatidylinositol 4,5-bisphosphate (PIP2) in the visual excitation process of microvillar receptors. J Biol Chem. 2005;280:16784–89. doi: 10.1074/jbc.M414538200. [DOI] [PubMed] [Google Scholar]
- 136.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, et al. Brady-kinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–62. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 137.Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–36. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 138.Huang FD, Matthies HJ, Speese SD, Smith MA, Broadie K. Rolling blackout, a newly identified PIP2-DAG pathway lipase required for Drosophila phototransduction. Nat Neurosci. 2004;7:1070–78. doi: 10.1038/nn1313. [DOI] [PubMed] [Google Scholar]
- 139.Hisatsune C, Nakamura K, Kuroda Y, Nakamura T, Mikoshiba K. Amplification of Ca2+ signaling by diacylglycerol-mediated inositol 1,4,5-trisphosphate production. J Biol Chem. 2005;280:11723–30. doi: 10.1074/jbc.M409535200. [DOI] [PubMed] [Google Scholar]
- 140.Mirnikjoo B, Brown SE, Kim HF, Marangell LB, Sweatt JD, Weeber EJ. Protein kinase inhibition by ω -3 fatty acids. J Biol Chem. 2001;276:10888–96. doi: 10.1074/jbc.M008150200. [DOI] [PubMed] [Google Scholar]
- 141.Arslan P, Corps AN, Hesketh TR, Metcalfe JC, Pozzan T. cis-Unsaturated fatty acids uncouple mitochondria and stimulate glycolysis in intact lymphocytes. Biochem J. 1984;217:419–25. doi: 10.1042/bj2170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–20. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 143.Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994;12:1257–67. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 144.Hochstrate P. Lanthanum mimicks the trp photoreceptor mutant of Drosophila in the blowfly Calliphora. J Comp Physiol A. 1989;166:179–87. doi: 10.1007/BF00193462. [DOI] [PubMed] [Google Scholar]
- 145.Suss-Toby E, Selinger Z, Minke B. Lanthanum reduces the excitation efficiency in fly photoreceptors. J Gen Physiol. 1991;98:849–68. doi: 10.1085/jgp.98.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Singh BB, Lockwich TP, Bandyopadhyay BC, Liu X, Bollimuntha S, et al. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell. 2004;15:635–46. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 147.Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem. 2004;279:7241–46. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- 148.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–70. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]


