Introduction
J.F. Gudernatsch, an anatomist at Cornell Medical School, traveled by ship to the Naples Zoological Station in the summer of 1910. He took along various mammalian organs because he planned to study the affect of normal and cancerous mammalian organ extracts on the development of fish and frogs. These extracts did disturb normal development but Gudernatsch attributed the result to the fact that the organs had been without cold storage on the long trip from New York. Undeterred he changed his protocol the following summer when he visited the histological laboratory in Prague. This time he used fresh extracts from newly killed animals. His subjects were tadpoles of the local frog, Rana temporaria. “As food were tried: thyroid, liver, adrenal, hypophysis, and muscle from horse, thymus from calf, testicle and ovary from dog or cat. Some organs from rabbits and pigs also were given. With each experiment one group was left unfed as control to test how much nourishment the animals could take from the tap-water which in Prague is very rich in organisms.” (Gudernatsch, 1912). The extract from thyroid glands caused the tadpoles to turn into frogs. This observation was confirmed when Allen (1925) removed the tadpole’s thyroid gland and inhibited metamorphosis. The discovery of the major product of the thyroid gland, thyroxine (T4) (Harington, 1926), was followed by the identification of its more active metabolite, the hormone 3,5,3′-l-triiodothyronine (T3) (Gross and Pitt-Rivers, 1952).
Thyroid hormone (TH) was the first developmental morphogen ever discovered. The advantage of having unlimited amounts of a chemical that just by adding to the rearing water induces the dramatic biological changes of amphibian metamorphosis stimulated the research of generations of anatomists, endocrinologists, physiologists, and biochemists. Traditional forward genetics has not been applied to amphibian metamorphosis. The classic book by Nieuwkoop and Faber (1956) remains the most complete source of morphological changes of all stages of Xenopus laevis development including embryogenesis and metamorphosis. A comprehensive review of early work in this field is by Dodd and Dodd (1976). Yoshizato’s (1989, 1996) reviews summarize the death and loss of tadpole organs. Shi (2000) wrote the first book devoted exclusively to amphibian metamorphosis. Recent reviews concentrate on the molecular aspects of metamorphosis (Furlow and Neff, 2006; Buchholz et al., 2006; Tata, 2006). The important roles of the deiodinases in amphibian metamorphosis have been reviewed (Brown, 2005).
Important unsolved questions
Amphibian metamorphosis provides a wonderful set of biological questions. How can a single hormone control so many diverse developmental programs? The same cell type can have a different fate depending upon its location. Tail muscle is induced by TH to die; limb muscle is induced by the same hormone to grow and differentiate. Organs respond autonomously to TH. For instance, amputated tails can be induced by TH to resorb in organ culture (Weber, 1962). The individual cell types that comprise an organ can themselves be direct targets of the hormone with independent cell autonomous programs (Yaoita and Nakajima, 1997; Rowe et al., 2002; Brown et al., 2005).
Metamorphosis is a model system to study vertebrate organogenesis. For example, the tadpole intestinal tract is a long simple tube with a rudimentary stomach and large intestine. Within 5 days at the climax of metamorphosis it shortens 75% in length, a stomach is formed with new secretory glands (Ishizuya-Oka and Shi, 2005). The small intestine develops the familiar crypts and villi that characterize a typical adult vertebrate small intestine. The pronephros is a tadpole structure that regresses and disappears at the end of metamorphosis (Fox, 1970). Remodeling from preexisting functional tadpole organs includes the skin (Yoshizato, 1996), the respiratory organs (Dodd and Dodd,1976), liver (Atkinson et al., 1998), the immune system (Rollins-Smith, 1998), the brain and spinal cord (Kollros, 1981), the eye (Hoskins, 1986; Mann and Holt, 2001), the nose (Higgs and Burd, 2001), the pituitary (Kikuyama et al., 1993; Buckbinder and Brown, 1993; Huang et al., 2001), the hematopoetic system (Weber, 1996), and much of the skeleton (Trueb and Hanken, 1992). These remodeling events invariably result in a typical vertebrate organ with TH having insinuated itself into the developmental programs. In addition, TH controls the formation of new organs and cell types for use by the frog after metamorphosis that either do not exist in the tadpole or have no function in tadpoles. These include the limbs, bone marrow, and skin and stomach glands. Not all frog organs require TH to develop. One way to observe these TH independent changes is to raise tadpoles for many months on methimazole, an inhibitor that blocks the synthesis of TH in the thyroid gland. Occasionally a tadpole will arrest development spontaneously. Both kinds of tadpoles grow to very large size and can live as long as 2 years. Normally, gonads with visible eggs and sperm appear several months after metamorphosis. However, in time these arrested tadpoles form primary oocytes and spermatagonia (Dodd and Dodd, 1976; Rot-Nikcevic and Wassersug, 2003). Remodeling of the tadpole skeleton, especially the skull, requires TH. However arrested tadpoles do not remodel their skeleton. Instead they ossify the tadpole skull and vertebral column (Brown, unpublished data). TH action potentiates the liver to respond to estrogen and synthesize vitellogenin (Kawahara et al., 1989; Rabelo et al., 1994). Androgen regulates male vocalization but only after a TH-dependent stage (Robertson and Kelley, 1996). Underlying many of these changes is a massive loss of water. A tadpole loses more than half of its wet weight in the one-week period from NF59 (Nieuwkoop and Faber, 1956) to the completion of metamorphosis. This dramatic water loss has never been explained.
The origin and loss in evolution of an intermediate TH-dependent developmental stage in the vertebrate life cycle are problems of extraordinary interest. Many varieties of fish (Youson, 1988) undergo a TH-controlled larval to juvenile transition attesting to the ancient origins of metamorphosis. Just as different insect species exhibit variations in their life cycle ranging from direct development to complete metamorphosis so are there amphibian strategies ranging from direct developing frogs (Callery et al., 2001) and entirely pedomorphic salamanders (animals that are totally resistant to TH and breed as larva) (Wakahara, 1996) to anurans that metamorphose completely like Rana, Bufo, and Xenopus. TH is required to complete the life cycle of many higher vertebrates from birds to man, but this intervention occurs after hatching or birth. TH deficiency after birth in humans results in cretinism characterized by mental retardation, shortness of stature and many other abnormalities. Although TH plays a crucial role in the maturation of the newborn mammalian brain, only a few candidate genes have been identified (Thompson and Bottcher, 1997; Bernal, 2005). TH induces larger gene expression changes in tadpoles than it does in mammalian systems judging from published microarray experiments (Yen et al., 2003; Das et al., 2006). The thyroid receptors and their RXR partners are conserved from man to amphibians to fish (Bertrand et al., 2004). The same DNA binding site for the TR-RXR heterodimer has been identified in various vertebrates. Many of the coactivators and corepressors of nuclear receptors that have been discovered in mammalian systems have been conserved in amphibians and implicated in TH-induced metamorphosis (Furlow and Neff, 2006). However, to date the only physiological pathway that is known to be regulated by TH in both mammals and amphibians is the negative feedback loop between the thyroid gland and the anterior pituitary in which thyrotropin (TSH) stimulates the thyroid gland to synthesize TH and excess TH down-regulates TSH (Dodd and Dodd, 1976; Huang et al., 2001). Therefore even though the TH receptors, their cofactors, and their DNA recognition sites are the same, most of the physiological responses to TH are different in anurans compared to mammals. Are there many more genes under TH control in anurans compared to mammals to account for the much greater biological effect of the hormone on amphibians? A survey of thyroid hormone response elements (TRE) in the respective genomes will be instructive. Organogenesis in vertebrates ends up with similar adult structures. However, TH controls many of these developmental programs in amphibians.
The Demeneix laboratory (Morvan Dubois et al., 2006; Havis et al., 2006) has raised the possibility that sensitivity to TH and its receptors might be important in embryogenesis. The thyroid hormone receptors (Eliceiri and Brown, 1994; Havis et al., 2006) and various deiodinases (Brown, 2005; Morvan Dubois et al., 2006) are present in embryos. In addition Morvan Dubois et al. (2006) report a high level of TH in X. laevis eggs and embryos. The location of the deiodinases and thyroid receptors in embryos has led these workers to propose a role for thyroid hormone signaling in neural and eye development during embryogenesis. We have grown frogs in methimazole or added high doses of TH to frogs for months with no apparent effect. The sensitivity of tadpoles compared to frogs suggests a global inactivation of the TH sensitive state of the genome after metamorphosis.
Tools available for research in amphibian metamorphosis
The two most popular amphibians used to study metamorphosis have been X. laevis and the bullfrog Rana catesbeiana. For most of the 20th century the bullfrog was favored for biochemical studies because of the availability and large size of the mature tadpoles that can be purchased from supply houses. Bullfrogs remain as tadpoles for 3 years and then require additional years to reach sexual maturity. X. laevis has replaced the bullfrog because it is easy to breed and raise in the laboratory and has a shorter life cycle. Embryogenesis takes 1 week. The tadpole stage (from NF46 to 59) is the most variable period of the life cycle ranging from 6 weeks to much longer periods depending upon the husbandry. The climax of metamorphosis (NF59 to 66) is reproducible and lasts for 8±1 days (Fig. 1). Males and females are fertile in 6 months and a year, respectively.
Fig. 1. Schematic representation of some key events during X. laevis tadpole development.
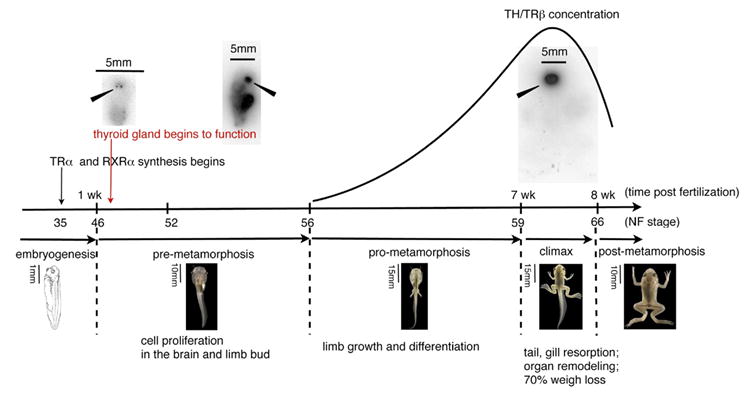
The time post fertilization is on the x axis. Above the line are “tadpole” blots of animals incubated for 24 h with Na 125I, fixed with formaldehyde, and washed to lower background (see Brown, 1997for the method). The tadpoles were dried on filter paper and filmed. X. laevis has 2 thyroid glands located on either side of the midline (solid arrow). The first incorporation of iodine into the thyroid gland occurs 10 days after fertilization (NF46). The solid line represents the concentrations of both TH and TRβ during development. TRβ is a direct response gene of TH.
TH added directly to tadpole rearing water induces metamorphic changes precociously. Pharmacological agents developed for the control of thyroid hormone synthesis and metabolism in mammals function identically in amphibians. One class of compounds inhibits iodination of thyroglobulin, the protein precursor of TH, in the thyroid gland. The two most useful chemicals in this class are methimazole (Cooper et al., 1984) and sodium perchlorate. X. laevis tadpoles grown in the presence of 1 mM methimazole for over a year will not advance in development beyond a stage equivalent to NF52 (Brown et al., 2005). These developmentally arrested tadpoles ultimately ossify their cartilaginous vertebrae and skull and form immature eggs and sperm, a developmental feature that normally happens many months after metamorphosis is completed. These tadpoles develop goiters just like humans testifying to the disruption of the negative feedback loop between the thyroid gland and the pituitary (Fig. 2). Iopanoic acid is a drug that inhibits the action of all classes of deiodinases (Galton, 1989). Tadpoles grown in the presence of iopanoic acid synthesize T4 but cannot destroy it or convert it into the more active T3. The high level of endogenous T4 induces development to climax, but the tadpoles arrest because the more active hormone T3 is needed to complete metamorphosis (Huang et al., 2001).
Fig. 2. The negative feedback loop between the thyroid gland and the anterior pituitary regulates the circulating levels of T4.
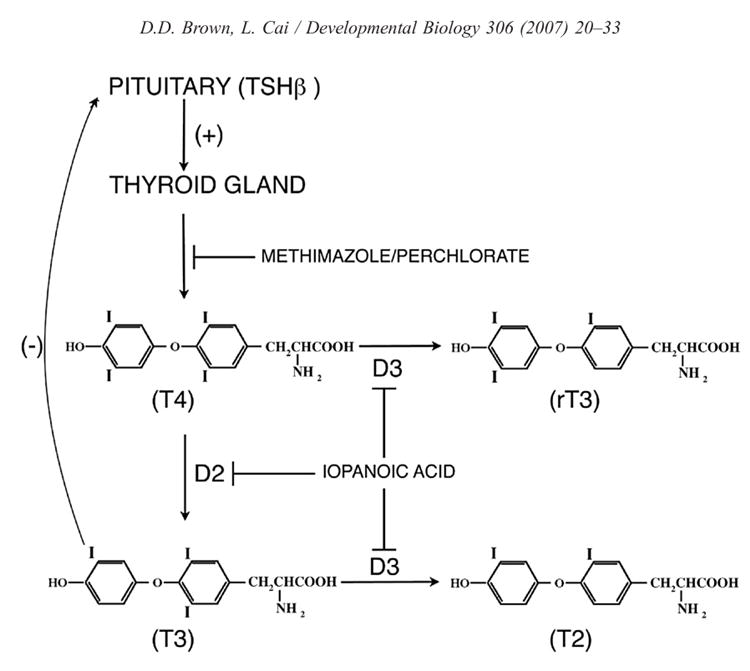
Throughout tadpole growth TSH is secreted by the anterior pituitary and stimulates the thyroid gland to synthesize increasing amounts of T4. However, TSH production is not shut down until D2 is up-regulated at metamorphic climax in the anterior pituitary (Huang et al., 2001) and converts local T4 to the active hormone T3. This figure shows where pharmacological inhibitors interfere with the formation or inactivation of thyroid hormone.
Recently Scanlan and his colleagues have synthesized the first TRβ specific agonist (GC-1) and antagonist (NH-3) (Lim et al., 2002). The agonist has helped to confirm that the latest event in metamorphosis, tail resorption, is mediated by TRβ (Furlow et al., 2004).
Several methods have been developed to alter gene expression in living X. laevis. Genes have been injected into tadpole tail muscle by intramuscular injection (de Luze et al., 1993) and into brain cells by injection into the ventricles (Haas et al., 2002). In both systems electroporation increases the number of transformed cells. These methods are equivalent to transient transfection assays since the genes are not integrated, but they reliably monitor and influence the developmental state of the cells. A co-injected plasmid that expresses GFP marks the transfected cells (Nakajima and Yaoita, 2003).
The Kroll and Amaya (1996) technique for transformation of X. laevis before first cleavage introduced the first genetic tool for amphibian research. Some of the modifications to transgenic procedures that have been pioneered in other organisms work in X. laevis. These include placing transgenes under the control of small molecules like tetracycline or RU486 (Das and Brown, 2004), control by the heat shock promoter (Marsh-Armstrong et al., 1999a), and use of the cre-lox system (Gargioli and Slack, 2004). Two new and efficient methods to produce Xenopus transgenics have been described. In both methods the DNA construct is injected directly into the fertilized egg avoiding the use of demembranated sperm that has been the most difficult barrier to success with the original Kroll Amaya procedure. One method that was developed first for Medaka (Thermes et al., 2002) co-injects a “meganuclease” (I-SceI) with a plasmid in which the transgene is flanked by the nuclease recognition sites (Ogino et al., 2006; Pan et al., 2006). The second technique co-injects the mRNA encoding a bacteriophage integrase (phiC31) with the transgene (Allen and Weeks, 2005). The transgene is bracketed by a chicken β globin insulator sequence and contains a 34 bp attachment site that will integrate into one of several related 39 bp sites in the genomic DNA. This method invariably results in just a single integration and the insulator sequences improve the specificity of the promoter. These new methods will enhance the efficiency of transgenesis especially with X. tropicalis that has a much smaller egg and is more difficult to transfect than X. laevis by the original sperm mediated method.
Transgenesis is suitable for over expression experiments. No traditional knockout or gene replacement experiments have been reported in amphibians. Transgenes are inherited through the germ line (Marsh-Armstrong et al., 1999a) as expected, and breeding colonies of mature transgenic X. laevis have been produced. The methods that have been so successful for studying X. laevis embryogenesis have little use for metamorphosis projects since the earliest affect on metamorphosis cannot be assayed until the second week after fertilization. The reliability at that late time of the action of a morpholino or mRNA that was injected into the fertilized egg has not been demonstrated. Naked cDNAs injected alone into the fertilized egg of X. laevis are expressed with a mosaic distribution, and there is no evidence that they are integrated.
While it is easy to establish cell lines from adult organs of X. laevis only one permanent cell line that responds to TH by undergoing a change in morphology has been reported. This muscle cell line was derived from tadpole tail and dies as a response to added TH (Yaoita and Nakajima, 1997). Other established X. laevis cell lines can be induced by TH to up-regulate the expression of response genes (Kanamori and Brown, 1993). However TH induction of these cell lines does not change their morphology. Nishikawa and Yoshizato (1986) prepared primary cultures of bullfrog tadpole epidermal cells that remained alive for 2 weeks. These cells died after several days of T3 treatment. The application of rapid screening methods such as RNAi will be needed to elucidate TH-induced pathways.
The first metamorphosis-related experiments have been reported for X. tropicalis (Rowe et al., 2002) the species for which there is high expectations for future genetic research. The life cycle of X. tropicalis is shorter than that of X. laevis, and there is every reason to believe that the details of metamorphosis will be the same in the two species justifying the use of the sequenced X. tropicalis genome for molecular analysis of experiments with X. laevis. Currently there are 28,000 and 30,000 Unigene entries for X. laevis and X. tropicalis, respectively, in the database. Microarray analyses of TH-induced gene expression changes in X. laevis with all of the X. laevis Unigene entries have been described (Das et al., 2006; Buchholz et al., 2007; Cai et al., 2007). An earlier set of experiments used 420 cDNAs collected from X. laevis and R. catesbeiana to study gene expression changes in the resorbing tail of X. laevis (Veldhoen et al., 2002; Helbing et al., 2003).
The roles of TH and the thyroid hormone receptors in metamorphosis
The research direction on the actions of TH changed in 1986 when the laboratories of Ronald Evans (Weinberger et al., 1986) and Bjorn Vennstrom (Sap et al., 1986) independently cloned the thyroid hormone receptors (TR) and identified them as members of the nuclear receptor transcription factor family. Until that time most investigators believed that TH acted by altering metabolism. Following the discovery that TRs are transcription factors, the focus of research changed to the influence of TH on gene expression. Two decades earlier Tata (1966) had anticipated a role for gene expression changes in TH-induced metamorphosis. He suppressed the response of tadpoles to TH with actinomycin D, an inhibitor of RNA synthesis. Nevertheless no gene relevant to amphibian metamorphosis was cloned until Brooks et al. (1989) cloned a cDNA for TRα from X. laevis. Since X. laevis is pseudotetraploid, there are two closely related copies of each TR isoform in the genome. The cDNAs of the isoforms of TRα and TRβ (Yaoita et al., 1990) were cloned and their genomic structure elucidated (Shi et al., 1992). Three forms of RXR (α, β, and γ), the known heterodimeric partner of TRs, have been cloned from X. laevis (Blumberg et al., 1992; Marklew et al., 1994). The isoforms of TR and RXR have been conserved evolutionarily throughout vertebrates from humans to fish (Escriva et al., 2004). Alternate splicing of TRβ results in additional forms of this receptor (Yaoita et al., 1990), but as yet no differential expression of a splice variant has been found (Kanamori and Brown, 1992).
Proof that the TRs control most if not all of the varied programs of metamorphosis comes from transgenic experiments in which a dominant negative form of the TR is expressed with a ubiquitous promoter (Schreiber et al., 2001; Buchholz et al., 2003). At least five TH-induced visible changes are inhibited in these animals: tail resorption, gill resorption, intestinal remodeling, limb bud growth, and DNA replication in the brain, nose, and limb buds. We conclude that TRs are required for these varied programs involved in metamorphosis. This same phenotype can be obtained in transgenics that over express the enzyme deiodinase 3 that inactivates TH. In these animals the TH cannot build up to a high enough level to affect the late metamorphic changes (Huang et al., 1999). Although the biochemical interaction of X. laevis RXR and TR with a typical TH response element has been demonstrated by mobility shift experiments (Ranjan et al., 1994; Wong and Shi, 1995; Furlow and Brown, 1999; Furlow and Kanamori, 2002), and the developmental expression pattern of at least RXRα is the same as TRα there has been no formal proof for a role of RXRs in metamorphosis. TR coactivators and corepressors have been cloned from X. laevis and implicated in metamorphosis (Furlow and Neff, 2006), and they are as conserved in sequence as the receptors themselves. TRs are located in the nucleus and chromatin immune precipitation (CHIP) experiments indicate that they are bound to their cognate TREs even in an unliganded state along with corepressors (Sachs et al., 2002; Buchholz et al., 2005). This observation is the basis of the “dual function” model (Wong and Shi, 1995). According to this hypothesis the unliganded receptor represses its cognate genes early in metamorphosis when TH concentration is low by binding corepressors. Buchholz et al. (2003) demonstrated by CHIP experiments that dominant negative receptors that block gene activation do not release a corepressor when TH is added. Over expression of a dominant negative form of the steroid receptor coactivator-3 (SRC3) (Paul et al., 2005) inhibits metamorphosis with a phenotype similar to that of the dominant negative TR. In a test of the importance of the unliganded TR, Havis et al. (2006) prepared transgenic X. laevis expressing a TR mutant with an impaired corepressor binding site. Although the resulting embryos died the investigators noted an inhibition of eye development. They postulate that the unliganded receptor is important for eye development during embryogenesis.
While most of the attention of researchers in the field of thyroid hormone action now concentrates on effects mediated through its receptors that influence gene expression there is a steady flow of papers that emphasize the “nongenomic” effects of TH (see Hiroi et al. 2006). In addition there is clear evidence of translational control of TRα receptor protein synthesis in the tail at metamorphic climax (Eliceiri and Brown, 1994). This latter effect may be mediated by a highly conserved sequence in the 5′ UTR of TRα that encodes a short open reading frame.
The developmental appearance of TH, its receptors, and the deiodinases and their role in the heterochronic response of metamorphosis
In X. laevis the expression of TRα begins at tail bud stage (NF35) (Eliceiri and Brown, 1994; Yaoita and Brown, 1990). A functional thyroid gland is detected 10 days post fertilization (NF46) (Fig. 1). Endogenous TH concentration was not quantified before NF56 until recently. TH synthesis increases as the tadpole grows reaching a peak at the climax of metamorphosis (NF60 to 63) (Leloup and Buscaglia, 1977; Regard et al., 1978). However, Morvan Dubois et al. (2006) report substantial levels of TH in eggs and embryos as measured by RIA. Confirmation by chemical identification that RIA reacting material is bona fide TH has not been carried out. As is the case in all vertebrates the less active precursor hormone thyroxine (T4) is released into the circulation (Huang et al., 2001). Conversion of T4 to the active form of the hormone occurs locally in cells that are expressing the outer ring deiodinase (D2) (Becker et al., 1997). The rising concentration of TH triggers a sequence of heterochronic developmental changes. At very low TH concentrations (NF52–55) there is extensive induction of DNA replication in the limb buds and the cells that line the ventricle of the brain (Schreiber et al., 2001). The limb and brain cells that are induced early to replicate have high constitutive levels of TRα, RXRα, and the enzyme D2 (Brown et al., 2005; Cai and Brown, 2004). The local conversion of T4 to the active hormone T3 catalyzed by D2 plays a role in the sensitivity of limb and brain to low levels of circulating TH (Becker et al., 1997; Cai and Brown, 2004). It requires high levels of TH to induce tail resorption, the last organ to change in metamorphosis. Tail cells have low concentrations of TR (Eliceiri and Brown, 1994) and no detectable D2 until climax (Cai and Brown, 2004). The high TH concentration that occurs at metamorphic climax up-regulates D2 in the late responding tissues and organs (tail, intestine, and anterior pituitary) (Cai and Brown, 2004). The maturation of an effective negative feedback loop requires the up-regulation of D2 in the anterior pituitary at climax (Huang et al., 2001). TRβ is itself a direct response gene of TH (Kanamori and Brown, 1992) and is at its highest levels in the tail at metamorphic climax (Yaoita and Brown, 1990; Kawahara et al., 1991; Eliceiri and Brown, 1994). These correlations have prompted the conclusion that TRα mediates the early events of metamorphosis that are mainly growth programs. When the TH concentration is high enough it induces TRβ, which then contributes to the late changes that involve death and remodeling.
The gene that encodes the enzyme deiodinase 3 (D3) also plays a role in the heterochrony of a cell’s response to TH. A dramatic example is the localized constitutive expression of D3 in the dorsal marginal zone of the tadpole retina (Marsh-Armstrong et al., 1999b). D3 inhibits the TH response in those cells that express the enzyme by destroying the hormone. The result is that only the ventral marginal cells respond to TH by dividing. The asymmetrical growth of the retina leads later in tadpole growth to the formation of ipsilateral projections to the optic tectum. Inhibition of D3 interferes with both asymmetric replication and the ipsilateral projections. A low constitutive expression of D3 throughout the growth of the tadpole tail helps to protect it against the ever-increasing endogenous TH levels (Wang and Brown, 1993). Finally at the climax of metamorphosis in the tail there is a sharp drop in D3 activity and a rapid rise in both D2 (Cai and Brown, 2004; Wang and Brown, 1993) and TRβ. All of these factors play a role in the timing of tail resorption.
The amounts of the thyroid receptors, the endogenous concentration of TH, and the localized activities of deiodinases that synthesize and inactivate TH play important roles in the timing of metamorphic events.
The programs of metamorphosis
Metamorphosis has been studied as a series of transcriptional programs controlled by TH. The first attempts to identify genes responsive to the hormone applied a subtractive hybridization method (Wang and Brown, 1991) to tail resorption (Wang and Brown, 1993), limb development (Buckbinder and Brown, 1992), intestinal remodeling (Shi and Brown, 1993), brain (Denver et al., 1997), and a cultured cell line (Kanamori and Brown, 1993). Control and TH-induced transcription levels were compared after various times of hormone treatment. The general features of TH induction include the kinetics of up-regulation and the specification of direct versus delayed gene expression using cycloheximide resistance. The tail resorption program consists of 2 waves of up-regulation (Wang and Brown, 1993). The direct response genes have a lag of about 2 to 4 h followed by an increase in mRNA for the next 12 h. Removal of the hormone inducer at 24 h results in a decrease of these mRNAs. These genes are direct response genes since their TH-induced up-regulation does not require protein synthesis. Table 1 lists some of the direct response genes that have been identified in various metamorphic programs. Another group of genes are induced in the second day after hormone addition. When cycloheximide is added to tails after 48 h of TH exposure, the tail will still resorb to some extent (Wang and Brown, 1993). The conclusion from this observation was that two waves of gene expression are sufficient to begin the process of tail resorption. Subsequent array studies applied to tails at climax (Das et al., 2006) found many more TH regulated genes than had been identified by subtractive hybridization. Terminally differentiated muscle and skin genes in the limb are not expressed for 4 to 5 days after the addition of TH indicating that multiple waves of gene expression are needed for limb growth (Buckbinder and Brown, 1992). The question remains. If most (all) of the varied developmental programs begin with the same required TR at what point do the programs diverge to account for their differences?
Table 1.
Some direct response genes of thyroid hormone
| Gene name | Method a | Tissue | Reference |
|---|---|---|---|
| Thyroid receptor beta | CHX, TRE | Multiple | Wang and Brown, 1993; Wong and Shi, 1995 |
| TH bZip | CHX, TRE | Tail fibroblast, intestine epithelium | Wang and Brown, 1993; Furlow and Brown, 1999 |
| BETB | CHX, TRE | Multiple | Wang and Brown, 1993; Furlow and Kanamori, 2002 |
| Deiodinase 3 | CHX | Multiple | Wang and Brown, 1993 |
| Stromelysin-3 | CHX | Tail fibroblast, intestine mesenchyme | Wang and Brown, 1993 |
| Gene 12 | CHX | Multiple | Wang and Brown, 1993 |
| MMP 9 TH | TRE | Tail fibroblast | Fujimoto et al., 2006 |
| SHH | CHX | Intestine epithelium | Stolow and Shi, 1995 |
| Notch | Kinetics | Brain ventricle cells | Das et al., 2006 |
| C/EBP delta-2 | Kinetics | Multiple | Das et al., 2006 |
| Enhancer of zeste | Kinetics | Multiple | Das et al., 2006 |
| c-myc II | Kinetics | Multiple | Das et al., 2006 |
CHX is cycloheximide resistant;
TRE, demonstrated thyroid response element in the promoter; kinetics.
The tail resorption program
TH-induced tadpole tail resorption was probably the earliest recognized example of hormone controlled programmed cell death. A series of experiments have concluded that the two major cell types in the tail, fibroblast and muscle, respond independently to TH. Muscle dies by apoptosis as a result of TH. Yaoita and Nakajima (1997) prepared a permanent cell line from tail muscle that dies as a response to TH. Tadpoles treated with mammalian prolactin (Dodd and Dodd, 1976) or transgenic tadpoles that over express prolactin do not resorb their tails at metamorphosis (Huang and Brown, 2000). The resistant tail has large fins and a notochord but it has lost its muscle. Muscle-specific expression of the dominant negative form of the TR protects the tail muscle from TH-induced cell death when the transgene is delivered by transgenesis (Das et al., 2002) or by direct injection into the tail muscle (Nakajima and Yaoita, 2003). The injection of an antiapoptotic gene, Bcl-Xl, into tail muscle protects muscle cells while the tail is resorbing (Rowe et al., 2002). A microarray analysis of tail resorption identified several cytoplasmic proteases that are up-regulated in tail muscle at climax in the same dying muscle cells that activate caspase-3 (Das et al., 2006). The tail fibroblasts in these tadpoles with protected muscle respond normally to the hormone. These experiments demonstrate that tail muscle is a cell autonomous target of TH. Tails contain four rows of muscle fibers called the “cords” (Elinson et al., 1999). These muscle fibers contract the tail as it shortens and persist until the very end of metamorphosis when the tail is practically dissolved. The cords do not die by cell autonomous death but are digested ultimately by the enzymes that degrade the bulk of the tail.
Gross and LaPiere (1962) identified the first collagenase activity in extracts of resorbing tadpole tails. In fact the important history of collagenases and related proteolytic enzymes dates from these discoveries on tail resorption (Gross, 2004). Fibroblasts in the growing tail especially those that surround the notochord actively synthesize various kinds of collagens (Das et al., 2006). TH induces these fibroblasts to down-regulate the synthesis of extra cellular matrix proteins and up-regulate the synthesis of many proteases that dissolve the structural component of the tail, the notochord, as well as the dorsal and ventral fins (Berry et al., 1998a). This change in gene expression occurs without DNA replication (Cai and Brown, unpublished data). Since the tail actually dissolves completely it is not surprising that gene expression screens have identified a wide variety of TH-induced proteolytic enzymes. TH-induced matrix metalloproteases (MMP) include stromelysin-3, collagenase-3 (Wang and Brown, 1993; Brown et al., 1996), gelatinase A (Jung et al., 2002), and collagenase 9 (Fujimoto et al., 2006). Intracellular hydrolases, serine proteases, and fibroblast activation factor are up-regulated by TH in tail fibroblasts (Berry et al., 1998a). Array analyses have augmented the list of TH-induced hydrolases including genes encoding a variety of lysosomal hydrolases (Das et al., 2006). These genes are expressed in fibroblasts. A synthetic inhibitor of MMPs inhibits tail resorption further confirming the importance of these proteases (Jung et al., 2002).
There is a sharp demarcation between the tail and the body (Berry et al., 1998b). For example, the larval skin genes are down-regulated throughout the tadpole, and the formation of adult skin is uniform throughout the body, but this transition only occurs in patches in the resorbing tail. The presumption is that no dermis forms under most of the tail epidermis. In situ hybridization of various genes demonstrates the sharp change in expression at the border of the tail and the body.
TH stimulates DNA replication in the early brain and limb growth programs
The limb axes are determined during embryogenesis (Cameron and Fallon, 1977). In the absence of TH a limb bud will not develop beyond an oblong structure (NF52) that has no terminally differentiated muscle or cartilage (Brown et al., 2005). The earliest TH-induced change in the limb and brain is a stimulation of DNA replication (Schreiber et al., 2001; Schlosser et al., 2002; Cai and Brown, 2004). TH induces all cell types in the limb bud and cells lining the brain ventricles to divide during premetamorphosis. Then TH induces limb growth followed by differentiation of the major cell types. Newly formed spinal cord cells give rise to the lumbar and cervical motor neurons. These cells reach a peak in number at about NF52 and then decrease to about 20% of their peak value by NF56 (Hughes, 1961). The early rise in motor neuron number requires TH (Marsh-Armstrong et al., 2004). As in all vertebrates the excess motor neurons that do not innervate their peripheral targets are lost, but TH does not induce this drop in the number of motor neurons.
The kinetics of TH-induced DNA replication is the same in the limb and brain. A microarray comparison confirmed that the same set of cell cycle genes, encoding proteins involved in all stages of the cell cycle, are up-regulated by TH in both tissues (Das et al., 2006). The cell cycle genes that are most dramatically up-regulated include 4 of the 5 genes that encode the minichromosome maintenance (MCM) complex. The TH-induced incorporation of Brdu occurs within the second 24 h but requires protein synthesis. A number of TH direct response genes encoding transcription factors that might be candidates to activate the cell cycle pathway have been identified by microarray studies of the limb and brain (Das et al., 2006).
Limb development
In X. laevis tadpoles the limb grows and specific cell types are formed through the period of pre- (NF52–56) and pro-(NF57–58) metamorphosis. By the onset of the climax of metamorphosis (NF59) hind limb development is complete and the front limbs become external. From this time to the completion of tail resorption (NF66) takes about 8 days (Fig. 1). The animal switches from tail to leg swimming at NF60 as the gills are beginning to resorb. In contrast to the importance of cell–cell interactions that determine the limb axes (Cameron and Fallon, 1977), the differentiated limb cell types develop in a cell autonomous fashion (Brown et al., 2005). Tadpoles that are transgenic for a dominant negative form of the receptor (TRDN) have been prepared using four different promoters (Fig. 3). Tadpoles transgenic for the TRDN transgene driven by a neural specific promoter (neuro-beta tubulin) develop normal appearing limbs in all respects except they are paralyzed (Fig. 3B) (Marsh-Armstrong et al., 2004). Tadpoles transgenic for the TRDN driven by a collagen promoter develop stunted limbs (Fig. 3C) that nonetheless can function (Brown et al., 2005). The change from tadpole to adult skin occurs in limbs at climax after the limb has completely formed. This is the same stage that the epidermis changes over the rest of the body. A constitutive promoter (CMV) that is expressed in all cell types of the limb inhibits TH-induced DNA replication (Schreiber et al., 2001) in all of the cell types. A late muscle promoter (“cardiac actin”) that is expressed in all kinds of muscle inhibits formation of limb muscle (Fig. 3D) (Das et al., 2002). These transgenic limbs grow and differentiate to climax but lack muscle. Their bone/cartilage development is normal. Therefore the transformation of the skin, the development of the long bones, and both the formation and the innervation of limb muscle have separate cell autonomous TH-controlled programs.
Fig. 3. Limb phenotypes at climax (NF62).

(A) Control. A dominant negative form of the thyroid receptor is expressed in (B) neural tissue; (C) collagen and skeletal structures; (D) muscle. This figure was taken from Brown et al. (2005). Scale bar: 2 mm.
When the TRDN transgene driven by the muscle promoter is placed under the control of the tetracycline system, the transgene can be induced at any stage of limb development. If muscle fibers have begun to form in the limb (NF55 or later) within 2 days after induction of the TRDN transgene the muscle fibers begin to degenerate. A microarray analysis of this phenotype has identified candidate muscle genes whose down-regulation might account for the degeneration phenotype (Cai et al., 2007). Most of these candidate muscle genes are also down-regulated in the tail muscle at climax. TH up-regulates these same genes in control limb muscle. We have concluded that TR is bound to the same set of muscle gene promoters in the limb and tail muscle. Their opposite regulatory response to TH might be caused by differences in chromatin structure or cofactor mobilization.
Muscle remodeling
The fate of tadpole muscle and the formation of adult muscle have been studied in some detail over the past decade. There are regional differences in muscle-specific gene expression patterns (Nishikawa and Hayashi, 1994; Gaillard et al., 1999), including the myogenic regulatory factors (Nicolas et al., 1998). Tail muscle is not the only muscle that is destined to die as a response to TH. Nishikawa and Hayashi (1994) identified tadpole and frog-specific isoforms of myosin heavy chain and β-tropomyosin and concluded that the change involved death of tadpole fibers and proliferation and growth of adult-type myoblasts. Shizu-Nishikawa et al. (2000) found separate progenitor larval and adult myoblasts in trunk muscle. Tail muscle only contains larval muscle. Mixed heterokaryon myotubes containing the two kinds of cells died or lived depending upon the proportions of the larval and adult nuclei in the syncytium. This switch in muscle programs resembles closely that observed by expressing the dominant negative TR in muscle (Cai et al., 2007).
Skin remodeling
Of all the tissues that remodel at metamorphosis the most extreme changes in gene expression occur in the skin (Yoshizato, 1992). The tadpole skin is very similar to mammalian fetal skin (Holbrook, 1983). It consists of three cell layers that all replicate with a thin collagen lamella separating it from the underlying muscle (Fig. 4). The outer “apical” layer is analogous to the mammalian “periderm”. Subepithelial fibroblasts line the collagen layer. There is no dermis. TH induces this skin to die cell autonomously (Schreiber and Brown, 2003) and the basal cells to form a typical germinative epithelium that is characteristic of adult vertebrate skin. A novel set of genes that are expressed only in the apical cells of the tadpole epidermis are down-regulated by TH at metamorphosis (Furlow et al., 1997). The genes encoding keratins change at metamorphosis (Mathisen and Miller, 1987; Miyatani et al., 1986; Suzuki et al., 2001; Tazawa et al., 2006; Watanabe et al., 2001). The more complex frog skin has an underlying dermis and two kinds of skin glands that appear for the first time at climax. These glands each secrete multiple unique products (Bevins and Zasloff, 1990). The X. laevis cDNA libraries and microarray analyses reveal many genes that are expressed in one or the other of these skin glands.
Fig. 4. Skin remodeling.

The tadpole epidermis (upper) has 3 cell layers covering a collagen lamella and contains no glands. The cells in all three layers replicate. At climax (middle) a dermis forms, and two different kinds of skin glands appear for the first time. The skin becomes a germinative epithelium with replication only in the basal cell layer.
Intestine remodeling
One of the most dramatic changes that occur at the climax of metamorphosis is the remodeling of the intestine. The tadpole intestine is a simple long coiled tube with one involution at its proximal end called the typhlosole. It is lined with a single endothelial cell layer surrounded by a sparse mesenchyme, a single inner radial, and an outer longitudinal muscle cell layer. Within 5 days at climax the intestinal tract shortens about 75% along its entire length and forms a typical vertebrate stomach and small intestine (McAvoy and Dixon, 1978; Ishizuya-Oka and Shi, 2005) (Fig. 5). The shortening happens rapidly in several days and heaps the epithelium into a thick temporary multi cellular lining. Although the epithelium is continually replicating throughout tadpole growth, the level of DNA synthesis is enhanced greatly at climax when remodeling occurs even as the innermost epithelial cells undergo apoptosis. DNA synthesis occurs throughout the epithelium as it remodels. Even when the crypts and villi have been formed at the end of metamorphosis DNA replication continues throughout the epithelium. Months after metamorphosis is complete DNA replication becomes restricted to the epithelial crypts (Schreiber et al., 2005). The nature of this remodeling is disputed. One group of workers believes that the adult epithelium differentiates from a select population of adult progenitor cells that can be identified before climax (Ishizuya-Oka et al., 2003). We concluded from our experiments that the functional tadpole epithelial cells are the progenitors of the functional adult epithelium (Schreiber et al., 2005). Subtractive hybridization identified genes that are regulated by TH (Shi and Brown, 1993). Down-regulation of specialized genes occurs at the climax of metamorphosis in the epithelium of the intestine (Shi and Hayes, 1994; Ishizuya-Oka et al., 1997) and the stomach (Ikuzawa et al., 2004). At the end of metamorphosis, these genes are expressed again.
Fig. 5. Intestine remodeling.

Left, before climax (NF58); right, froglet (NF66). Below each picture is an H&E cross section of the anterior intestine (red line on upper pictures). In the 8 days of climax the intestine shrinks 75% in length and undergoes changes from a simple tube with one involution to the crypts and villi of a typical vertebrate intestine. Throughout metamorphosis there is extensive DNA replication of all of the endothelial cells. Even at the end of metamorphosis (lower right) all of the endothelial cells are replicating (Schreiber et al., 2005). Several months later replication becomes specific to the epithelial crypts. The stomach differentiates during climax. We are grateful to Alex Schreiber for these pictures.
Cell–cell interaction plays a role in intestinal remodeling. By dissociating and recombining the intestinal epithelium and mesenchyme in organ culture, Ishizuya-Oka and Shimozawa (1994) concluded that TH induces a change in the mesenchyme that controls the transformation of the epithelium. We have extended this observation recently by demonstrating that a dominant negative form of the TR expressed exclusively in the epithelium also inhibits metamorphic changes in the intestinal mesenchyme and the muscle layers (Schreiber and Brown, unpublished data). Whereas TRDN expressed in the epithelium inhibits the changes that are seen in a cross section of the small intestine, it does not inhibit intestinal shortening. The genes encoding sonic hedgehog and bone morphogenetic protein have been implicated in these TH-induced changes (Ishizuya-Oka et al., 2006) just as they are in the formation of the intestine during embryogenesis. Therefore remodeling may reutilize important genes that were involved in forming the organ during embryogenesis, but in the tadpole the genes come under the control of TH. Many of the same genes are induced by TH in the tail and intestine (Buchholz et al., 2006) suggesting that the remodeling and resorption programs share gene pathways.
Pancreas remodeling
The specialized exocrine genes of the pancreas are expressed at very high levels in tadpoles. At metamorphic climax the pancreas shrinks in size and changes its shape so that at the end of metamorphosis it forms the typical elongated shape adjacent to the duodenum that characterizes other vertebrate pancreases (Leone et al., 1976). As it changes shape, the exocrine cells down-regulate the terminally differentiated genes of the exocrine pancreas (Leone et al., 1976; Shi and Brown, 1990). Similar to the intestinal epithelium this “extinction” is reversed at the end of metamorphosis and the same genes are expressed again. Exogenous TH induces the down-regulation of these genes precociously. While the expression of exocrine products is changing, the synthesis of endocrine specific gene products remains constant (Maake et al., 1998). However the structure of the insulin-producing cells is remodeled. The beta cells that synthesize insulin are mainly single or small clusters of cells in tadpoles. At the end of metamorphosis, insulin is produced by aggregates of cells that more closely resemble islets. The endocrine cells that produce somatostatin and glucagon remain mainly as single cells that become more tightly clustered as the pancreas decreases in size at climax.
Liver remodeling
The liver is remodeled at metamorphosis. Paik and Cohen (1960) recognized that when Rana tadpoles metamorphose to land dwelling frogs they change from NH3 to urea excretion. The liver is the site of the urea cycle, and its enzymes are up-regulated at metamorphosis in Rana species and to a lesser extent Xenopus. Atkinson et al. (1996) showed that these genes are regulated at the transcriptional level. Albumin synthesis is up-regulated in the liver late in tadpole life (Moskaitis et al., 1989) as it is at the end of fetal life in mammalian liver. Exogenous TH induces these changes in liver parenchymal cells.
Brain remodeling
TH controls DNA replication in the brain ventricular cells as the tadpole grows. By the climax of metamorphosis DNA replication stops and extensive differentiation and remodeling take place. Very little is known about the molecular basis of these changes although morphological changes have been documented. TH-induced cell death of specific neurons such as the Rohon-Beard or Mauthner neurons has been demonstrated to occur by an apoptotic pathway (Coen et al., 2001). The most comprehensive account of changes in the brain at metamorphosis remains the morphological description by Nieuwkoop and Faber (1956).
Erythrocyte remodeling
As is the case with many other vertebrates frogs express different globin genes in their red cells at different stages of their life cycle (Weber, 1996). The unique feature of metamorphosing amphibians and other vertebrates is that TH induces tadpole red cells to undergo apoptosis and adult progenitor cells to differentiate (Nishikawa and Hayashi, 1999). The site of synthesis of both kinds of red cells in the tadpole is the liver (Maniatis and Ingram, 1971). The synthesis of erythrocytes shifts to the bone marrow after metamorphosis. An unanswered cell biological question remains. Does the same progenitor cell differentiate into a tadpole erythrocyte when the endogenous TH is low and then change its fate in the presence of TH to produce an adult erythrocyte or are there separate progenitor cells? One group of researchers claim that frog and tadpole globin mRNAs are never found in the same cell (Maniatis and Ingram, 1971; Weber, 1996) while others claim to have found both mRNAs in the same cell (Benbassat, 1974; Jurd and Maclean, 1970; Widmer et al., 1983). Our own in situ hybridization experiments (Cai and Brown, unpublished) have never observed tadpole and frog globin mRNAs in the same cell. Regardless, TH must have at least two separate effects on the erythrocyte program at metamorphosis. It stimulates apoptosis of tadpole red cells and the differentiation of adult (frog) erythrocyte progenitor cells.
Remodeling the immune system
Skin transplants from X. laevis tadpoles even to syngeneic frogs are rejected (Izutsu and Yoshizato, 1993). Some but not all of the immunological memory of tadpole proteins is lost at metamorphosis because the spleen and thymus lose most of their B and T cells (Du Pasquier et al., 2000). The major types of heavy and light chains are expressed in tadpoles and the VDJ rearrangements take place even during embryogenesis (Du Pasquier et al., 2000), but the diversity of humoral antibodies generated by tadpoles to an antigenic stimulus is less complex than that of a frog. Tadpole antibodies have lower affinity to their cognate antigen (Hsu and Du Pasquier, 1992) than the frog ones. This increase in complexity at metamorphosis has been attributed to the appearance at climax of the enzyme terminal deoxynucleotidyl transferase (Lee and Hsu, 1994) that generates diversity in the “N” region of frog antibodies. This junctional diversity also is absent in fetal mice (Benedict and Kearney, 1999). There are major changes in expression of genes that encode the major histocompatibility complex at metamorphosis (Du Pasquier and Flajnik, 1990).
What is needed
About 25 years ago genetics and molecular biology came to the rescue of a third world science called embryology. What will encourage the modern generation of scientists to study the wonderful biological problems presented by amphibian metamorphosis? There is no problem identifying differentially regulated genes. However, placing them into correct TH-induced pathways will require the development of rapid functional assays. This review has tried to point out how worthwhile that effort will be.
Acknowledgments
The National Institutes of Health and the G. Harold and Leila Y. Mathers Charitable Trust support our research. We thank Sandeep Mukhi for critically reading the manuscript and Rejeanne Juste for expert technical assistance.
References
- Allen BM. The effects of extirpation of the thyroid and pituitary glands upon the limb development of anurans. J Exp Zool. 1925;42:13–30. [Google Scholar]
- Allen BG, Weeks DL. Transgenic Xenopus laevis embryos can be generated using phiC31 integrase. Nat Methods. 2005;2:975–979. doi: 10.1038/nmeth814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson BG, Helbing C, Chen Y. Reprogramming of genes expressed in amphibian liver during metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis. Academic Press; New York: 1996. pp. 539–566. [Google Scholar]
- Atkinson BG, Warkman AS, Chen Y. Thyroid hormone induces a reprogramming of gene expression in the liver of premetamorphic Rana catesbeiana tadpoles. Wound Repair Regen. 1998;6:323–337. doi: 10.1046/j.1524-475x.1998.60408.x. [DOI] [PubMed] [Google Scholar]
- Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA. The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeiana tadpoles. Endocrinology. 1997;138:2989–2997. doi: 10.1210/endo.138.7.5272. [DOI] [PubMed] [Google Scholar]
- Benbassat J. The transition from tadpole to frog hemoglobin during natural amphibian metamorphosis. J Cell Sci. 1974;15:347–357. doi: 10.1242/jcs.15.2.347. [DOI] [PubMed] [Google Scholar]
- Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122. doi: 10.1016/S0083-6729(05)71004-9. [DOI] [PubMed] [Google Scholar]
- Berry D, Scwartzman R, Brown DD. The expression pattern of thyroid hormone response genes in the tadpole tail identifies multiple resorption programs. Dev Biol. 1998a;203:12–23. doi: 10.1006/dbio.1998.8974. [DOI] [PubMed] [Google Scholar]
- Berry D, Rose C, Remo B, Brown DD. The expression pattern of thyroid hormone genes in remodeling tadpole tissue defines distinct growth and resorption gene expression programs. Dev Biol. 1998b;203:24–35. doi: 10.1006/dbio.1998.8975. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Brunet FG, Escriva H, Parmentier G, Laudet V, Robinson-Rechavi M. Evolutionary genomics of nuclear receptors: from twenty-five ancestral genes to derived endocrine systems. Mol Biol Evol. 2004;21:1923–1937. doi: 10.1093/molbev/msh200. [DOI] [PubMed] [Google Scholar]
- Bevins CL, Zasloff M. Peptides from frog skin. Ann Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Mangelsdorf DJ, Dyck JA, Bittner DA, Evans RM, De Robertis EM. Multiple retinoid-responsive receptors in a single cell: families of retinoid “X” receptors and retinoic acid receptors in the Xenopus egg. Proc Natl Acad Sci U S A. 1992;89:2321–2325. doi: 10.1073/pnas.89.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AR, Sweeney G, Old RW. Structure and functional expression of a cloned Xenopus thyroid hormone receptor. Nucleic Acids Res. 1989;17:9395–9405. doi: 10.1093/nar/17.22.9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci U S A. 1997;94:13011–13016. doi: 10.1073/pnas.94.24.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD. The role of deiodinases in amphibian metamorphosis. Thyroid. 2005;15:815–821. doi: 10.1089/thy.2005.15.815. [DOI] [PubMed] [Google Scholar]
- Brown DD, Wang Z, Furlow JD, Kanamori A, Schwartzman RA, Remo BF, Pinder A. The thyroid hormone-induced tail resorption program during Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 1996;93:1924–1929. doi: 10.1073/pnas.93.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Cai L, Das B, Marsh-Armstrong N, Schreiber AM, Juste R. Thyroid hormone controls multiple independent programs required for limb development in Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2005;102:12455–12458. doi: 10.1073/pnas.0505989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Hsia SC, Fu L, Shi Y-B. A dominant-negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol. 2003;23:6750–6758. doi: 10.1128/MCB.23.19.6750-6758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Shi Y-B. Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. Implications for developmental gene regulation. J Biol Chem. 2005;280:41222–41228. doi: 10.1074/jbc.M509593200. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi Y-B. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol. 2006;145:1–19. doi: 10.1016/j.ygcen.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol. 2007;303:576–590. doi: 10.1016/j.ydbio.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Buckbinder L, Brown DD. Thyroid hormone-induced gene expression changes in the developing frog limb. J Biol Chem. 1992;267:25786–25791. [PubMed] [Google Scholar]
- Buckbinder L, Brown DD. Cloning and developmental expression of Xenopus laevis prolactin and thyrotropin genes. Proc Natl Acad Sci U S A. 1993;90:3820–3824. doi: 10.1073/pnas.90.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Brown DD. Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol. 2004;266:87–95. doi: 10.1016/j.ydbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cai L, Das B, Brown DD. Changing a limb muscle growth program into a resorption program. Dev Biol. 2007;304:260–271. doi: 10.1016/j.ydbio.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callery EM, Fang H, Elinson RP. Frogs without polliwogs: evolution of anuran direct development. BioEssays. 2001;23:233–241. doi: 10.1002/1521-1878(200103)23:3<233::AID-BIES1033>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Cameron JA, Fallon JF. Evidence for polarizing zone in the limb buds of Xenopus laevis. Dev Biol. 1977;55:320–330. doi: 10.1016/0012-1606(77)90175-0. [DOI] [PubMed] [Google Scholar]
- Coen L, du Pasquier D, Le Mevel S, Brown S, Tata J, Mazabraud A, Demeneix BA. Xenopus Bcl-X(L) selectively protects Rohon-Beard neurons from metamorphic degeneration. Proc Natl Acad Sci U S A. 2001;98:7869–7874. doi: 10.1073/pnas.141226798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DS, Kiefer JD, Saxe V, Mover H, Maloof F, Ridgway EC. Methimazole pharmacology in man: studies using a newly developed radioimmunoassay for methimazole. Endocrinology. 1984;114:786–793. doi: 10.1210/endo-114-3-786. [DOI] [PubMed] [Google Scholar]
- Das B, Brown DD. Controlling transgene expression to study Xe-nopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2004;101:4839–4842. doi: 10.1073/pnas.0401011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Schreiber AM, Huang H, Brown DD. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc Natl Acad Sci U S A. 2002;99:12230–12235. doi: 10.1073/pnas.182430599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Cai L, Carter MG, Piao Y-L, Sharov AA, Ko MSH, Brown DD. Gene expression changes at metamorphosis induced by thyroid hormone in Xenopus laevis tadpoles. Dev Biol. 2006;291:342–355. doi: 10.1016/j.ydbio.2005.12.032. [DOI] [PubMed] [Google Scholar]
- de Luze A, Sachs L, Demeneix B. Thyroid hormone-dependent transcriptional regulation of exogenous genes transferred into Xenopus tadpole muscle in vivo. Proc Natl Acad Sci U S A. 1993;90:7322–7326. doi: 10.1073/pnas.90.15.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver RJ, Pavgi S, Shi Y-B. Thyroid hormone-dependent gene expression program for Xenopus neural development. J Biol Chem. 1997;272:8179–8188. doi: 10.1074/jbc.272.13.8179. [DOI] [PubMed] [Google Scholar]
- Dodd MHI, Dodd JM. The biology of metamorphosis. In: Lofts B, editor. Physiology of the Amphibia. Vol. 3. Academic Press; New York: 1976. pp. 467–599. [Google Scholar]
- Du Pasquier L, Flajnik M. Expression of MHC Class II antigens during Xenopus development. Dev Immunol. 1990;1:85–95. doi: 10.1155/1990/67913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L, Robert J, Courtet M, Mussmann R. B-cell development in the amphibian Xenopus. Immunol Rev. 2000;175:201–213. doi: 10.1111/j.1600-065x.2000.imr017501.x. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Brown DD. Quantitation of endogenous thyroid hormone receptors α and β during embryogenesis and metamorphosis in Xenopus laevis. J Biol Chem. 1994;269:24459–24465. [PubMed] [Google Scholar]
- Elinson RP, Remo B, Brown DD. Novel structural elements during tail resorption in Xenopus metamorphosis: lessons from tailed frogs. Dev Biol. 1999;215:243–252. doi: 10.1006/dbio.1999.9481. [DOI] [PubMed] [Google Scholar]
- Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- Fox H. Tissue degeneration: an electron microscope study of thepronephros of Rana temporaria. J Embryol Exp Morphol. 1970;24:139–157. [PubMed] [Google Scholar]
- Fujimoto K, Nakajima K, Yaoita Y. One of the duplicated matrix metalloproteinase-9 genes is expressed in regressing tail during anuran metamorphosis. Dev Growth Differ. 2006;48:223–241. doi: 10.1111/j.1440-169X.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Brown DD. In vivo and in vitro analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol Endocrinol. 1999;13:2076–2089. doi: 10.1210/mend.13.12.0383. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Kanamori A. The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinology. 2002;143:3295–3305. doi: 10.1210/en.2002-220126. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Neff ES. A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis. Trends Endocrinol Metab. 2006;17:40–47. doi: 10.1016/j.tem.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Berry DL, Wang Z, Brown DD. A set of novel tadpole specific genes expressed only in the epidermis are down-regulated by thyroid hormone during Xenopus laevis metamorphosis. Dev Biol. 1997;182:284–298. doi: 10.1006/dbio.1996.8478. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Yang HY, Hsu M, Lim W, Ermio DJ, Chiellini G, Scanlan TS. Induction of larval tissue resorption in Xenopus laevis tadpoles by the thyroid hormone receptor agonist GC-1. J Biol Chem. 2004;279:26555–26562. doi: 10.1074/jbc.M402847200. [DOI] [PubMed] [Google Scholar]
- Galton V. The role of 3,5,3′-triiodothyronine in the physiological action of thyroxine in the premetamorphic tadpole. Endocrinology. 1989;124:2427–2433. doi: 10.1210/endo-124-5-2427. [DOI] [PubMed] [Google Scholar]
- Gargioli C, Slack JMW. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- Gaillard C, Levivray H, Theze N, Cooper B, Lepetit D, Mohun T, Thiebaud P. Differential expression of two skeletal muscle beta-tropomyosin mRNAs during Xenopus laevis development. Int J Dev Biol. 1999;43:175–178. [PubMed] [Google Scholar]
- Gross J. How tadpoles lose their tails: pathto discovery of the first matrix metalloproteinase. Matrix Biol. 2004;23:3–13. doi: 10.1016/j.matbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Gross J, LaPiere CM. Collagenolytic an amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Pitt-Rivers R. The identification of 3:5:3′-l-triiodothyronine in human plasma. Lancet. 1952;1:439–441. doi: 10.1016/s0140-6736(52)91952-1. [DOI] [PubMed] [Google Scholar]
- Gudernatsch JF. Feeding experiments on tadpoles: I. The influence of specific organs given as food on growth and differentiation. A contribution to the knowledge of organs with internal secretion. Wilhelm Roux Arch Entwicklungsmech Organismen. 1912;35:457–483. [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo—From single cells to the entire brain. Differentiation. 2002;70:148–154. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- Harington CR. Chemistry of thyroxine: isolation of thyroxine from the thyroid gland. Biochem J. 1926;20:293–299. doi: 10.1042/bj0200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havis E, Le Mevel S, Morvan Dubois G, Demeneix BA, Sachs LM. Unliganded thyroid hormone receptor is essential for Xenopus laevis eye development. EMBO J. 2006;25:4943–4951. doi: 10.1038/sj.emboj.7601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbing CC, Werry K, Crump D, Domanski D, Veldhoen N, Bailey CM. Expression profiles of novel thyroid hormone-responsive genes and proteins in the tail of Xenopus laevis tadpoles undergoing precocious metamorphosis. Mol Endocrinol. 2003;17:1395–1409. doi: 10.1210/me.2002-0274. [DOI] [PubMed] [Google Scholar]
- Higgs DM, Burd GD. Neuronal turnover in the Xenopus laevis olfactory epithelium during metamorphosis. J Comp Neurol. 2001;433:124–130. doi: 10.1002/cne.1130. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Liao SY. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:14104–14149. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook KA. In: Structure and function of the developing human skin. Goldsmith LA, editor. Oxford Univ. Press; Oxford: 1983. pp. 64–101. [Google Scholar]
- Hoskins SG. Control of the development of the ipsilateral retinothalamic projection in Xenopus laevis by thyroxine: results and speculation. J Neurobiol. 1986;17:203–229. doi: 10.1002/neu.480170306. [DOI] [PubMed] [Google Scholar]
- Hsu E, Du Pasquier L. Changes in the amphibian antibody repertoire are correlated with metamorphosis and not with age and size. Dev Immunol. 1992;2:1–6. doi: 10.1155/1992/28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Brown DD. Prolactin is not a juvenile hormone in Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2000;97:195–199. doi: 10.1073/pnas.97.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Marsh-Armstrong N, Brown DD. Metamorphosis is inhibited in transgenic Xenopus laevis tadpoles that over-express type III deiodinase. Proc Natl Acad Sci U S A. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Cai L, Remo B, Brown DD. Timing of metamorphosis and the onset of the negative feedback loop between the thyroid gland and the pituitary is controlled by type II iodothyronine deiodinase in Xenopus laevis. Proc Natl Acad Sci U S A. 2001;98:7348–7353. doi: 10.1073/pnas.131198998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. Cell degeneration in the larval ventral horn of Xenopus laevis (Daudin) J Embryol Exp Morphol. 1961;9:269–284. [PubMed] [Google Scholar]
- Ikuzawa M, Yasumasu S, Kobayashi K, Inokuchi T, Iuchi I. Stomach remodeling-associated changes of H+/K+-ATPase beta subunit expression in Xenopus laevis and H+/K+-ATPase-dependent acid secretion in tadpole stomach. J Exp Zool A Comp Exp Biol. 2004;301:992–1002. doi: 10.1002/jez.a.127. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shi Y-B. Molecular mechanisms for thyroid hormone-induced remodeling in the amphibian digestive tract: a model for studying organ regeneration. Dev Growth Differ. 2005;47:601–607. doi: 10.1111/j.1440-169X.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Inductive action of epithelium on differentiation of intestinal connective tissue of Xenopus laevis tadpoles during metamorphosis in vitro. Cell Tissue Res. 1994;277:427–436. doi: 10.1007/BF00300215. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Stolow MA, Ueda S, Shi Y-B. Temporal and spatial expression of an intestinal Na1/PO432 cotransporter correlates with epithelial transformation during thyroid hormone-dependent frog metamorphosis. Dev Genet. 1997;20:53–66. doi: 10.1002/(SICI)1520-6408(1997)20:1<53::AID-DVG7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimizu K, Sakakibara S, Okano H, Ueda S. Thyroid hormone-upregulated expression of Musashi-1 is specific for progenitor cells of the adult epithelium during amphibian gastrointestinal remodeling. J Cell Sci. 2003;116:3157–3164. doi: 10.1242/jcs.00616. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Hasebe T, Shimizu K, Suzuki K, Ueda S. Shh/ BMP-4 signaling pathway is essential for intestinal epithelial development during Xenopus larval-to-adult remodeling. Dev Dyn. 2006;235:3240–3249. doi: 10.1002/dvdy.20969. [DOI] [PubMed] [Google Scholar]
- Izutsu Y, Yoshizato K. Metamorphosis-dependent recognition of larval skin as non-self by inbred adult frogs (Xenopus laevis) J Exp Zool. 1993;266:163–167. doi: 10.1002/jez.1402660211. [DOI] [PubMed] [Google Scholar]
- Jung JC, Leco KJ, Edwards DR, Fini ME. Matrix metalloproteinases mediate the dismantling of mesenchymal structures in the tadpole tail during thyroid hormone-induced tail resorption. Dev Dyn. 2002;223:402–413. doi: 10.1002/dvdy.10069. [DOI] [PubMed] [Google Scholar]
- Jurd RD, Maclean N. An immunofluorescent study of the haemoglobins in metamorphosing Xenopus laevis. J Embryol Exp Morphol. 1970;23:299–309. [PubMed] [Google Scholar]
- Kanamori A, Brown DD. The regulation of thyroid hormone receptor b genes by thyroid hormone in Xenopus laevis. J Biol Chem. 1992;267:739–745. [PubMed] [Google Scholar]
- Kanamori A, Brown DD. Cultured cells as a model for amphibian metamorphosis. Proc Natl Acad Sci U S A. 1993;90:6013–6017. doi: 10.1073/pnas.90.13.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Kohara S, Amano M. Thyroid hormone directly induces hepatocyte competence for estrogen-dependent vitellogenin synthesis during the metamorphosis of Xenopus laevis. Dev Biol. 1989;132:73–80. doi: 10.1016/0012-1606(89)90206-6. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Baker BS, Tata JR. Developmental and regional expression of thyroid hormone receptor genes during Xenopus metamorphosis. Development. 1991;112:933–943. doi: 10.1242/dev.112.4.933. [DOI] [PubMed] [Google Scholar]
- Kikuyama S, Kawamura K, Tanaka S, Yamamoto K. Aspects of amphibian metamorphosis: hormonal control. Int Rev Cytol. 1993;145:105–148. doi: 10.1016/s0074-7696(08)60426-x. [DOI] [PubMed] [Google Scholar]
- Kollros JJ. Transitions in the nervous system during amphibian metamorphosis. In: Gilbert LI, Frieden E, editors. Metamorphosis. 2. Plenum Press; New York: 1981. pp. 445–459. [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Lee A, Hsu E. Isolation and characterization of the Xenopus terminal deoxynucleotidyl transferase. J Immunol. 1994;152:4500–4507. [PubMed] [Google Scholar]
- Leloup J, Buscaglia M. La triiodothyronine: hormone de la metamorphose des amphibians. C R Acad Sci. 1977;284:2261–2263. [Google Scholar]
- Leone F, Lambert-Gardini S, Sartori C, Scapin S. Ultrastructural analysis of some functional aspects of Xenopus laevis pancreas during development andmetamorphosis. J Embryol Exp Morphol. 1976;36:711–724. [PubMed] [Google Scholar]
- Lim W, Nguyen NH, Yang HY, Scanlan TS, Furlow JD. A thyroid hormone antagonist that inhibits thyroid hormone action in vivo. J Biol Chem. 2002;277:35664–35670. doi: 10.1074/jbc.M205608200. [DOI] [PubMed] [Google Scholar]
- Maake C, Hanke W, Reinecke M. An immunohistochemical and morphometric analysis of insulin, insulin-like growth factor I, glucagon, somatostatin, and PP in the development of the gastro-entero-pancreatic system of Xenopus laevis. Gen Comp Endocrinol. 1998;110:182–195. doi: 10.1006/gcen.1998.7064. [DOI] [PubMed] [Google Scholar]
- Maniatis GM, Ingram VM. Erythropoiesis during amphibian metamorphosis: 1. Site of maturation of erythrocytes in Rana catesbeiana. J Cell Biol. 1971;49:372–378. doi: 10.1083/jcb.49.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Holt CE. Control of retinal growth and axon divergence at the chiasm: lessons from Xenopus. BioEssays. 2001;23:319–326. doi: 10.1002/bies.1046. [DOI] [PubMed] [Google Scholar]
- Marklew S, Smith DP, Mason CS, Old RW. Isolation of a novel RXR from Xenopus that most closely resembles mammalian RXR beta and is expressed throughout early development. Biochim Biophys Acta. 1994;1218:267–272. doi: 10.1016/0167-4781(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Berry DL, Brown DD. Germline transmission of transgenes in Xenopus laevis. Proc Natl Acad Sci. 1999a;96:14389–14393. doi: 10.1073/pnas.96.25.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Liu TT, Brown DD. Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type-III deiodinase. Neuron. 1999b;24:871–878. doi: 10.1016/s0896-6273(00)81034-x. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Cai L, Brown DD. Thyroid hormone controls the development of connections between the spinal cord and limbs during Xenopus laevis metamorphosis. Proc Nat Acad Sci. 2004;101:165–170. doi: 10.1073/pnas.2136755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathisen PM, Miller L. Thyroid hormone induction of keratin genes: a two-step activation of gene expression during development. Genes Dev. 1987;1:1107–1117. doi: 10.1101/gad.1.10.1107. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Dixon KE. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: structural aspects. J Anat. 1978;125:155–169. [PMC free article] [PubMed] [Google Scholar]
- Miyatani S, Winkles JA, Sargent TD, Dawid IB. Stage-specific keratins in Xenopus laevis embryos and tadpoles: the XK81 gene family. J Cell Biol. 1986;103:1957–1965. doi: 10.1083/jcb.103.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan Dubois G, Sebillot A, Kuiper GGJM, Verhoelst CHJ, Darras VM, Visser TJ, Demeneix BA. Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology. 2006;147:4941–4949. doi: 10.1210/en.2006-0609. [DOI] [PubMed] [Google Scholar]
- Moskaitis JE, Sargent TD, Smith LH, Jr, Pastori RL, Schoenberg DR. Xenopus laevis serum albumin: sequence of the complementary deoxyribonucleic acids encoding the 68- and 74-kilodalton peptides and the regulation of albumin gene expression by thyroid hormone during development. Mol Endocrinol. 1989;3:464–473. doi: 10.1210/mend-3-3-464. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yaoita Y. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn. 2003;227:246–255. doi: 10.1002/dvdy.10300. [DOI] [PubMed] [Google Scholar]
- Nicolas N, Gallien C-L, Chanoine C. Expression of myogenic regulatory factors during muscle development of Xenopus: myogenin mRNA accumulation is limited strictly to secondary myogenesis. Dev Dyn. 1998;213:309–321. doi: 10.1002/(SICI)1097-0177(199811)213:3<309::AID-AJA7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Elsevier/North-Holland; New York: 1956. [Google Scholar]
- Nishikawa A, Hayashi H. Isoform transition of contractile proteins related to muscle remodeling with an axial gradient during metamorphosis in Xenopus laevis. Dev Biol. 1994;165:86–94. doi: 10.1006/dbio.1994.1236. [DOI] [PubMed] [Google Scholar]
- Nishikawa A, Hayashi H. T3-hydrocortisone synergism on adult-type erythroblast proliferation and T3-mediated apoptosis of larval-type erythroblasts during erythropoietic conversion in Xenopus laevis. Histochem Cell Biol. 1999;111:325–334. doi: 10.1007/s004180050364. [DOI] [PubMed] [Google Scholar]
- Nishikawa A, Yoshizato K. Hormonal regulation of growth and life span of bullfrog tadpole tail epidermal cells cultured in vitro. J Exp Zool. 1986;237:221–230. doi: 10.1002/jez.1402370208. [DOI] [PubMed] [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mech Dev. 2006;123:103–113. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Paik WK, Cohen PP. Biochemical studies on amphibian metamorphosis: I. The effect of thyroxine on protein synthesis in the tadpole. J Gen Physiol. 1960;43:683–696. doi: 10.1085/jgp.43.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev Dyn. 2006;235:247–252. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Paul BD, Fu L, Buchholz DR, Shi Y-B. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol Cell Biol. 2005;25:5712–5724. doi: 10.1128/MCB.25.13.5712-5724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo EM, Baker BS, Tata JR. Interplay between thyroid hormone and estrogen in modulating expression of their receptor and vitellogenin genes during Xenopus metamorphosis. Mech Dev. 1994;45:49–57. doi: 10.1016/0925-4773(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Regard E, Taurog A, Nakashima T. Plasma thyroxine and triiodothyronine levels in spontaneously metamorphosing Rana catesbeiana tadpoles and in adult anuran amphibia. Endocrinology. 1978;102:674–684. doi: 10.1210/endo-102-3-674. [DOI] [PubMed] [Google Scholar]
- Ranjan M, Wong J, Shi Y-B. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269:24699–24705. [PubMed] [Google Scholar]
- Robertson JC, Kelley DB. Thyroid hormone controls the onset of androgen sensitivity in Xenopus laevis. Dev Biol. 1996;176:108–123. doi: 10.1006/dbio.1996.9990. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA. Metamorphosis and the amphibian immune system. Immunol Rev. 1998;166:221–230. doi: 10.1111/j.1600-065x.1998.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Rot-Nikcevic I, Wassersug RJ. Tissue sensitivity to thyroid hormone in athyroid Xenopus laevis larvae. Dev Growth Differ. 2003;45:321–325. doi: 10.1046/j.1440-169x.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- Rowe I, Coen L, Le Blay K, Le Mevel S, Demeneix BA. Autonomous regulation of muscle fibre fate during metamorphosis in Xenopus tropicalis. Dev Dyn. 2002;224:381–390. doi: 10.1002/dvdy.10117. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi Y-B. Nuclear receptor corepressor recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol Cell Biol. 2002;22:8527–8538. doi: 10.1128/MCB.22.24.8527-8538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Koyano-Nakagawa N, Kintner C. Thyroid hormone promotes neurogenesis in the Xenopus spinal cord. Dev Dyn. 2002;225:485–498. doi: 10.1002/dvdy.10179. [DOI] [PubMed] [Google Scholar]
- Schreiber AM, Brown DD. Tadpole skin dies autonomously in response to thyroid hormone at metamorphosis. Proc Natl Acad Sci U S A. 2003;100:1769–1774. doi: 10.1073/pnas.252774999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Cai L, Brown DD. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci U S A. 2005;102:3720–3725. doi: 10.1073/pnas.0409868102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by expressing a dominant negative thyroid hormone receptor. Proc Natl Acad Sci U S A. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-B. From Morphology to Molecular Biology. Wiley-Liss; New York: 2000. Amphibian metamorphosis; p. 288. [Google Scholar]
- Shi Y-B, Brown DD. Developmental and thyroid hormone-dependent regulation of pancreatic genes in Xenopus laevis. Genes Dev. 1990;4:1107–1113. doi: 10.1101/gad.4.7.1107. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Brown DD. The earliest changes in gene expression in tadpole intestine induced by thyroid hormone. J Biol Chem. 1993;268:20312–20317. [PubMed] [Google Scholar]
- Shi Y-B, Hayes WP. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol. 1994;161:48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Yaoita Y, Brown DD. Genomic organization and alternative promoter usage of the two thyroid hormone receptor genes in Xenopus laevis. J Biol Chem. 1992;267:733–738. [PubMed] [Google Scholar]
- Shizu-Nishikawa K, Shibota Y, Takei A, Kuroda M, Nishikawa A. Regulation of specific developmental fates of larval- and adult-type muscles during metamorphosis of the frog Xenopus. Dev Biol. 2002;251:91–104. doi: 10.1006/dbio.2002.0800. [DOI] [PubMed] [Google Scholar]
- Stolow MA, Shi Y-B. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res. 1995;23:2555–2562. doi: 10.1093/nar/23.13.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sato K, Katsu K, Hayashita H, Kristensen DB, Yoshizato K. Novel Rana keratin genes and their expression during larval to adult epidermal conversion in bullfrog tadpoles. Differentiation. 2001;68:44–54. doi: 10.1046/j.1432-0436.2001.068001044.x. [DOI] [PubMed] [Google Scholar]
- Tata JR. Requirement for RNA and protein synthesis for induced regression of the tadpole tail in organ culture. Dev Biol. 1966;13:77–94. doi: 10.1016/0012-1606(66)90050-9. [DOI] [PubMed] [Google Scholar]
- Tata JR. Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol. 2006;246:10–20. doi: 10.1016/j.mce.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Tazawa I, Shimizu-Nishikawa K, Yoshizato K. A novel Xenopus laevis larval keratin gene, xlk2: its gene structure and expression during regeneration and metamorphosis of limb and tail. Biochim Biophys Acta. 2006;1759:216–224. doi: 10.1016/j.bbaexp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002;118:91–98. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Thompson CC, Bottcher MC. The product of a thyroid hormone-responsive gene interacts with thyroid hormone receptors. Proc Natl Acad Sci U S A. 1997;94:8527–8532. doi: 10.1073/pnas.94.16.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueb L, Hanken J. Skeletal development in Xenopus laevis. J Mophol. 1992;214:1–41. doi: 10.1002/jmor.1052140102. [DOI] [PubMed] [Google Scholar]
- Veldhoen N, Crump D, Werry K, Helbing CC. Distinctive gene profiles occur at key points during natural metamorphosis in the Xenopus laevis tail. Dev Dyn. 2002;225:457–468. doi: 10.1002/dvdy.10175. [DOI] [PubMed] [Google Scholar]
- Wakahara M. Heterochrony and neotenic salamanders: possible clues for the understanding the animal development and evolution. Zool Sci. 1996;13:765–776. doi: 10.2108/zsj.13.765. [DOI] [PubMed] [Google Scholar]
- Wang Z, Brown DD. A gene expression screen. Proc Natl Acad Sci U S A. 1991;88:11505–11509. doi: 10.1073/pnas.88.24.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Brown DD. The thyroid hormone-induced gene expression program for amphibian tail resorption. J Biol Chem. 1993;268:16270–16278. [PubMed] [Google Scholar]
- Watanabe Y, Kobayashi H, Suzuki K, Kotani K, Yoshizato K. New epidermal keratin genes from Xenopus laevis: hormonal and regional regulation of their expression during anuran skin metamorphosis. Biochim Biophys Acta. 2001;1517:339–350. [Google Scholar]
- Weber R. Induced metamorphosis in isolated tails of Xenopus larvae. Experientia. 1962;15:84–85. doi: 10.1007/BF02138272. [DOI] [PubMed] [Google Scholar]
- Weber R. Switching of globin genes during anuran metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis. Academic Press; New York: 1996. pp. 567–597. [Google Scholar]
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Widmer HJ, Hosbach HA, Weber R. Globin gene expression in Xenopus laevis: anemia induces precocious globin transition and appearance of adult erythroblasts during metamorphosis. Dev Biol. 1983;99:50–60. doi: 10.1016/0012-1606(83)90253-1. [DOI] [PubMed] [Google Scholar]
- Wong J, Shi Y-B. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem. 1995;270(1):8479–8483. doi: 10.1074/jbc.270.31.18479. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990;4:1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Nakajima K. Induction of apoptosis and CPP32 expression by thyroid hormone in a myoblastic cell line derived from tadpole tail. J Biol Chem. 1997;272:5122–5127. doi: 10.1074/jbc.272.8.5122. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Shi Y-B, Brown DD. Xenopus laevis α and β thyroid hormone receptors. Proc Natl Acad Sci U S A. 1990;87:7090–7094. doi: 10.1073/pnas.87.18.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM, Feng X, Flamant F, Chen Y, Walker RL, Weiss RE, Chassande O, Samarut J, Refetoff S. Effects of ligand and thyroid hormone receptor isoforms on hepatic gene expression profiles of thyroid hormone receptor knockout mice. EMBO Rep. 2003;4:581–587. doi: 10.1038/sj.embor.embor862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizato K. Biochemistry and cell biology of amphibian metamorphosis with a special emphasis on the mechanism of removal of larval organs. Int Rev Cytol. 1989;119:97–149. doi: 10.1016/s0074-7696(08)60650-6. [DOI] [PubMed] [Google Scholar]
- Yoshizato K. Death and transformation of larval cells during metamorphosis of anura. Dev Growth Differ. 1992;34:607–612. doi: 10.1111/j.1440-169X.1992.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Yoshizato K. Cell death and histolysis in amphibian tail during metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis. Academic Press; New York: 1996. pp. 647–671. [Google Scholar]
- Yousson JH. First metamorphosis in fish. In: Hoar WS, Randall DJ, editors. Fish Physiology XI Part B. Academic Press; San Diego: 1988. pp. 135–184. [Google Scholar]


