Eukaryotic cells extend their polypeptide diversity beyond the constraints of the encoded amino acids by means of posttranslational modifications. These modifications regulate the stability, localization, activity, and probably other as-yet-uncharacterized protein functions. Methylated derivatives of arginines were identified 40 years ago from acidic lysates of nuclear extracts (69). However, the first genes encoding protein arginine methyltransferases (PRMTs) were identified only a decade ago (29, 37, 54). Since this important discovery, the study of protein arginine methylation has been a rapidly expanding field, and PRMT-encoding genes have been identified from the sequenced genomes of yeasts, worm, fly, plants, and mammals but not prokaryotes (7, 28, 60). Here, I will focus on the current knowledge of PRMTs predicted to be expressed in several unicellular eukaryotic species. References and comparisons to the functional roles played by these evolutionarily conserved PRMTs in higher eukaryotes, especially humans, will also be discussed.
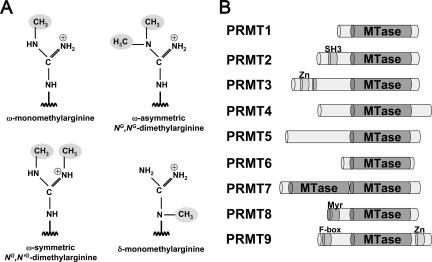
PRMTs are divided into four major classes depending on the type of methylarginine they generate (Fig. 1A). Type I and II PRMTs both catalyze monomethylation of the guanidinium (ω) nitrogen of specific arginine residues in proteins; yet, they differ on the type of dimethylarginine they generate (Fig. 1A). Type I PRMTs modify proteins by the catalysis of asymmetric NG,NG-dimethylarginine, whereas type II PRMTs catalyze the formation of symmetric NG,N′G-dimethylarginine. Type III enzymes catalyze only the monomethylation of arginine residues in proteins (64). It remains unclear, however, whether such enzymes are dedicated for the catalysis of ω-NG-monomethylarginine or if the modified monomethylarginine is an intermediate to additional steps of methylation. A less common form of methylarginine is one in which the delta (δ) nitrogen atom of arginine residues is modified by type IV PRMTs (Fig. 1A). As yet, protein arginine methylation by the type IV PRMTs has been characterized only in Saccharomyces cerevisiae (67) and is less abundant than protein arginine methylation of the guanidinium nitrogen.
FIG. 1.
Methylated arginine derivatives and PRMTs. (A) The structure of methylated arginine derivatives. (B) Schematic representation of the nine mammalian PRMTs, all containing a conserved methyltransferase (MTase) domain. SH3, Src-homology 3; Zn, zinc finger; Myr, myristoylation; and F-box motifs of specific PRMTs are shown.
In humans, nine PRMTs have so far been identified (Fig. 1B): the type I arginine methyltransferases PRMT1, PRMT3, PRMT4, PRMT6, and PRMT8, as well as those of type II, PRMT5, PRMT7, and PRMT9. Although PRMT2 demonstrates a significant degree of similarity to PRMT1, no enzymatic activity has yet been described for human PRMT2 (83). As can be seen in Fig. 1B, the primary structure of each PRMT shares a conserved methyltransferase domain that includes subdomains for binding to the methyl donor, S-adenosyl-l-methionine (SAM) and substrate proteins.
PROTEIN ARGININE METHYLATION IN UNICELLULAR EUKARYOTES
Although evidence for protein arginine methylation was reported for Leishmania (71) and Trypanosoma (103) species, the evolutionary conservation of human PRMTs in unicellular eukaryotes has not been thoroughly investigated. With the recent sequencing and annotation of genomes from several unicellular eukaryotes, it is now possible to identify and characterize homologs of the different human PRMTs in various species. Table 1 presents an overview of the evolutionary conservation of each human PRMT in 10 unicellular eukaryotes, including yeasts, molds, amoebae, and protozoa. This analysis reveals that PRMT1, PRMT3, and PRMT5 are the arginine methyltransferase-encoding genes that were most strictly conserved throughout eukaryotic evolution. Furthermore, it can be seen that PRMT2, PRMT8, and PRMT9 do not appear to have homologs in any of the unicellular eukaryotes shown in Table 1. These three methyltransferases may have evolved as a requirement for tissue-specific functions in multicellular organisms. Accordingly, Northern blotting analyses of PRMT2 and PRMT8 demonstrate tissue-specific mRNA expression (46, 83). Conversely, the PRMT1, PRMT3, PRMT4, PRMT5, and PRMT6 mRNAs are more widely expressed in human tissues, and proteins with homology to some of these methyltransferases can be identified in unicellular eukaryotes (Table 1). Gene products with low amino acid sequence similarity to human PRMT7 were also found in Leishmania major and Trypanosoma brucei (Table 1). The current state of knowledge about the biology of the highly conserved methyltransferases PRMT1, PRMT3, and PRMT5 will be discussed in the next sections.
TABLE 1.
Conservation of type I and II PRMTs across simple eukaryotesa
| Organism | % Of identity to human PRMTb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PRMT1 | PRMT2 | PRMT3 | PRMT4 | PRMT5 | PRMT6 | PRMT7 | PRMT8 | PRMT9 | |
| Cryptococcus neoformans | 50 | ND | 32 | ? | 38 | ND | ND | ND | ND |
| Schizosaccharomyces pombe | 49 | ND | 33 | ND | 33 | ND | ND | ND | ND |
| Saccharomyces cerevisiae | 38 | ND | ND | ND | 27 | ND | ND | ND | ND |
| Candida albicans | 41 | ND | ND | ND | ND | ND | ND | ND | ND |
| Aspergillus nidulans | 46 | ND | 36 | ND | 37 | ND | ND | ND | ND |
| Neurospora crassa | 46 | ND | 33 | ND | 33 | ND | ND | ND | ND |
| Dictyostelium discoideum | 50 | ND | ND | ND | 46 | ? | ND | ND | ND |
| Leishmania major | 41 | ND | ND | ND | 23 | ? | ? | ND | ND |
| Trypanosoma brucei | 43 | ND | ND | ND | 21 | ? | ? | ND | ND |
| Toxoplasma gondii | 45 | ND | 30 | ? | 32 | ND | ND | ND | ND |
The amino acid sequences of human PRMT1 to PRMT9 were used as bait for BLAST searches. The search and collection of related information was performed at the following websites: NCBI (www.ncbi.nlm.nih.gov), the Wellcome Trust Sanger Institute (www.sanger.ac.uk), the BROAD Institute (www.broad.mit.edu/tools/data/seq.html), the ToxoDB (www.toxodb.org/toxo/home.jsp), and the Saccharomyces Genome Database (www.yeastgenome.org).
The percentages of identity were derived after amino acid sequence alignments using ClustalW (www.ebi.ac.uk/services). ND, not determined. ?, a gene product with low amino acid sequence similarity to this human PRMT has been found in this organism (see text).
PROTEIN ARGININE METHYLTRANSFERASE 1
The first studies reporting a gene product with protein arginine methyltransferase activity were published in 1996 after the identification of the budding yeast heterogeneous nuclear ribonucleoprotein (hnRNP) methyltransferase 1 (HMT1, or RMT1) (29, 37) and of human PRMT1 (54). Amino acid analysis by ion exchange chromatography indicates that PRMT1 is the methyltransferase responsible for the majority of asymmetric dimethylation (aDMA) in budding yeast (29), trypanosomes (73), and human cells (72). Consistently, yeast PRMT1 is an abundant protein with approximately 37,600 molecules/cell as determined by quantitative immunoblotting (30). Such a level is comparable to the number of specific translation initiation factors and proteins involved in ribosome assembly (30). Structures of PRMT1 from budding yeast and humans have also been resolved by X-ray crystallography (99, 107). Both crystals reveal the formation of a dimer ring that is characterized by a large acidic cavity at the dimer interface. This acidic cleft forms a binding surface that targets arginines of positively charged arginine-rich regions of substrate proteins for methylation. Consistently, structure-function analyses reveal that dimerization is essential for PRMT1 function, as substitutions that replace the helix-loop-helix domain involved in dimer formation lead to a loss of catalytic activity and function in vivo (99). The oligomerization of PRMTs has also been proposed to favor enzymatic processivity whereby monomethylated arginines could be dimethylated by adjacent active sites without substrate release (107).
PRMT1 is found in the cytoplasm and the nucleus of both yeast and human cells (5, 30, 93). The pathway by which it is imported into the nucleus, however, remains to be determined, as PRMT1 appears to lack a nuclear localization signal. One possibility is that PRMT1 is indirectly imported into the cell nucleus via association with unmethylated substrates. Such a model is supported by mobility assays of live human cells that demonstrate nuclear accumulation of PRMT1 upon the inhibition of cellular methylation (38).
Our knowledge about the biochemistry and biology of PRMT1 is best understood from studies of the budding yeast Saccharomyces cerevisiae. In this organism, two main functions have been ascribed to PRMT1: mRNA biogenesis and heterochromatin formation. One of the first demonstrations of the functional consequence of PRMT1-dependent arginine methylation was on the kinetics of nucleocytoplasmic transport. Using a temperature-sensitive strain with a substitution in a nucleoporin that blocks only nuclear import, it was demonstrated that nuclear export of the yeast RNA-binding proteins Npl3, Hrp1, and Nab2 is perturbed in PRMT1-null cells (34, 85). Subsequent studies with humans showed that PRMT1 affects the subcellular localization of a number of PRMT1 substrates (21, 66, 89). Additional insights into the biological role of PRMT1 have recently been obtained from genome-wide studies of yeast. A combination of chromatin immunoprecipitation assays (ChIP) and whole-genome microarrays indicate that PRMT1 is recruited to actively transcribed genes and that arginine methylation modulates the recruitment of different PRMT1 substrates during mRNA synthesis (104). Accordingly, the kinetics of transcript release from sites of transcription are delayed in PRMT1-null cells (104). Since an important biochemical effect of arginine methylation is the regulation of protein-protein interactions (59, 100, 104), these studies suggest that arginine methylation by PRMT1 is involved in modulating the architecture of mRNA synthesis for the proper release of messenger RNPs from sites of transcription and subsequent nuclear export. Therefore, the nuclear accumulation of specific RNA-binding proteins in PRMT1-null cells may be explained in part by the effect of PRMT1 on mRNA production, as several PRMT1 substrates exit the nucleus in association with polyadenylated mRNAs (34, 49).
Regulation of RNA-binding proteins by PRMT1-dependent arginine methylation was also described in fission yeast. In this organism, PRMT1 modulates the oligomerization level and the growth inhibitory effect of the S. pombe homolog of the human nuclear poly(A)-binding protein (PABP2) (74). These findings are significant, given the suspected role of PABP2 oligomerization in the formation of nuclear aggregates found in oculopharyngeal muscular dystrophy, a syndrome linked to mutations in the human PABP2 gene. Therefore, fission yeast should prove to be a useful model system with which to understand the biological significance of PABP2 methylation at arginines.
Recently, a role for PRMT1 in the establishment and maintenance of silent chromatin was demonstrated with budding yeast (105). Transcription profiles from PRMT1-null cells indicate upregulation of RNAs expressed near telomere-proximal regions, mating loci, and ribosomal DNA repeats. Since these chromosomal regions are normally condensed into silent chromatin (80), these data suggest defects in the formation of such chromatin structure in PRMT1-null cells. Accordingly, a survey of silent loci occupancy by ChIP assays reveals a reduction in the recruitment of the silent information regulator 2 (Sir2) as well as a corresponding increase in acetylated histone H4 in a strain that expresses a catalytically inactive version of PRMT1 (105). The role of histone H4 arginine-3 methylation in PRMT1-dependent heterochromatin formation has been proposed (105); yet, it remains unclear whether PRMT1 is the methyltransferase responsible for this specific modification of histone H4 in yeast (45). A role for PRMT1 in the maintenance of genomic integrity in humans was also reported. From a proteomic screen for arginine methylated substrates in human cells (11), it was demonstrated that the arginine-rich domain of the human DNA endonuclease, MRE11, is arginine methylated by PRMT1 (12). Significantly, a decrease in MRE11 methylation results in defective S-phase checkpoint control (12). The evolutionary conservation of this PRMT1 function in unicellular eukaryotes seems unlikely, however, as arginine-rich domains do not appear to be present in yeast homologs of MRE11.
Given the important cellular roles played by PRMT1 and the conservation of the PRMT1 gene in eukaryotes (Table 1), it is surprising to learn that expression of this gene is not required for cell viability in yeasts and mammals (5, 29, 37, 72). Nevertheless, PRMT1 is essential during development as mice in which PRMT1 expression has been significantly reduced die during embryogenesis (72). PRMT1 also appears to play an important role in the Trypanosoma life cycle. A recent chromosome-wide screen for gene function in Trypanosoma brucei indicates growth impairment and nuclear defects in the bloodstream form of the parasites after depletion of PRMT1 expression via RNA interference (90). However, this growth defect is not observed in the procyclic form of the parasites (73). It appears unlikely that this stage-specific growth defect is explained by the role of PRMT1 in T. brucei mitochondrial gene expression (33), as mitochondrial activity is significantly reduced in the bloodstream form of T. brucei (8, 9).
PROTEIN ARGININE METHYLTRANSFERASE 3
The conserved methyltransferase core domain of PRMT3 was the first PRMT structure obtained (108). This structure suggested that a putative dimer interface is involved in the mechanism for the methylation reaction (108). As mentioned previously, the formation of a dimer ring was later confirmed after the structure of PRMT1 was resolved (99, 107). Human PRMT3 was originally identified from a two-hybrid screen that used PRMT1 as a bait protein (93). The formation of PRMT1/PRMT3 oligomers is unlikely, however, as these methyltransferases do not appear to cofractionate as determined by gel filtration chromatography (93). Searches for arginine methyltransferase homologs in eukaryotic lineages reveal that PRMT3 is not as strictly conserved as PRMT1 (see Table 1). Genes with significant homology to the PRMT3 gene are found in S. pombe and Drosophila melanogaster but not in S. cerevisiae and Caenorhabditis elegans (5). Homologs of PRMT3 can also be identified in other unicellular eukaryotes, such as Cryptococcus, Aspergillus, Neurospora, and Toxoplasma species (Table 1).
PRMT3 is a cytosolic protein that contains a single C2H2-type zinc finger domain in the amino-terminal part of the protein that is conserved among rats and humans (93). The cytoplasmic localization and the zinc finger domain are also features of the fission yeast homolog of mammalian PRMT3 (5). Truncation of the amino-terminal region of PRMT3, including its zinc finger motif, results in an enzyme that can methylate arginine residues of a recombinant substrate but cannot methylate substrates from a total cell extract (25). Based on these results, it was concluded that the zinc finger domain is key for PRMT3 substrate selectivity. Interestingly, amino acid sequence alignments between PRMT3 homologs indicate that the zinc finger domain of PRMT3 is not strictly conserved. As can be seen in Fig. 2, the canonical cysteine and histidine residues that constitute the zinc finger motif are not conserved in PRMT3 from Neurospora, Aspergillus, and Cryptococcus species. Based on the lack of conservation in the zinc finger domain, the Aspergillus nidulans RmtB methyltransferase was previously classified in a separate group within the PRMT family (95). Despite the lack of conservation in the zinc-binding cysteine and histidine residues of the predicted PRMT3 homologs from N. crassa, A. nidulans, and C. neoformans, their amino-terminal domains demonstrate significant homology in terms of amino acid identity and spacing for two blocks of highly conserved residues. These blocks are referred to as conserved region 1 (CR1) and CR2 (Fig. 2). In addition, the overall percentages of identity of PRMT3 between humans and N. crassa, A. nidulans, and C. neoformans (33, 36, and 32%, respectively) are similar to the identity calculated after alignments of human PRMT3 and its characterized S. pombe homolog (33%). Based on the strong similarities of their amino-terminal domains and the overall percentage of identity, it is therefore likely that the discussed methyltransferases from N. crassa, A. nidulans, and C. neoformans are closely related to the PRMT3 methyltransferase.
FIG. 2.
Amino acid sequence alignment of the amino-terminal domains of PRMT3 from multiple species. Identical amino acids are shown in black, and similar amino acids are shown in gray. The predicted zinc finger domain, the conserved region 1 (CR1) and CR2, and the PRMT-specific motif I are boxed. Asterisks are present under the canonical cysteine and histidine residues of the zinc finger motif. Sequence are from Neurospora crassa (Nc), Aspergillus nidulans (An), Homo sapiens (Hs), Drosophila melanogaster (Dm), Schizosaccharomyces pombe (Sp), Cryptococcus neoformans (Cn), and Toxoplasma gondii (Tg). Alignment and shading were generated using ClustalW and Boxshade software.
The first insight into the biological role of PRMT3 was obtained from studies of fission yeast. Tandem affinity purification coupled with mass spectrometric protein identification indicates that specific components of the translation machinery copurify with S. pombe PRMT3. Accordingly, the 40S ribosomal protein S2 (rpS2) was the first physiological substrate of PRMT3 to be identified (5). This role of PRMT3 in rpS2 methylation is evolutionarily conserved (91) and likely suggests a significant role for PRMT3 in ribosome function among eukaryotes. Indeed, the deletion of the PRMT3 coding sequence in fission yeast produces unmethylated rpS2 and yields an imbalance in the 40S/60S ribosomal subunit ratio (5). Significant changes at the levels of monosomes and polysomes are not seen, however, consistent with the absence of growth defects in PRMT3-null cells. In a recent study, the genome-wide biological response of PRMT3-null cells to the ribosomal subunit imbalance was investigated. Whereas hardly any changes were noted at the transcriptional level in PRMT3Δ cells, nearly all of the 40S ribosomal protein-encoding mRNAs showed increased ribosome density in PRMT3-null cells (4). Sucrose gradient analysis also indicated that PRMT3Δ cells have reduced levels of small ribosomal subunits (4). These findings thus suggest that PRMT3-null cells respond to a small ribosomal subunit deficit by upregulating the translational efficiency of 40S ribosomal protein mRNAs. Accordingly, such a compensatory mechanism could explain the viable phenotype of PRMT3-null cells by providing a sufficient number of small subunits, and thus functional ribosomes, to accommodate global protein synthesis and survival. Future studies are needed to determine the nature of the 40S ribosomal subunit deficit in the absence of PRMT3 expression in fission yeast.
Ribosomal proteins are subject to a variety of posttranslational modifications including acetylation, ubiquitination, phosphorylation, and methylation (50, 56). In fact, asymmetric dimethylarginine is the predominant methylated amino acid in both the eukaryotic 40S and 60S ribosomal subunits (15). Arginine methylation of ribosomal proteins is evolutionarily conserved among eukaryotes (44, 51, 78) and fluctuates during the cell cycle (14). Ribosomal proteins can also be modified by lysine methylation, and methyltransferases that catalyze such modifications in ribosomal proteins were recently identified in eukaryotes (75, 76). Interestingly, internal ribosome entry site (IRES)-bound ribosomes contain a different methylation pattern than native ribosomes (106), suggesting a role for methylation in translation control. It will be of great interest to study the role of ribosomal protein methylation, given the evolutionary conservation of this modification. Similar to posttranslational modifications of nucleosomal histones (41), ribosome heterogeneity via combinations of ribosomal protein modifications may represent a key layer of gene regulation by controlling translation of specific cellular transcripts.
PROTEIN ARGININE METHYLTRANSFERASE 5
Every multicellular eukaryote from which the genome has been sequenced and annotated appears to encode the type II methyltransferase PRMT5. As can be seen in Table 1, the evolutionary conservation of the PRMT5 gene among unicellular eukaryotes is significant. The fungus Candida albicans appears to be an exception, however, as a gene with homology to PRMT5 could not be found (96 and Table 1). Given the significant conservation of PRMT5 across eukaryotic species, it is possible that C. albicans uses another gene product to accomplish the evolutionarily conserved function of the PRMT5 methyltransferase.
Although this evolutionary conservation suggests a key functional role for PRMT5, deciphering the biological function of this methyltransferase has been challenging. A number of studies of PRMT5 have been published, using different model organisms, including budding and fission yeasts, Drosophila, Xenopus, and human; yet, an evolutionarily conserved function for this methyltransferase remains elusive. Whereas the apparent budding and fission yeast homologs of PRMT5, histone synthetic lethal 7 (Hsl7) and Shk1-binding protein 1 (Skb1), respectively, demonstrate catalytic activity in vitro (6, 47, 94), a physiological substrate for PRMT5 has yet to be identified in these unicellular eukaryotes. Furthermore, recent evidence indicates that Hsl7 catalyzes only the formation of monomethylarginine in vitro, as the formation of symmetrical dimethylarginine was not detected using a variety of artificial substrates (64). This is in contrast to mammalian cells where evidence strongly supports the PRMT5-dependent formation of symmetrical dimethylarginine in several proteins, including specific Sm proteins of the small nuclear ribonucleoprotein core particle (10, 27, 63), the Coilin protein (10, 36), histones H3 and H4 (70), and the methyl-DNA binding domain protein 2 (92). It is unclear whether any of these proteins represent evolutionarily conserved PRMT5 substrates such as rpS2 for PRMT3 (5, 91) and PABP2/PABPN1 (74, 88) or fibrillarin/Nop1 (53, 101) for PRMT1. Therefore, the search for endogenous substrates of the PRMT5 methyltransferase in unicellular eukaryotes is ongoing. Alternatively, PRMT5 homologs in some unicellular eukaryotes may have evolved functional roles independent of methyltransferase activity, as appears to be the case with S. cerevisiae (see discussion below).
Functionally, biochemical and genetic evidence link S. cerevisiae Hsl7 and S. pombe Skb1 to regulatory steps of the cell cycle at the G2/M transition. However, both genes appear to control different molecular events such that Hsl7 and Skb1 have been reported as positive and negative regulators, respectively, of the G2/M transition. The HSL7 gene was originally identified in a genetic screen designed to identify secondary mutations that are lethal when combined with a deletion of the amino-terminal tail of histone H3 (57). HSL7-null cells are viable but show elongated buds and a delay in the G2 phase of the cell cycle (57). In S. cerevisiae, mitotic entry is promoted by the inactivation of the Swe1 kinase, which phosphorylates and inhibits the cyclin-dependent kinase Cdc28 (48). The current data on Hsl7 suggest the following model: a direct interaction between the Hsl1 kinase (identified in the same synthetic lethal screen as Hsl7) and Hsl7 leads to the Hsl1-dependent localization of Hsl7 to bud necks (17, 87). Phosphorylation of Hsl7 by Hsl1 also appears to be important for tethering Hsl7 to bud necks, as a kinase-dead version of Hsl1 perturbs this specific localization of Hsl7 (94). A physical interaction between Swe1 and Hsl7 at the G2/M stage would recruit increasing concentrations of Swe1 to bud necks (17, 55, 61, 62) to trigger sequential phosphorylation of Swe1 by bud neck-localized kinases, including Cla4 and the polo-like kinase, Cdc5 (3, 81, 87). This highly phosphorylated form of Swe1 undergoes efficient degradation (3, 81), presumably via the ubiquitin-dependent proteasome pathway (42). Therefore, deletion of HSL7 results in a constitutively activated morphogenesis checkpoint control in budding yeast due to Swe1 accumulation at the G2/M phase and a consequent impairment in budding.
Significantly, catalytically inactive versions of Hsl7, as confirmed by in vitro methyltransferase assays, rescue the elongated bud phenotype of HSL7-null cells (17, 94). This is in contrast to a report suggesting that the protein methyltransferase activity of human PRMT5 is required in yeast for complementation of the budding defect of HSL7-null cells (47). The expression of the catalytically inactive version of human PRMT5 was not confirmed in this study, however. Therefore, the available data for the role of Hsl7 in the morphogenesis checkpoint control would strongly suggest that the methyltransferase activity of Hsl7 is not required.
Although the role of Hsl7 in cell cycle control is fairly well understood, the molecular basis for the genetic interaction between HSL7 and histones has remained unclear for over a decade after its discovery. A recent study shed some light on these poorly understood genetic interactions. It was shown that a number of genes encoding chromatin-modifying enzymes, including the acetyltransferases Gcn5 and Esa1, the deacetylase Rpd3, and the lysine methyltransferase Set1, result in synthetic lethality when combined with either HSL7Δ or HSL1Δ (79). Whereas the enzymatic activity of Rpd3 and Gcn5 are required in HSL7-null cells, the methyltransferase activity of Hsl7 is dispensable in RPD3Δ, SET1Δ, and GCN5Δ cells. Furthermore, deletion of the SWE1 gene suppresses all of the synthetic lethal interactions between HSL7 and the above-mentioned chromatin modifiers. These data suggest that the molecular basis for the synthetic lethality between Hsl7 and histones results from the inability to trigger a transcriptional response via specific chromatin modifications to circumvent the constitutive morphogenesis checkpoint that is activated in HSL7-null cells. This transcriptional response would require chromatin modifications at the amino-terminal tail of histones H3/H4 that are mediated, at least in part, by Gcn5, Rpd3, Esa1, and Set1 but do not appear to involve histone methylation by Hsl7. This is consistent with the lack of Hsl7-dependent methylation observed upon amino acid analyses of endogenous yeast histones (64). Therefore, whether Hsl7 acts enzymatically in S. cerevisiae remains to be established.
In the fission yeast S. pombe, the homolog of human PRMT5 was originally identified as an Shk1-binding protein (Skb1) via a two-hybrid screen (32). Shk1 is the fission yeast homolog of the S. cerevisiae Ste20 and of the mammalian p21-activated kinase (58), two kinases that are involved in Ras-dependent signaling cascades. As for S. cerevisiae Hsl7, Skb1 expression is not essential for cell viability. Compared to normal fission yeast, however, SKB1-null cells have a less elongated morphology (32). Conversely, whereas overexpression of Skb1 in fission yeast does not appear to affect growth, it leads to hyperelongated cells (31, 32). These phenotypes are consistent with Skb1 being a negative regulator of mitosis. Accordingly, Gilbreth et al. (31) demonstrated that deletion of the SKB1 gene partly suppresses the cell cycle block in G2 that normally occurs upon inactivation of Cdc25, the phosphatase targeting Wee1 (Wee1 is the homolog of the S. cerevisiae Swe1). Furthermore, the hyperelongated cell phenotype observed upon increasing SKB1 gene dosage is dependent on the mitotic regulator Wee1 (31). Because catalytically inactive versions of Skb1 were not assayed in these experiments, it remains unknown whether the methyltransferase activity of Skb1 is required for cell cycle control in fission yeast. Therefore, despite the observations where Skb1 and Hsl7 both regulate the transition between the G2 and M phases of the cell cycle, they appear to have evolved to control opposite checkpoint mechanisms of mitotic entry. In Xenopus laevis, PRMT5 was also shown to regulate the stability of Xenopus Swe1 in a methyltransferase-independent fashion (102). As opposed to budding yeast, where Hsl7 regulates the morphogenesis checkpoint, Xenopus PRMT5 is involved in the DNA replication checkpoint. Collectively, the available data indicate that the PRMT5-Wee1 regulatory module appears evolutionarily conserved, but has evolved to function in different cell cycle-dependent checkpoints in a species-specific manner.
The Hsl7-Swe1 module of budding yeast also appears to be sensitive to stress signals, including high osmolarity. Under osmostress conditions, Hsl1 phosphorylation by the stress-activated Hog1 kinase prevents Hsl7 localization to bud necks, leading to Swe1 accumulation and cell cycle arrest at G2 (19). Similarly, it was previously demonstrated that Skb1 expression in fission yeast is important for cell survival under conditions of hyperosmolarity (6). Although the molecular mechanism by which Skb1 provides an adaptive response in S. pombe under osmostress conditions remains to be determined, evidence suggest that it may involve the methyltransferase activity of Skb1. In vitro assays using Skb1 that was immunopurified from extracts of cells that were previously subjected to high osmolarity indicate that the methyltransferase activity of Skb1 is stimulated under hyperosmotic conditions. As yet, this is the only report providing evidence that the methyltransferase activity of a PRMT is regulated in unicellular eukaryotes. In humans, stimulation of cells in culture by the nerve growth factor has been reported to increase the catalytic activity of PRMT1 (18).
TYPE IV PROTEIN ARGININE METHYLTRANSFERASES
Most of the characterized protein arginine methyltransferases mediate the addition of methyl groups onto the guanidino (ω) nitrogens of arginine residues of proteins (Fig. 1A). Nevertheless, an unusual PRMT that catalyzes methylation of the delta (δ) nitrogen atom of arginines within proteins has been identified in S. cerevisiae (67, 109). This arginine methyltransferase, designated Rmt2, is not essential for cell viability and accumulates in granulated and punctuated structures in both the nucleus and the cytoplasm of yeast cells, as determined by fluorescence microscopy (67, 68). Interestingly, whereas the ability of budding yeast PRMT1 to modify protein substrates is not affected by translational inhibitors (101), Rmt2 appears to act on protein substrates in a cotranslational manner (67). Therefore, it remains to be determined whether the nuclear pool of Rmt2 acts enzymatically. In vitro methylation assays using recombinantly expressed Rmt2 identified the large ribosomal subunit protein rpL12 as a substrate of this methyltransferase (16). However, the association of Rmt2 with rpL12 and/or ribosomes, as well as the role of Rmt2 in ribosome function, remains elusive. The association between Rmt2 and specific nucleoporins was also recently demonstrated (68). Without any functional data, however, the role of Rmt2 in biological activities related to the ribosome and the nuclear pore complex remains to be established.
Genes encoding products with significant amino acid identity to budding yeast Rmt2 are found in the genomes of Schizosaccharomyces, Aspergillus, Candida, Neurospora, and Cryptococcus species. Rmt2 does not appear to be conserved in protozoa, however, as genes similar to RMT2 were not found in Leishmania major, Trypanosoma brucei, Dictyostelium discoideum, and Toxoplasma gondii. Similarly, a protein with significant homology to yeast Rmt2 is not found in the human genome. However, Rmt2 does share a low degree of sequence similarity to the human guanidinoacetate N-methyltransferase (GMAT). The enzymatic activity of GMAT is responsible for SAM-dependent methylation of the δ-nitrogen atom of guanidinoacetate into creatine, and therefore, the shortage in GMAT activity leads to creatine deficiency syndromes in humans (82). Despite the existence of a human enzyme such as GMAT that specifically modifies the δ-nitrogen atom of guanidine derivatives, the absence of a strong candidate for an Rmt2 homolog in protozoa and mammals suggests that a protein arginine methyltransferase with such an enzymatic activity has not been evolutionarily conserved.
PREDICTED PROTEIN ARGININE METHYLTRANSFERASES
Sequence alignments have permitted the identification and the characterization of several new PRMTs in different biological systems (5, 20, 26, 46, 83, 95). Although there are inherent dangers in characterizing homologous proteins based solely on sequence alignments, such analyses are useful for the development of experimental validation to test for methyltransferase activity and function. This can lead to the potential identification of novel PRMT-like proteins in unicellular eukaryotes.
In addition to PRMT1, PRMT3, and PRMT5, other arginine methyltransferase-encoding genes can be identified in the genomes of certain unicellular eukaryotes. In both of the protozoans Leishmania major and Trypanosoma brucei, three genes (LmjF16.0030, LmjF06.0870, and LmjF03.0600 in Leishmania and Tb927.5.3960, Tb927.7.5490, and Tb10.70.3860 in Trypanosoma) encode putative PRMTs that demonstrate low sequence similarity to human PRMT6, PRMT7, and PRMT8, respectively. The genome of the soil-living amoeba Dictyostelium discoideum also contains two predicted protein arginine methyltransferase-encoding genes (DDB0219438 and DDB0217760) in addition to the genes for its PRMT1 and PRMT5 homologs (Table 1). Interestingly, the amino-terminal domains of the putative PRMTs encoded by the Trypanosoma Tb10.70.3860 and the Dictyostelium DDB0217760 genes are predicted to contain a signal peptide sequence and a transmembrane helix, respectively. Because human PRMT8 is targeted to the plasma membrane via N-terminal myristoylation (46), it appears that the Trypanosoma Tb10.70.3860, the Dictyostelium DDB0217760, and human PRMT8 genes have evolved different mechanisms for membrane localization. Predicted methyltransferases showing 31% and 26% amino acid sequence identity to human PRMT4 can also be found in the genomes of Cryptococcus neoformans and Toxoplasma gondii, respectively. Future studies with these predicted PRMTs will undoubtedly provide significant insights into the evolution of arginine methyltransferases and the roles performed by these enzymes in the biology of unicellular eukaryotes.
IS PROTEIN ARGININE METHYLATION DYNAMIC IN UNICELLULAR EUKARYOTES?
Circumstantial evidence over the past 40 years depicted arginine methylation as an irreversible posttranslational modification. The experimental evidence includes data that demonstrate similar half-lives of bulk histones and their methylarginine derivatives (13, 24) as well as the substantial metabolic cost of arginine methylation (12 ATPs for catalysis), making this modification energetically unfavorable if rapid turnover is needed (28). Recent studies, however, have reported the identification of an enzymatic activity in mammalian cells that can convert methylarginines within histones to citrullines (22, 98), consistent with the previous identification of citrullinated histones (35, 65). Peptidylarginine deiminases (PADs), a family of enzymes that were previously characterized for the conversion of arginines within proteins to citrullines, are responsible for such modifications. Structures of PAD4 as determined by X-ray crystallography (1, 2) also suggest that the active site of this enzyme can accommodate arginine or monomethylarginine but not dimethylarginines. There are conflicting data about the ability of PADs to use monomethylarginine-containing substrates, however. A number of studies have indeed reported that arginine-methylated peptides, including methylated peptides derived from the N-terminal tail of histones, are poor substrates for PAD enzymes in vitro (39, 43, 77). Hence, the reversibility of histone arginine methylation and the underlying mechanism by which it is turned over remain elusive.
PADs are apparently not evolutionarily conserved in unicellular eukaryotes. BLAST searches reveal PAD-encoding genes in mammals and some nonmammalian vertebrates (97), including Xenopus and zebrafish, but not in unicellular eukaryotes. Therefore, it appears unlikely that PAD-dependent regulation of arginine methylation occurs in unicellular eukaryotes. In contrast, lysine demethylases have recently been identified in budding and fission yeasts that can reverse mono-, di-, and trimethyl lysines in histones (52, 84, 86). Given the recent evidence that histone lysine methylation is regulated in a dynamic fashion in yeast, it is likely that protein arginine methylation is also actively controlled in unicellular eukaryotes.
PERSPECTIVES FOR FUTURE STUDIES
Future studies of PRMTs in unicellular eukaryotes will hopefully resolve some of the outstanding questions about the mechanism of action of these methyltransferases. First, it remains unclear whether the catalytic activity of PRMTs is regulated or constitutive. Although proteins with regulatory action toward PRMT1 in yeast (40) and mammals (54) have been reported, the biological significance of these proteins in any PRMT1-dependent function remains to be determined. The amenability of some unicellular eukaryotic model systems to genetic screens may also reveal novel regulatory pathways that control the methylation status of arginine methylated proteins. Last, whereas several methyl lysine-reading proteins have been identified (23), much remains to be elucidated about effectors for arginine-methylated proteins. Such studies will surely further our understanding of the biological significance of protein arginine methylation and provide additional challenges and surprises about this evolutionarily conserved posttranslational modification.
Acknowledgments
I thank Stéphane Richard, Anne McBride, Michael Yu, and Laurie Read for critical reading of the manuscript. I also apologize to those whose work was not cited due to space limitation.
Work from my laboratory is supported by a grant from the Canadian Institutes of Health Research (CIHR). F.B. is a Chercheur-Boursier of the Fonds de la Recherche en Santé du Québec (FRSQ) and a New Investigator of the CIHR.
Footnotes
Published ahead of print on 27 April 2007.
REFERENCES
- 1.Arita, K., H. Hashimoto, T. Shimizu, K. Nakashima, M. Yamada, and M. Sato. 2004. Structural basis for Ca(2+)-induced activation of human PAD4. Nat. Struct. Mol. Biol. 11:777-783. [DOI] [PubMed] [Google Scholar]
- 2.Arita, K., T. Shimizu, H. Hashimoto, Y. Hidaka, M. Yamada, and M. Sato. 2006. Structural basis for histone N-terminal recognition by human peptidylarginine deiminase 4. Proc. Natl. Acad. Sci. USA 103:5291-5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano, S., J. E. Park, K. Sakchaisri, L. R. Yu, S. Song, P. Supavilai, T. D. Veenstra, and K. S. Lee. 2005. Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast. EMBO J. 24:2194-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachand, F., D. H. Lackner, J. Bahler, and P. A. Silver. 2006. Autoregulation of ribosome biosynthesis by a translational response in fission yeast. Mol. Cell. Biol. 26:1731-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachand, F., and P. A. Silver. 2004. PRMT3 is a ribosomal protein methyltransferase that affects the cellular levels of ribosomal subunits. EMBO J. 23:2641-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao, S., Y. Qyang, P. Yang, H. Kim, H. Du, G. Bartholomeusz, J. Henkel, R. Pimental, F. Verde, and S. Marcus. 2001. The highly conserved protein methyltransferase, Skb1, is a mediator of hyperosmotic stress response in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 276:14549-14552. [DOI] [PubMed] [Google Scholar]
- 7.Bedford, M. T., and S. Richard. 2005. Arginine methylation an emerging regulator of protein function. Mol. Cell 18:263-272. [DOI] [PubMed] [Google Scholar]
- 8.Bienen, E. J., E. Hammadi, and G. C. Hill. 1981. Trypanosoma brucei: biochemical and morphological changes during in vitro transformation of bloodstream- to procyclic-trypomastigotes. Exp. Parasitol. 51:408-417. [DOI] [PubMed] [Google Scholar]
- 9.Bienen, E. J., G. C. Hill, and K. O. Shin. 1983. Elaboration of mitochondrial function during Trypanosoma brucei differentiation. Mol. Biochem. Parasitol. 7:75-86. [DOI] [PubMed] [Google Scholar]
- 10.Boisvert, F. M., J. Cote, M. C. Boulanger, P. Cleroux, F. Bachand, C. Autexier, and S. Richard. 2002. Symmetrical dimethylarginine methylation is required for the localization of SMN in Cajal bodies and pre-mRNA splicing. J. Cell Biol. 159:957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boisvert, F. M., J. Cote, M. C. Boulanger, and S. Richard. 2003. A proteomic analysis of arginine-methylated protein complexes. Mol. Cell. Proteomics 2:1319-1330. [DOI] [PubMed] [Google Scholar]
- 12.Boisvert, F. M., U. Dery, J. Y. Masson, and S. Richard. 2005. Arginine methylation of MRE11 by PRMT1 is required for DNA damage checkpoint control. Genes Dev. 19:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byvoet, P., G. R. Shepherd, J. M. Hardin, and B. J. Noland. 1972. The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch. Biochem. Biophys. 148:558-567. [DOI] [PubMed] [Google Scholar]
- 14.Chang, F. N., I. J. Navickas, C. Au, and C. Budzilowicz. 1978. Identification of the methylated ribosomal proteins in HeLa cells and the fluctuation of methylation during the cell cycle. Biochim. Biophys. Acta 518:89-94. [DOI] [PubMed] [Google Scholar]
- 15.Chang, F. N., I. J. Navickas, C. N. Chang, and B. M. Dancis. 1976. Methylation of ribosomal proteins in HeLa cells. Arch. Biochem. Biophys. 172:627-633. [DOI] [PubMed] [Google Scholar]
- 16.Chern, M. K., K. N. Chang, L. F. Liu, T. C. Tam, Y. C. Liu, Y. L. Liang, and M. F. Tam. 2002. Yeast ribosomal protein L12 is a substrate of protein-arginine methyltransferase 2. J. Biol. Chem. 277:15345-15353. [DOI] [PubMed] [Google Scholar]
- 17.Cid, V. J., M. J. Shulewitz, K. L. McDonald, and J. Thorner. 2001. Dynamic localization of the Swe1 regulator Hsl7 during the Saccharomyces cerevisiae cell cycle. Mol. Biol. Cell 12:1645-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimato, T. R., J. Tang, Y. Xu, C. Guarnaccia, H. R. Herschman, S. Pongor, and J. M. Aletta. 2002. Nerve growth factor-mediated increases in protein methylation occur predominantly at type I arginine methylation sites and involve protein arginine methyltransferase 1. J. Neurosci. Res. 67:435-442. [DOI] [PubMed] [Google Scholar]
- 19.Clotet, J., X. Escote, M. A. Adrover, G. Yaakov, E. Gari, M. Aldea, E. de Nadal, and F. Posas. 2006. Phosphorylation of Hsl1 by Hog1 leads to a G2 arrest essential for cell survival at high osmolarity. EMBO J. 25:2338-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook, J. R., J. H. Lee, Z. H. Yang, C. D. Krause, N. Herth, R. Hoffmann, and S. Pestka. 2006. FBXO11/PRMT9, a new protein arginine methyltransferase, symmetrically dimethylates arginine residues. Biochem. Biophys. Res. Commun. 342:472-481. [DOI] [PubMed] [Google Scholar]
- 21.Cote, J., F. M. Boisvert, M. C. Boulanger, M. T. Bedford, and S. Richard. 2003. Sam68 RNA binding protein is an in vivo substrate for protein arginine N-methyltransferase 1. Mol. Biol. Cell 14:274-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuthbert, G. L., S. Daujat, A. W. Snowden, H. Erdjument-Bromage, T. Hagiwara, M. Yamada, R. Schneider, P. D. Gregory, P. Tempst, A. J. Bannister, and T. Kouzarides. 2004. Histone deimination antagonizes arginine methylation. Cell 118:545-553. [DOI] [PubMed] [Google Scholar]
- 23.Daniel, J. A., M. G. Pray-Grant, and P. A. Grant. 2005. Effector proteins for methylated histones: an expanding family. Cell Cycle 4:919-926. [DOI] [PubMed] [Google Scholar]
- 24.Duerre, J. A., and C. T. Lee. 1974. In vivo methylation and turnover of rat brain histones. J. Neurochem. 23:541-547. [DOI] [PubMed] [Google Scholar]
- 25.Frankel, A., and S. Clarke. 2000. PRMT3 is a distinct member of the protein arginine N-methyltransferase family. Conferral of substrate specificity by a zinc-finger domain. J. Biol. Chem. 275:32974-32982. [DOI] [PubMed] [Google Scholar]
- 26.Frankel, A., N. Yadav, J. Lee, T. L. Branscombe, S. Clarke, and M. T. Bedford. 2002. The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specificity. J. Biol. Chem. 277:3537-3543. [DOI] [PubMed] [Google Scholar]
- 27.Friesen, W. J., S. Paushkin, A. Wyce, S. Massenet, G. S. Pesiridis, G. Van Duyne, J. Rappsilber, M. Mann, and G. Dreyfuss. 2001. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol. Cell. Biol. 21:8289-8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gary, J. D., and S. Clarke. 1998. RNA and protein interactions modulated by protein arginine methylation. Prog. Nucleic Acid Res. Mol. Biol. 61:65-131. [DOI] [PubMed] [Google Scholar]
- 29.Gary, J. D., W. J. Lin, M. C. Yang, H. R. Herschman, and S. Clarke. 1996. The predominant protein-arginine methyltransferase from Saccharomyces cerevisiae. J. Biol. Chem. 271:12585-12594. [DOI] [PubMed] [Google Scholar]
- 30.Ghaemmaghami, S., W. K. Huh, K. Bower, R. W. Howson, A. Belle, N. Dephoure, E. K. O'Shea, and J. S. Weissman. 2003. Global analysis of protein expression in yeast. Nature 425:737-741. [DOI] [PubMed] [Google Scholar]
- 31.Gilbreth, M., P. Yang, G. Bartholomeusz, R. A. Pimental, S. Kansra, R. Gadiraju, and S. Marcus. 1998. Negative regulation of mitosis in fission yeast by the shk1 interacting protein skb1 and its human homolog, Skb1Hs. Proc. Natl. Acad. Sci. USA 95:14781-14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbreth, M., P. Yang, D. Wang, J. Frost, A. Polverino, M. H. Cobb, and S. Marcus. 1996. The highly conserved skb1 gene encodes a protein that interacts with Shk1, a fission yeast Ste20/PAK homolog. Proc. Natl. Acad. Sci. USA 93:13802-13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goulah, C. C., M. Pelletier, and L. K. Read. 2006. Arginine methylation regulates mitochondrial gene expression in Trypanosoma brucei through multiple effector proteins. RNA 12:1545-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green, D. M., K. A. Marfatia, E. B. Crafton, X. Zhang, X. Cheng, and A. H. Corbett. 2002. Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p. J. Biol. Chem. 277:7752-7760. [DOI] [PubMed] [Google Scholar]
- 35.Hagiwara, T., K. Nakashima, H. Hirano, T. Senshu, and M. Yamada. 2002. Deimination of arginine residues in nucleophosmin/B23 and histones in HL-60 granulocytes. Biochem. Biophys. Res. Commun. 290:979-983. [DOI] [PubMed] [Google Scholar]
- 36.Hebert, M. D., K. B. Shpargel, J. K. Ospina, K. E. Tucker, and A. G. Matera. 2002. Coilin methylation regulates nuclear body formation. Dev. Cell 3:329-337. [DOI] [PubMed] [Google Scholar]
- 37.Henry, M. F., and P. A. Silver. 1996. A novel methyltransferase (Hmt1p) modifies poly(A)+-RNA-binding proteins. Mol. Cell. Biol. 16:3668-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrmann, F., J. Lee, M. T. Bedford, and F. O. Fackelmayer. 2005. Dynamics of human protein arginine methyltransferase 1(PRMT1) in vivo. J. Biol. Chem. 280:38005-38010. [DOI] [PubMed] [Google Scholar]
- 39.Hidaka, Y., T. Hagiwara, and M. Yamada. 2005. Methylation of the guanidino group of arginine residues prevents citrullination by peptidylarginine deiminase IV. FEBS Lett. 579:4088-4092. [DOI] [PubMed] [Google Scholar]
- 40.Inoue, K., T. Mizuno, K. Wada, and M. Hagiwara. 2000. Novel RING finger proteins, Air1p and Air2p, interact with Hmt1p and inhibit the arginine methylation of Npl3p. J. Biol. Chem. 275:32793-32799. [DOI] [PubMed] [Google Scholar]
- 41.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser, P., R. A. Sia, E. G. Bardes, D. J. Lew, and S. I. Reed. 1998. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 12:2587-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kearney, P. L., M. Bhatia, N. G. Jones, L. Yuan, M. C. Glascock, K. L. Catchings, M. Yamada, and P. R. Thompson. 2005. Kinetic characterization of protein arginine deiminase 4: a transcriptional corepressor implicated in the onset and progression of rheumatoid arthritis. Biochemistry 44:10570-10582. [DOI] [PubMed] [Google Scholar]
- 44.Kruiswijk, T., A. Kunst, R. J. Planta, and W. H. Mager. 1978. Modification of yeast ribosomal proteins. Methylation. Biochem. J. 175:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacoste, N., R. T. Utley, J. M. Hunter, G. G. Poirier, and J. Cote. 2002. Disruptor of telomeric silencing-1 is a chromatin-specific histone H3 methyltransferase. J. Biol. Chem. 277:30421-30424. [DOI] [PubMed] [Google Scholar]
- 46.Lee, J., J. Sayegh, J. Daniel, S. Clarke, and M. T. Bedford. 2005. PRMT8, a new membrane-bound tissue-specific member of the protein arginine methyltransferase family. J. Biol. Chem. 280:32890-32896. [DOI] [PubMed] [Google Scholar]
- 47.Lee, J. H., J. R. Cook, B. P. Pollack, T. G. Kinzy, D. Norris, and S. Pestka. 2000. Hsl7p, the yeast homologue of human JBP1, is a protein methyltransferase. Biochem. Biophys. Res. Commun. 274:105-111. [DOI] [PubMed] [Google Scholar]
- 48.Lee, K. S., S. Asano, J. E. Park, K. Sakchaisri, and R. L. Erikson. 2005. Monitoring the cell cycle by multi-kinase-dependent regulation of Swe1/Wee1 in budding yeast. Cell Cycle 4:1346-1349. [DOI] [PubMed] [Google Scholar]
- 49.Lee, M. S., M. Henry, and P. A. Silver. 1996. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 10:1233-1246. [DOI] [PubMed] [Google Scholar]
- 50.Lee, S. W., S. J. Berger, S. Martinovic, L. Pasa-Tolic, G. A. Anderson, Y. Shen, R. Zhao, and R. D. Smith. 2002. Direct mass spectrometric analysis of intact proteins of the yeast large ribosomal subunit using capillary LC/FTICR. Proc. Natl. Acad. Sci. USA 99:5942-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lhoest, J., Y. Lobet, E. Costers, and C. Colson. 1984. Methylated proteins and amino acids in the ribosomes of Saccharomyces cerevisiae. Eur. J. Biochem. 141:585-590. [DOI] [PubMed] [Google Scholar]
- 52.Liang, G., R. J. Klose, K. E. Gardner, and Y. Zhang. 2007. Yeast Jhd2p is a histone H3 Lys4 trimethyl demethylase. Nat. Struct. Mol. Biol. 14:243-245. [DOI] [PubMed] [Google Scholar]
- 53.Lin, C. H., H. M. Huang, M. Hsieh, K. M. Pollard, and C. Li. 2002. Arginine methylation of recombinant murine fibrillarin by protein arginine methyltransferase. J. Protein Chem. 21:447-453. [DOI] [PubMed] [Google Scholar]
- 54.Lin, W. J., J. D. Gary, M. C. Yang, S. Clarke, and H. R. Herschman. 1996. The mammalian immediate-early TIS21 protein and the leukemia-associated BTG1 protein interact with a protein-arginine N-methyltransferase. J. Biol. Chem. 271:15034-15044. [DOI] [PubMed] [Google Scholar]
- 55.Longtine, M. S., C. L. Theesfeld, J. N. McMillan, E. Weaver, J. R. Pringle, and D. J. Lew. 2000. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:4049-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Louie, D. F., K. A. Resing, T. S. Lewis, and N. G. Ahn. 1996. Mass spectrometric analysis of 40 S ribosomal proteins from Rat-1 fibroblasts. J. Biol. Chem. 271:28189-28198. [DOI] [PubMed] [Google Scholar]
- 57.Ma, X. J., Q. Lu, and M. Grunstein. 1996. A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev. 10:1327-1340. [DOI] [PubMed] [Google Scholar]
- 58.Marcus, S., A. Polverino, E. Chang, D. Robbins, M. H. Cobb, and M. H. Wigler. 1995. Shk1, a homolog of the Saccharomyces cerevisiae Ste20 and mammalian p65PAK protein kinases, is a component of a Ras/Cdc42 signaling module in the fission yeast Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 92:6180-6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McBride, A. E., J. T. Cook, E. A. Stemmler, K. L. Rutledge, K. A. McGrath, and J. A. Rubens. 2005. Arginine methylation of yeast mRNA-binding protein Npl3 directly affects its function, nuclear export, and intranuclear protein interactions. J. Biol. Chem. 280:30888-30898. [DOI] [PubMed] [Google Scholar]
- 60.McBride, A. E., and P. A. Silver. 2001. State of the arg: protein methylation at arginine comes of age. Cell 106:5-8. [DOI] [PubMed] [Google Scholar]
- 61.McMillan, J. N., M. S. Longtine, R. A. Sia, C. L. Theesfeld, E. S. Bardes, J. R. Pringle, and D. J. Lew. 1999. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol. Cell. Biol. 19:6929-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McMillan, J. N., C. L. Theesfeld, J. C. Harrison, E. S. Bardes, and D. J. Lew. 2002. Determinants of Swe1p degradation in Saccharomyces cerevisiae. Mol. Biol. Cell 13:3560-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meister, G., C. Eggert, D. Buhler, H. Brahms, C. Kambach, and U. Fischer. 2001. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 11:1990-1994. [DOI] [PubMed] [Google Scholar]
- 64.Miranda, T. B., J. Sayegh, A. Frankel, J. E. Katz, M. Miranda, and S. Clarke. 2006. Yeast Hsl7 (histone synthetic lethal 7) catalyses the in vitro formation of omega-N(G)-monomethylarginine in calf thymus histone H2A. Biochem. J. 395:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakashima, K., T. Hagiwara, and M. Yamada. 2002. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J. Biol. Chem. 277:49562-49568. [DOI] [PubMed] [Google Scholar]
- 66.Nichols, R. C., X. W. Wang, J. Tang, B. J. Hamilton, F. A. High, H. R. Herschman, and W. F. Rigby. 2000. The RGG domain in hnRNP A2 affects subcellular localization. Exp. Cell Res. 256:522-532. [DOI] [PubMed] [Google Scholar]
- 67.Niewmierzycka, A., and S. Clarke. 1999. S-Adenosylmethionine-dependent methylation in Saccharomyces cerevisiae. Identification of a novel protein arginine methyltransferase. J. Biol. Chem. 274:814-824. [DOI] [PubMed] [Google Scholar]
- 68.Olsson, I., J. Berrez, A. Leipus, C. Ostlund, and A. Mutvei. 2007. The arginine methyltransferase Rmt2 is enriched in the nucleus and co-purifies with the nuclear porins Nup49, Nup57 and Nup100. Exp. Cell Res. 313:1778-1789. [DOI] [PubMed] [Google Scholar]
- 69.Paik, W. K., and S. Kim. 1967. Enzymatic methylation of protein fractions from calf thymus nuclei. Biochem. Biophys. Res. Commun. 29:14-20. [DOI] [PubMed] [Google Scholar]
- 70.Pal, S., S. N. Vishwanath, H. Erdjument-Bromage, P. Tempst, and S. Sif. 2004. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol. Cell. Biol. 24:9630-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paolantonacci, P., F. Lawrence, F. Lederer, and M. Robert-Gero. 1986. Protein methylation and protein methylases in Leishmania donovani and Leishmania tropica promastigotes. Mol. Biochem. Parasitol. 21:47-54. [DOI] [PubMed] [Google Scholar]
- 72.Pawlak, M. R., C. A. Scherer, J. Chen, M. J. Roshon, and H. E. Ruley. 2000. Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol. Cell. Biol. 20:4859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pelletier, M., D. A. Pasternack, and L. K. Read. 2005. In vitro and in vivo analysis of the major type I protein arginine methyltransferase from Trypanosoma brucei. Mol. Biochem. Parasitol. 144:206-217. [DOI] [PubMed] [Google Scholar]
- 74.Perreault, A., C. Lemieux, and F. Bachand. 2007. Regulation of the nuclear poly(A)-binding protein by arginine methylation in fission yeast. J. Biol. Chem. 282:7552-7562. [DOI] [PubMed] [Google Scholar]
- 75.Porras-Yakushi, T. R., J. P. Whitelegge, and S. Clarke. 2006. A novel SET domain methyltransferase in yeast: Rkm2-dependent trimethylation of ribosomal protein L12ab at lysine 10. J. Biol. Chem. 281:35835-35845. [DOI] [PubMed] [Google Scholar]
- 76.Porras-Yakushi, T. R., J. P. Whitelegge, T. B. Miranda, and S. Clarke. 2005. A novel SET domain methyltransferase modifies ribosomal protein Rpl23ab in yeast. J. Biol. Chem. 280:34590-34598. [DOI] [PubMed] [Google Scholar]
- 77.Raijmakers, R., A. J. Zendman, W. V. Egberts, E. R. Vossenaar, J. Raats, C. Soede-Huijbregts, F. P. Rutjes, P. A. van Veelen, J. W. Drijfhout, and G. J. Pruijn. 2007. Methylation of arginine residues interferes with citrullination by peptidylarginine deiminases in vitro. J. Mol. Biol. 367:1118-1129. [DOI] [PubMed] [Google Scholar]
- 78.Ramagopal, S. 1991. Covalent modifications of ribosomal proteins in growing and aggregation-competent Dictyostelium discoideum: phosphorylation and methylation. Biochem. Cell Biol. 69:263-268. [DOI] [PubMed] [Google Scholar]
- 79.Ruault, M., and L. Pillus. 2006. Chromatin-modifying enzymes are essential when the Saccharomyces cerevisiae morphogenesis checkpoint is constitutively activated. Genetics 174:1135-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72:481-516. [DOI] [PubMed] [Google Scholar]
- 81.Sakchaisri, K., S. Asano, L. R. Yu, M. J. Shulewitz, C. J. Park, J. E. Park, Y. W. Cho, T. D. Veenstra, J. Thorner, and K. S. Lee. 2004. Coupling morphogenesis to mitotic entry. Proc. Natl. Acad. Sci. USA 101:4124-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulze, A. 2003. Creatine deficiency syndromes. Mol. Cell Biochem. 244:143-150. [PubMed] [Google Scholar]
- 83.Scott, H. S., S. E. Antonarakis, M. D. Lalioti, C. Rossier, P. A. Silver, and M. F. Henry. 1998. Identification and characterization of two putative human arginine methyltransferases (HRMT1L1 and HRMT1L2). Genomics 48:330-340. [DOI] [PubMed] [Google Scholar]
- 84.Seward, D. J., G. Cubberley, S. Kim, M. Schonewald, L. Zhang, B. Tripet, and D. L. Bentley. 2007. Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat. Struct. Mol. Biol. 14:240-242. [DOI] [PubMed] [Google Scholar]
- 85.Shen, E. C., M. F. Henry, V. H. Weiss, S. R. Valentini, P. A. Silver, and M. S. Lee. 1998. Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev. 12:679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi, Y., F. Lan, C. Matson, P. Mulligan, J. R. Whetstine, P. A. Cole, and R. A. Casero. 2004. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941-953. [DOI] [PubMed] [Google Scholar]
- 87.Shulewitz, M. J., C. J. Inouye, and J. Thorner. 1999. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:7123-7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith, J. J., K. P. Rucknagel, A. Schierhorn, J. Tang, A. Nemeth, M. Linder, H. R. Herschman, and E. Wahle. 1999. Unusual sites of arginine methylation in poly(A)-binding protein II and in vitro methylation by protein arginine methyltransferases PRMT1 and PRMT3. J. Biol. Chem. 274:13229-13234. [DOI] [PubMed] [Google Scholar]
- 89.Smith, W. A., B. T. Schurter, F. Wong-Staal, and M. David. 2004. Arginine methylation of RNA helicase a determines its subcellular localization. J. Biol. Chem. 279:22795-22798. [DOI] [PubMed] [Google Scholar]
- 90.Subramaniam, C., P. Veazey, S. Redmond, J. Hayes-Sinclair, E. Chambers, M. Carrington, K. Gull, K. Matthews, D. Horn, and M. C. Field. 2006. Chromosome-wide analysis of gene function by RNA interference in the African trypanosome. Eukaryot. Cell 5:1539-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swiercz, R., M. D. Person, and M. T. Bedford. 2005. Ribosomal protein S2 is a substrate for mammalian PRMT3 (protein arginine methyltransferase 3). Biochem. J. 386:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan, C. P., and S. Nakielny. 2006. Control of the DNA methylation system component MBD2 by protein arginine methylation. Mol. Cell. Biol. 26:7224-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang, J., J. D. Gary, S. Clarke, and H. R. Herschman. 1998. PRMT 3, a type I protein arginine N-methyltransferase that differs from PRMT1 in its oligomerization, subcellular localization, substrate specificity, and regulation. J. Biol. Chem. 273:16935-16945. [DOI] [PubMed] [Google Scholar]
- 94.Theesfeld, C. L., T. R. Zyla, E. G. Bardes, and D. J. Lew. 2003. A monitor for bud emergence in the yeast morphogenesis checkpoint. Mol. Biol. Cell 14:3280-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trojer, P., M. Dangl, I. Bauer, S. Graessle, P. Loidl, and G. Brosch. 2004. Histone methyltransferases in Aspergillus nidulans: evidence for a novel enzyme with a unique substrate specificity. Biochemistry 43:10834-10843. [DOI] [PubMed] [Google Scholar]
- 96.Umeyama, T., A. Kaneko, Y. Nagai, N. Hanaoka, K. Tanabe, Y. Takano, M. Niimi, and Y. Uehara. 2005. Candida albicans protein kinase CaHsl1p regulates cell elongation and virulence. Mol. Microbiol. 55:381-395. [DOI] [PubMed] [Google Scholar]
- 97.Vossenaar, E. R., A. J. Zendman, W. J. van Venrooij, and G. J. Pruijn. 2003. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 25:1106-1118. [DOI] [PubMed] [Google Scholar]
- 98.Wang, Y., J. Wysocka, J. Sayegh, Y. H. Lee, J. R. Perlin, L. Leonelli, L. S. Sonbuchner, C. H. McDonald, R. G. Cook, Y. Dou, R. G. Roeder, S. Clarke, M. R. Stallcup, C. D. Allis, and S. A. Coonrod. 2004. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 306:279-283. [DOI] [PubMed] [Google Scholar]
- 99.Weiss, V. H., A. E. McBride, M. A. Soriano, D. J. Filman, P. A. Silver, and J. M. Hogle. 2000. The structure and oligomerization of the yeast arginine methyltransferase, Hmt1. Nat. Struct. Biol. 7:1165-1171. [DOI] [PubMed] [Google Scholar]
- 100.Xu, C., and M. F. Henry. 2004. Nuclear export of hnRNP Hrp1p and nuclear export of hnRNP Npl3p are linked and influenced by the methylation state of Npl3p. Mol. Cell. Biol. 24:10742-10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu, C., P. A. Henry, A. Setya, and M. F. Henry. 2003. In vivo analysis of nucleolar proteins modified by the yeast arginine methyltransferase Hmt1/Rmt1p. RNA 9:746-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada, A., B. Duffy, J. A. Perry, and S. Kornbluth. 2004. DNA replication checkpoint control of Wee1 stability by vertebrate Hsl7. J. Cell Biol. 167:841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yarlett, N., A. Quamina, and C. J. Bacchi. 1991. Protein methylases in Trypanosoma brucei brucei: activities and response to DL-alpha-difluoromethylornithine. J. Gen. Microbiol. 137:717-724. [DOI] [PubMed] [Google Scholar]
- 104.Yu, M. C., F. Bachand, A. E. McBride, S. Komili, J. M. Casolari, and P. A. Silver. 2004. Arginine methyltransferase affects interactions and recruitment of mRNA processing and export factors. Genes Dev. 18:2024-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu, M. C., D. W. Lamming, J. A. Eskin, D. A. Sinclair, and P. A. Silver. 2006. The role of protein arginine methylation in the formation of silent chromatin. Genes Dev. 20:3249-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu, Y., H. Ji, J. A. Doudna, and J. A. Leary. 2005. Mass spectrometric analysis of the human 40S ribosomal subunit: native and HCV IRES-bound complexes. Protein Sci. 14:1438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang, X., and X. Cheng. 2003. Structure of the predominant protein arginine methyltransferase PRMT1 and analysis of its binding to substrate peptides. Structure (Cambridge) 11:509-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang, X., L. Zhou, and X. Cheng. 2000. Crystal structure of the conserved core of protein arginine methyltransferase PRMT3. EMBO J. 19:3509-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zobel-Thropp, P., J. D. Gary, and S. Clarke. 1998. Delta-N-methylarginine is a novel posttranslational modification of arginine residues in yeast proteins. J. Biol. Chem. 273:29283-29286. [DOI] [PubMed] [Google Scholar]