Abstract
Muscarinic receptor-mediated cardiac parasympathetic activity is essential for regulating heart rate and heart rate variability (HRV). It has not been clear which Gi/Go protein is responsible for these effects. We addressed this question using knockout mice that lack G protein αi2, αi3, or αo specifically. Unlike previously reported, our αo-null mice had significantly more survivors with normal life span. Isolated hearts from αo-null mice demonstrated much less sensitivity to the negative chronotropic effects of muscarinic agonist carbachol to lower heart rate at baseline and more profound under the stimulation of β-adrenergic agonist isoproterenol. In the presence of parasympathetic activation indirectly produced by methoxamine, an α1-adrenergic agonist, αo-null mice showed markedly decreased HRV compared with wild type control mice. These differences in heart rate and HRV were not observed in αi2-null or αi3-null mice. Our findings establish an essential role for αo G protein in the anti-adrenergic effect of carbachol on heart rate regulation.
Keywords: G-proteins, heart rate, heart rate variability, parasympathetic
Introduction
Normal heart rate is controlled by cardiac sympathetic and parasympathetic nerves to achieve homeostasis [1, 2]. Cardiac sympathetic system is mediated by β-adrenergic receptors that are coupled primarily to αs G protein and responsible for cardiac activation including positive chronotropic, dromotropic, lusitropic, and inotropic effects. Cardiac parasympathetic system is mediated by muscarinic acetylcholine (M2) receptor that is coupled to αi/αo G proteins and responsible for negative chronotropic and dromotropic effects. In sinoatrial node pacemaker cells, αs and αo act simultaneously, with αo being more potent, suggesting that parasympathetic inhibition of heart rate is much greater on a background of sympathetic stimulation [3]. Mutations in either αi2 or αo to disrupt binding to regulator of G protein signaling results in enhanced muscarinic M2 receptor–mediated bradycardic responses in cardiocytes derived from embryonic stem cells [4]. However, how different αi/αo G proteins (αi2, αi3, and αo) control whole heart rate has not been determined.
The opposing effects of sympathetic and parasympathetic systems cause beat-to-beat alterations in heart rate, a phenomenon called heart rate variability (HRV) [5]. Because HRV is a reflection of parasympathetic tone it predicts survival after heart attack [6]. It is not clear how different αi/αo G proteins control HRV.
To investigate the specificity of αi/αo G proteins in controlling heart rate and HRV, we created αo-null mice and used an isolated heart perfusion method to study the alteration in muscarinic-induced chronotropic effects under basal conditions and in the presence of prior β-adrenergic stimulation, as well as telemetry implants to analyze their EKG. The results were compared with those from αi2-null mice and αi3-null mice previously reported [7, 8].
Materials and Methods
Animal Model
Production of gene targeted mice: Using previously published constructs for gene targeting [7, 8], the αo gene was inactivated in J1 cells cultured on mouse embryo fibroblasts. Targeted lines identified as previously described by Southern analysis [8], were injected into Balb/c blastocysts. Resulting chimeras passed the targeted mutation in the germline bred to Balb/c or 129SvEv. Heterozygotes were mated to obtain littermates that were either wild type or homozygous for the gene inactivation in order to control for genetic background.
Western analysis
Plasma membranes from mouse cardiac ventricles were prepared, subjected to electrophoresis, and transferred to PVDF membranes as described previously [9]. Membranes were incubated with primary antibodies specifically recognizing αo, αi2, αi3, βcomm, and αs. The membranes were then incubated in secondary antiserums conjugated with horseradish peroxidase and detected as before[9].
Isolated Heart Preparation
Hearts were excised, the aortic root cannulated to a 20-gauge needle and perfused retrograde with a modified Krebs-Henseleit buffer (in mM): 120 NaCl, 4.5 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 0.5 EDTA, 25 NaCO3, and 10 dextrose (pH 7.4 when gassed with 95%O2/5%CO2 at 37°C). The entrance of the pulmonary veins into the left atrium was then cannulated and the perfusion was subsequently switched to the working mode [10]. The pulmonary artery was cut to allow measurement of coronary flow. Left ventricular pressures were monitored continually through indwelling cannulae coupled to pressure transducers and linked to a MacLab A/D system and a Macintosh computer with the sampling rate set at 2 kHz. LVP and heart rate were monitored in the working heart mode for 5–10 minutes to ensure stability of the preparation before initiation of the experiment. After a 10-minute stabilization period, the dose-response to carbachol was determined in the absence or presence of 1 μM isoproterenol; stable beating rate was then quantified after each drug dose addition.
In Vivo EKG
Telemetry monitor implants (Data Science, model TA10ETA-F20) were placed in mice. After recovery for at least 24 hours, heart rate was monitored by telemetry using a MacLab digitization and a Power PC computer. Heart rate variability (HRV) was analyzed using MacLab software. Data was collected at baseline for at least 15 minutes and continuously following administration of methoxamine. Data were analyzed for HRV and heart rate when stabilized 5–15 minutes after addition of methoxamine.
Statistics
Mean ± standard error (SE) values were analyzed using Prism (GraphPad Software Inc.). Statistical comparisons between groups were performed by Student’s t test. Dose response curves were compared by Two-way ANOVA and Bonferroni post-tests. Groups were considered significantly different if P values were ≤ 0.05.
RESULTS
Generating αo-null mice
Inactivation of the αo gene was confirmed by western blot (Figure 1A). There was no apparent change of other G-protein subunits, including αi2, αi3, βcomm, and αs, in response to αo inactivation (Figure 1A). Mice homozygous for the αo targeted gene exhibited perinatal mortality, but in contrast to previous reports [11, 12], a significant percentage in the present study had normal life spans (Figure 1B). We were therefore able to study healthy, fertile, adult mice for alterations in heart rate control. Improved animal husbandry in decreasing stress is likely the reason for better survival rate, although difference in genetic background can not be ruled out.
Figure 1. Inactivation of αo in mice.
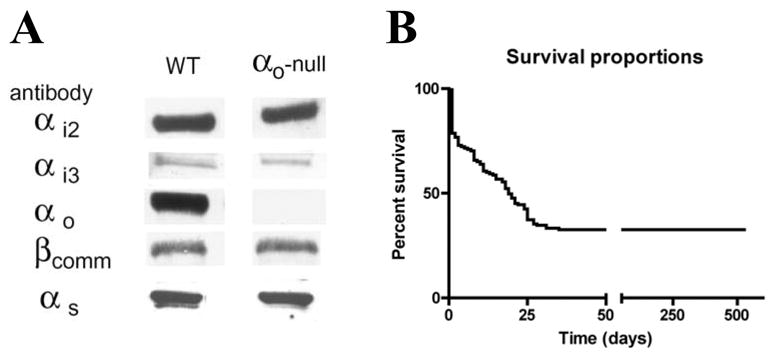
A. Western blots of hearts from αo-null mice and control wild type mice. Antibodies against αo, as well as αi2, αi3, βcomm, and αs were used to verify the deletion of αo and no compensatory changes in other G proteins. B. Survival of αo-null mice. Mice that survived the critical period lived nearly 2 years. The numbers of animals were n=25 at 100 days, 18 at 150 days, and 12 at 200 days. The decrease in number of animals was due to sacrifice for experiments.
Response to carbachol in heart rate is significantly reduced in mouse heart lacking αo
At baseline, carbachol dose response curve in αo-null mouse hearts demonstrated a shift to the right for a half log unit when compared with WT (Figure 2A, lower two curves), showing decreased response to carbachol. When isoproterenol was present, the extent of slowing was much reduced and the effect of lacking αo was not overcome even by very high doses of carbachol (Figure 2A, upper two curves). These results indicate that αo-mediated pathways are critical to negative chronotropic effects of carbachol, particularly in the presence of isoproterenol. To establish whether this is specific to αo, we tested αi2-null and αi3-null mice using the same set up. Neither αi2-null nor αi3-null mouse heart displayed significantly different responses to carbachol than WT at baseline or with isoproterenol stimulation (Figure 2B, C). Mutants lacking both αi2 and αi3 were not born implying embryonic lethality.
Figure 2. Hearts from αo-null mice show decreased sensitivity to muscarinic agonist.
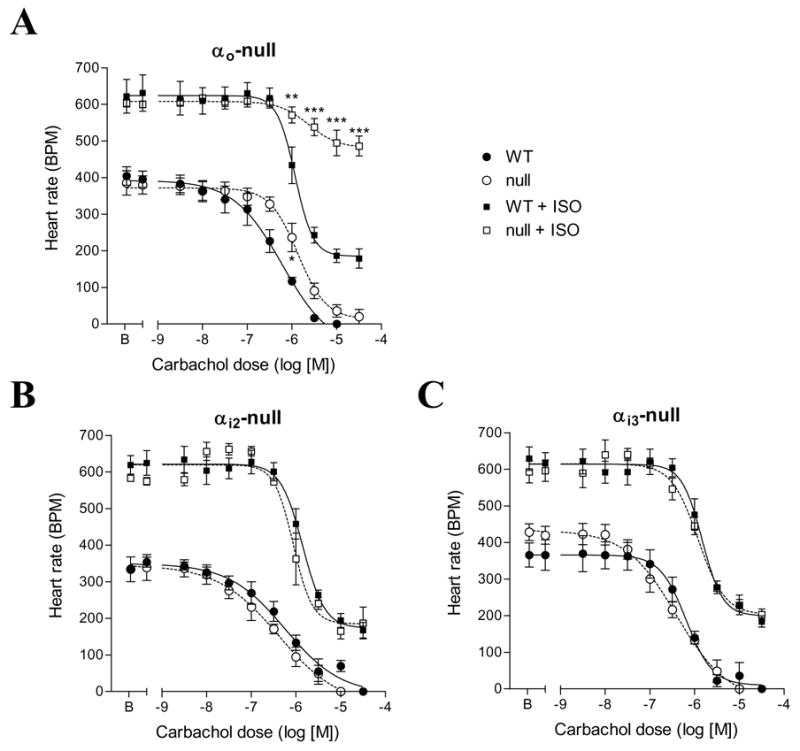
A. Negative chronotropic responses to carbachol in isolated hearts from αo-null and littermate control (WT) mice. Hearts were perfused ex vivo in working or ejecting mode as described in methods. Carbachol dose response curves in the absence and presence of isoproterenol (1 μM, squares) were determined by increasing addition of carbachol. n = 5 for each group. By Two-Way ANOVA, P = 0.0071 WT vs. null; P < 0.0001 WT+ISO vs. null+ISO. Bonferroni post-tests, * P < 0.05 vs. WT; ** P < 0.01 vs. WT+ISO; *** P < 0.001 vs. WT+ISO. B, C. Negative chronotropic responses to carbachol in isolated hearts from αi2-null and αi3-null mice respectively. No statistical significance was detected.
Decreased HRV in mice lacking αo
HRV was determined from at least 1000 R-R intervals between successive beats before and after administration of methoxamine to stimulate the parasympathetic system. This system was chosen in order to compare results with the study on IKACh inactivation [13]. As an α1-adrenergic agonist methoxamine causes vasoconstriction and increases blood pressure leading to activation of the carotid sinus baroreceptor and hence reflex activation of the parasympathetic system. The basal heart rate was not significantly different between αo-null animals and their WT littermates and methoxamine lowered the heart rate in both genotypes (Figure 3A), consistent with low parasympathetic tone in mice [14]. There was a markedly diminished response to methoxamine in HRV in the αo-null animals, but not in αi2-null or αi3-null mice (Figure 3B). Spectral analysis of HRV data indicated that the difference between αo-null and WT was significant after administration of methoxamine, in all 3 components, high frequency (HF), low frequency (LF), and very low frequency (VLF) (Figure 3C).
Figure 3. Diminished HRV in αo-null mice responding to methoxamine.
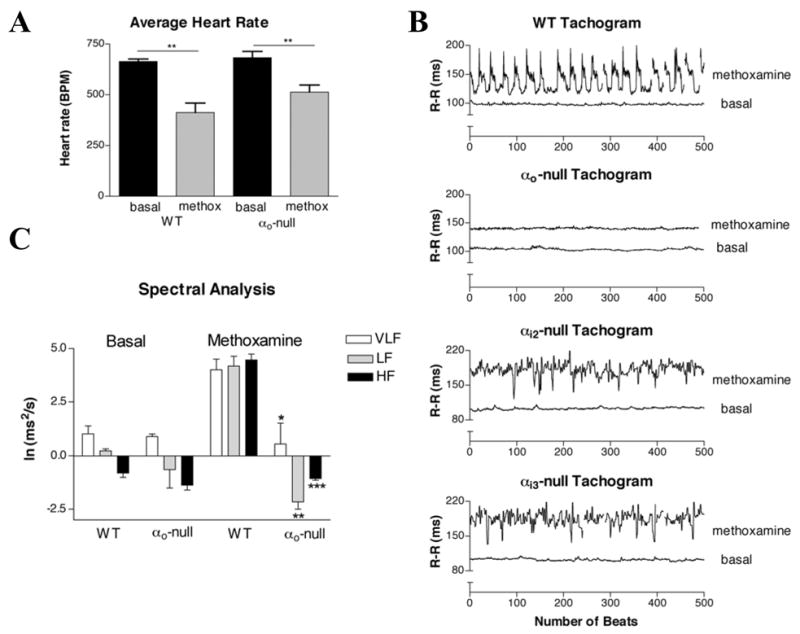
A. In vivo heart rates before and after methoxamine. Heart rates were measured using implantable EKG telemetry devices at baseline and after the injection of 6 mg/kg methoxamine. n=4 for each group. ** P < 0.01. B. Decreased HRV in the αo-null mice compared to their WT littermates after methoxamine stimulation. Mice were chronically maintained EKG telemetry implants as described in methods. Data of the R-R intervals between successive beats before and after addition of methoxamine were collected continuously. C. Quantification of HRV data in αo-null and WT mice. Measurement of the power spectra was broken into three components: high frequency (HF) 1.5–4 Hz; low frequency (LF) 0.4–1.5 Hz; and very low frequency (VLF) <0.4 Hz. * P < 0.02 vs. WT VLF; ** P < 0.002 vs. WT LF; *** P < 0.0001 vs. WT HF.
Discussion
Gene inactivation studies have established the requirement for the IKACh channel in parasympathetic slowing heart rate and increases in HRV. It was estimated that about 50% of the negative chronotropic effect required an intact IKACh channel [13]. We now show that αo-mediated pathways, which are not required for IKACh activation [7, 12] but do regulate If, are also required for normal negative chronotropy in heart rate and parasympathetic stimulated increases in HRV. These results indicate that activation of IKAch through the remaining pertussis toxin-sensitive G-proteins is not capable of maintaining normal negative chronotropic responses on its own. Therefore, at least two pathways are required, one leading to activation of IKACh mediated by αi2 and αi3 released βγ [7] and the other likely leading to inhibition of hyperpolarization-activated current If, which could require αo but not αi2 or αi3.
The exact contributions of these two pathways may differ depending on the system studied. In the intact heart, both pathways appear to be important in regulating heart rate and HRV. Both IKACh and αo inactivation (and hence effects of If), markedly affected the muscarinic negative chronotropic effects. Here, we show the importance of αo under ex vivo conditions where complicating effects on blood pressure and sympathetic or parasympathetic nerve activity have been eliminated. A detailed interpretation of the meaning of the whole animal experiments is difficult in that, like the previous study [13], alterations in blood pressure or effects of knockouts on central nervous system function were not evaluated. However, there is a clear decrease in the HRV in αo-null animals. Inactivation of IKACh nearly eliminated HRV at baseline whereas αo inactivation had no effect. When stimulated with methoxamine, both IKACh and αo inactivation caused marked decreases in HRV with IKACh effects being slightly more pronounced. The effect on HRV is consistent with the decreased sensitivity to muscarinic stimulation by carbachol seen in the isolated perfused hearts. The contribution of the αo dependent pathway may increase with high sympathetic activity or with other β-adrenergic stimulation compared to resting conditions.
These results establish a critical role for αo-containing heterotrimers in the parasympathetic regulation of heart rate and HRV.
Footnotes
Conflict-of-Interest: None.
Grants
This work was supported by grants NIH PO1 HL36028 (DSM), R01 HD04089 (DSM), and HL790902 (RMM).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006;12:4513–4523. doi: 10.2174/138161206779010468. [DOI] [PubMed] [Google Scholar]
- 3.Yatani A, Okabe K, Codina J, Birnbaumer L, Brown AM. Heart rate regulation by G proteins acting on the cardiac pacemaker channel. Science. 1990;249:1163–1166. doi: 10.1126/science.1697697. [DOI] [PubMed] [Google Scholar]
- 4.Fu Y, Huang X, Zhong H, Mortensen RM, D’Alecy LG, Neubig RR. Endogenous RGS proteins and Galpha subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ Res. 2006;98:659–666. doi: 10.1161/01.RES.0000207497.50477.60. [DOI] [PubMed] [Google Scholar]
- 5.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 6.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 7.Sowell MO, Ye C, Ricupero DA, Hansen S, Quinn SJ, Vassilev PM, Mortensen RM. Targeted inactivation of alphai2 or alphai3 disrupts activation of the cardiac muscarinic K+ channel, IK+Ach, in intact cells. Proc Natl Acad Sci U S A. 1997;94:7921–7926. doi: 10.1073/pnas.94.15.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AAT, Seidman JG. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain M, Lim CC, Nagata K, Davis VM, Milstone DS, Liao R, Mortensen RM. Targeted inactivation of Galpha(i) does not alter cardiac function or beta-adrenergic sensitivity. Am J Physiol Heart Circ Physiol. 2001;280:H569–575. doi: 10.1152/ajpheart.2001.280.2.H569. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers RL, McNeill JH. Effect of reserpine pretreatment on guinea pig ventricular performance and responsiveness to inotropic agents. Journal of Pharmacology & Experimental Therapeutics. 1982;221:721–730. [PubMed] [Google Scholar]
- 11.Jiang M, Gold MS, Boulay G, Spicher K, Peyton M, Brabet P, Srinivasan Y, Rudolph U, Ellison G, Birnbaumer L. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci U S A. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, Pfeffer J, Neer EJ, Fishman MC. G alpha(o) is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proc Natl Acad Sci U S A. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 14.Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol. 1997;272:H1053–1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]


