Abstract
Intermediate filaments have long been considered mechanical components of the cell that provide resistance to deformation stress. Practical experimental problems, including insolubility, lack of good pharmacological antagonists, and the paucity of powerful genetic models, have handicapped the research of other functions. In single-layered epithelial cells, keratin intermediate filaments are cortical, either apically polarized or apico-lateral. This review analyzes phenotypes of genetic manipulations of simple epithelial cell keratins in mice and C. elegans that strongly suggest a role of keratins in apico-basal polarization and membrane traffic. Published evidence that intermediate filaments can act as scaffolds for proteins involved in membrane traffic and signaling is also discussed. Such a scaffolding function would generate a highly polarized compartment within the cytoplasm of simple epithelial cells. While in most cases mechanistic explanations for the keratin-null or overexpression phenotypes are still missing, it is hoped investigators will be encouraged to study these as yet poorly understood functions of intermediate filaments.
Introduction
Polarity (asymmetry) is an ubiquitous property of eukaryotic cells. In particular, simple (single-layered) epithelial cells are polarized in apical (luminal) and basolateral domains. This polarity is essential for life in metazoans, as it enables absorption and secretion from or to the external (luminal) compartment. These properties permit the homeostasis of the internal millieu and are well exemplified in the functions of the gut, liver, kidney, genital organs, and exocrine glands. The mechanisms underlying the acquisition and maintenance of epithelial polarity have been extensively reviewed [1,2,3], and include membrane traffic and sorting, especially at the trans-Golgi network and specialized endosomal compartments, transport and fusion of vesicles, and a system of polarized signaling molecules that include, among others, aPKC-PAR3-PAR6 and PAR1-Lgl [3]. Central to the ability of cells to become polarized is the cytoskeleton. The cytoskeleton comprises three major groups of filamentous structures: microtubules, actin filaments, and intermediate filaments (IFs). It is widely recognized that cellular asymmetries are, in some way, based on at least one type of cytoskeletal structure. In fact, polarity may be sustained by different components of the cytoskeleton. For example, asymmetry is based mostly on F-actin in budding yeast [4] while organized by microtubules in fission yeast [5]. One can envision the contributions of each part of the cytoskeleton to cell polarity in two possible ways. First, if the cytoskeletal component is polarized itself, it can serve as an asymmetric scaffold for other cytoskeletal elements, membrane proteins or components, or signaling cascades. Second, if a cytoskeletal component sustains molecular motors and vectorial movements, it can carry vesicles, cytosolic components, or other cytoskeletal structures to either the apical or basolateral domains of the plasma membrane. The latter is a well known function of microtubules and actin filaments and has been reviewed before [6,7]. Intermediate filaments, on the other hand, do not sustain known molecular motors, and, conceivably, can only contribute to the polarization process by creating polarized scaffolds. While the role of microtubules and actin microfilaments in epithelial polarity has been long and widely recognized, little attention has been paid to the function of intermediate filaments in the generation of polarity and their participation in secretion. This review is intended to summarize current published evidence that indicates that intermediate filaments are an essential part of the complex machinery that generates and maintains epithelial asymmetry, albeit their roles are different from those of their more popular tubulin and actin counterparts.
Polarized subcellular distribution of cytoskeletal components in simple epithelia
1. Actin and tubulin
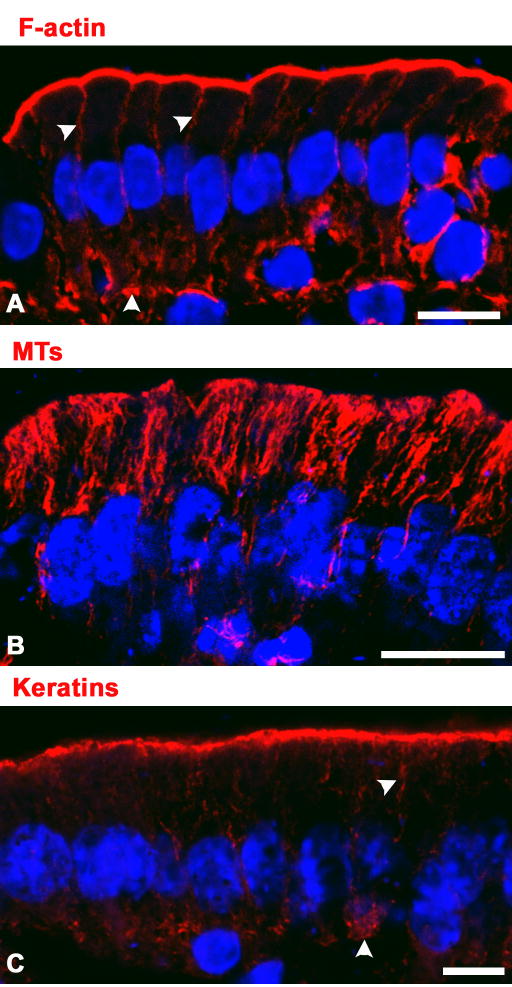
Like in other non-muscle cells, F-actin is strictly cortical (submembrane) in simple epithelia. Because it makes up the core of the apical microvilli, it is concentrated in the apical domain, but a uniform thin layer of F-actin also decorates the inner surface of the basolateral membrane [8] (Fig. 1A, arrowheads). While actin is not polarized, the organization of actin microfilaments, on the other hand, shows examples of striking polarization. Specifically, the actin cores of microvilli are uniquely apical. This is a consequence of highly polarized actin-bundling proteins such as villin, fimbrin and espin [9]. Likewise, other actin-binding proteins such as ezrin [10] and spectrin (fodrin) [11] also form very polarized scaffolds, attaching actin filaments to the membrane. The mechanisms involved in the polarized recruitment of these cytosolic proteins to a specific subdomain of the cortical F-actin network are poorly undestood. It has been proposed that interactions with polarized membrane proteins are responsible for the organization of these actin-based scaffolds [10]. In that case, their polarization should be considered a consequence rather than a determinant of membrane polarity. On the other hand, it has been shown that for some membrane proteins such as the apical anion transporter CFTR [12] and the basolateral ammonium transporter RhBG [13], these actin-based scaffolds represent true anchors that stabilize and enhance polarization.
Figure 1.
Subcellular localization of cytoskeletal components in mouse small intestine epithelium. Small intestines were fixed in formaldehyde, embedded and frozen in OCT and sectioned at −19°C. The sections were stained with fluorescent phalloidin (A), or specific antibodies against α-tubulin (B) or mouse K8 (Troma I, C), all of which are shown in the red channel. The sections were counter-stained with DAPI (blue channel), and villus enterocytes were imaged in a laser-scanning confocal microscope. Bars, 10 μm.
Microtubules (MTs) are asymmetric structures with a plus- and a minus-end [14]. The subcellular distribution of microtubules in simple epithelia is completely different from that of actin. MTs are distributed throughout the cytoplasm in a parallel array, with the minus-ends at the submembrane apical region [15,16] (Fig. 1B). The position of the plus-ends varies in different tissues. In the intestinal epithelium MTs end above the nucleus (Fig. 1B), while in kidney epithelia MTs circumvent the nucleus and the plus-ends are close to the basal aspect of the plasma membrane [17]. In kidney epithelia, a thin, cortical network of MTs is observed at the basolateral domain. These MTs, unlike the bulk of the apically-oriented MTs have both their plus and minus ends attached to the basolateral membrane [18]. In both cases, plus-end tethering molecules such as APC play an important role in polarizing the plus-ends to the basolateral domain [19]. MTs are generally thought to represent the “tracks” that polarized traffic uses to vectorially move to each one of the plasma membrane domains [20]. Because MT-based molecular motors move in specific directions respect to the polarity of the MT [21], the polarized positioning of plus- and minus-ends of MTs is of paramount importance in polarized membrane traffic. A role of IFs organizing the position of minus-ends of MTs will be discussed in the second part of this review.
2. Location, location, location: Apical distribution of intermediate filaments in simple epithelia
Almost 30 years ago, Franke and coworkers first recognized the apical distribution of IFs in the intestinal epithelium [22]. A thick layer of IFs lies immediately below and within the terminal web, at the rootlets of the microvilli [23]. Only thin, isolated bundles of IFs extend along the apical-most half of the lateral domain (Fig. 1C, arrowheads), where they connect with desmosomes [24]. Similar subcellular distributions have been shown in tissue culture cell lines including Madin Darby Canine Kidney cells [25], MCF-10A (human mammary) and CACO-2 (human colon carcinoma) cells [26] as well as in intestine, kidney, uterus, and vas deferens in vivo [27,28,29,30, 31]. In some cases, “cytoplasmic” (e.g. localized 1 μm or more away from the plasma membrane) IFs are observed in simple epithelia, for example in pacreatic acini [32] and in hepatocytes [33]. On the other hand, we have failed to observe “cytoplasmic” keratin IFs in enterocytes [34] (see also Fig. 1C), kidney epithelia [17], CACO-2 (human intestinal) and MCF-10A (human mammary) cells in tissue culture [26]. The apical localization of keratin (K) IFs (see [35] for current K nomenclature) in simple epithelial cells seems to be conserved in evolution, because it is also observed in the gut of Caenorhabditis elegans [36]. It must be emphasized that the subcellular distribution of IFs in simple epithelial cells is very different from that of keratin IFs in non-polarized, multi-layered epithelia, such as in keratinocytes. The non-polarized distribution of IFs in those cells corresponds to the well-known textbook perinuclear localization with IFs ubiquitously present throughout the cytoplasm. Interestingly, IFs are dynamic in these non-polarized cells, moving from the cortical region to the perinuclear area, possibly carried by MT-based motors [37]. Conversely, the molecular basis of the apical polarization of keratin IFs in simple epithelial cells is currently unknown. However, unlike in the case of keratinocyte IFs [37] or neurofilament IFs [38] that move on microtubules, the steady-state, polarized, distribution of keratin IF in simple epithelial cells is not affected by long and extensive depolymerization of MTs, such as in the case of ATP depletion in tissue culture cells, ischemia-reperfusion in kidney proximal tubules [17] or long incubations in anti-microtubular drugs [39]. Moreover, in simple epithelia IFs are not perinuclear, with the exception of a few lateral bundles coming in contact with the lateral aspect of the nucleus (Fig. 1C).
While in most simple epithelial tissues keratin IFs are highly polarized to the apical domain, some exceptions must be noted. Aside of the IFs extending along the lateral membrane, a faint but distinct basal network was originally shown by Franke and coworkers at the basal pole of enterocytes [22] (Fig. 1C, arrowhead), and may be related to the presence of hemidesmosomes [40]. Alternatively, it has been shown that focal adhesions may act polymerizing keratin IFs [41]. It is difficult to conceive how this mechanism may be related to the bulk of the apical IFs, but it may be related to the small basal population of IFs.
It is well known that keratins are obligate heterodymers of one type I and one type II keratin. In simple epithelia, the most common type I keratins are K18 and K19, and the most common type II is K8 [42]. It is worth noting that the subcellular distribution of K8-18 pairs is slightly different from that of K8-19 pairs. The former are less polarized and form the IFs present at the lateral domain, and, when present (e.g. in pancreatic acinar cells) cytoplasmic IFs as well [32]. The K8-19 pairs, on the other hand, are better polarized to either the apical domain in CACO-2 tissue culture cells [43], mouse enterocytes [44], or to the apico-lateral cortical region in acinar cells [32].
Simple epithelia are not always polarized. In tissue culture they acquire polarity after seeding, in vivo they polarize after proliferation from a pool of stem cells, such as in the intestine or they polarize during embryo development. In all three instances, examples are known to show that the apical distribution of IFs precedes the polarization of actin filaments or microtubules. In a tissue culture cell line that polarizes slowly through a period of several days, such as CACO-2 cells [45], keratin IFs are apically polarized by day 3 after seeding, while the brush border begins to be organized after day 5 [34]. Likewise, MTs acquire their polarized orientation only at a late stage of MDCK cell polarization [46]. In the small intestine, the epithelium is maintained by a small population of stem cells located near the bottom of the crypts. Epithelial cells migrate along the crypt and then along the villi to desquamate from the tip of villi 3-4 days after leaving the stem cell niche. During that period they polarize and differentiate [47]. All the stages of differentiation are, therefore, visible along the cryptvillus axis. In this system, keratin IFs are apically polarized from the very early stages at the bottom of the crypts, while the brush border organizes later, when the cells exit the crypts. In parietal cells in the stomach epithelium, IFs are polarized before the formation of apical actin-based canaliculi during fetal development [48].
Functional significance of intermediate filaments in epithelial polarity and exocytosis
The understanding of the functions of IFs has lagged behind our knowledge of microtubules or actin filaments because of three experimental difficulties inherent to IFs: 1) lack of good anti-IF pharmacological agents; 2) lack of mainstream biochemical approaches; and 3) lack of powerful genetic model systems such as yeast and Drosophila, and with the exception of C. elegans discussed below.
In the last decades of the 20th Century, the basic functions of microtubules and actin filaments were extensively explored using highly specific, powerful anti-microtubular drugs such as colchicine or nocodazole, or anti-F-actin drugs such as cytochalasins or latrunculin. In contrast, anti-IF agents, including anti-phosphatases [49] or acrylamide [50], have too broad or too restricted effects respectively to be useful for functional experiments.
Standard biochemical procedures such as immunoprecipitation, pull-down, co-sedimentation, and chromatography, are normally applied to soluble supernatants and not to insoluble pellets. Microtubules readily disassemble during standard cell or tissue extraction in the cold due to their dynamic instability [51] and, thus, MT-associated proteins become available to biochemical approaches. Likewise, at least 30% of total actin is soluble in epithelial cells and a fraction of cortical actin filaments depolymerize rapidly when ATP is depleted [52]. IFs, on the other hand, are highly insoluble [53] and resistant to ATP depletion [17]. As a consequence, most of the IF-associated proteins are expected to remain in pellets and to be unavailable for immunoprecipitation, pull-down, co-sedimentation, or chromatography in regular cell homogenates. Our laboratory developed a technique to immunoprecipitate large (up to 1500 S) multiprotein complexes from the cytoskeletal pellet by fine homogenization with ultrasound. The procedure is not quantitative, but we demonstrated that the complexes are a representative random sampling of the insoluble cytoskeletal fraction [43]. More importantly, although it does not provide information on whether co-immunoprecipitating proteins interact directly or indirectly, it allows to differentiate between proteins in the actin scaffolds from proteins in the IF-based scaffolds, specially when applied to cells treated with actin depolymerizing drugs [34].
It is well known that microtubules and actin filaments are conserved in evolution in all eukaryotes. Cytoplasmic IFs (i.e. non-lamins), on the other hand, are present only in metazoans, with the notable exception of arthropods [54]. In the last two decades, a bounty of knowledge about functions of microtubules and actin filaments, their mechanisms of polymerization and related proteins, was obtained in yeast and Drosophila. Conversely, functional experiments with cytoplasmic IFs have been restricted to the phenotype of transgenic mice and human disease resulting from IF protein mutations [55,56]. These experimental and observational analyses lack the power of genetics in model systems. In addition, and especially pertinent to epithelial IFs, keratins are encoded by a large family of genes [35] commonly expressed in redundant patterns [42] which, along with plasticity in the expression of keratins, may obscure the knock-out phenotype [57]. Therefore, phenotypes may not become appartent in transgenic knock-outs, even when the protein under study is, indeed, functionally important. Moreover, in transgenic systems it is often difficult to obtain detailed mechanistic information about the molecular events that result in a particular phenotype. Most of the information described in the next sections related to the functions of IFs in the subcellular localization of Microtubule Organizing Centers (MTOCs), the organization of the apical domain, and exocytosis, was obtained analyzing the phenotype of transgenic mice.
1. Role of keratin IFs in the subcellular localization of Microtubule Organizing Centers (MTOCs) and the polarized architecture of microtubules
MTs are known to facilitate apical exocytosis and secretion, possibly because vesicles involved in apically-bound membrane traffic use minus-end directed motors. KIFC3 carries Annexin XIIIb apically-bound vesicles for constitutive exocytosis [58]. Minus-end directed kinesins and dyneins are also involved in apical secretion [59,60,61]. Therefore, the apical localization of the minus-ends of microtubules is expected to be functionally more important for apical exocytosis and secretion than the apico-basal architecture of the microtubular apparatus, that has been shown to be dispensable for apical polarization [46]. Furthermore, it must be pointed out that the minus-ends of microtubules are unstable and need to be capped [62]. Although there is consensus that the microtubules in simple epithelial cells are non-centrosomal, how they are nucleated (i.e. what type of MTOC generates the minus-ends) and how the minus-ends are stabilized has been a matter of an as yet unsolved controversy. The original model proposed by Karsenti and coworkers based on very compelling evidence depicted epithelial microtubules nucleated away from the centrosomes, in multiple cortical nucleation sites [63]. Cytoplasmic, non-centrosomal nucleation of microtubules has been reported by other groups as well [64,65]. Such a cytoplasmic nucleation is thought to be carried out by isolated (i.e. non-centrosomal) γ–tubulin ring complexes (γ–TurC), multiprotein 27 S complexes that contain γ–tubulin as well as other proteins, and can nucleate one single microtubule [66]. In addition, γ–TurCs can also cap and stabilize the minus-ends of MTs [67]. An alternative model has been proposed, suggesting that acentrosomal MTs are released from the centrosome [68] and then the minus-ends are stabilized by ninein [69].
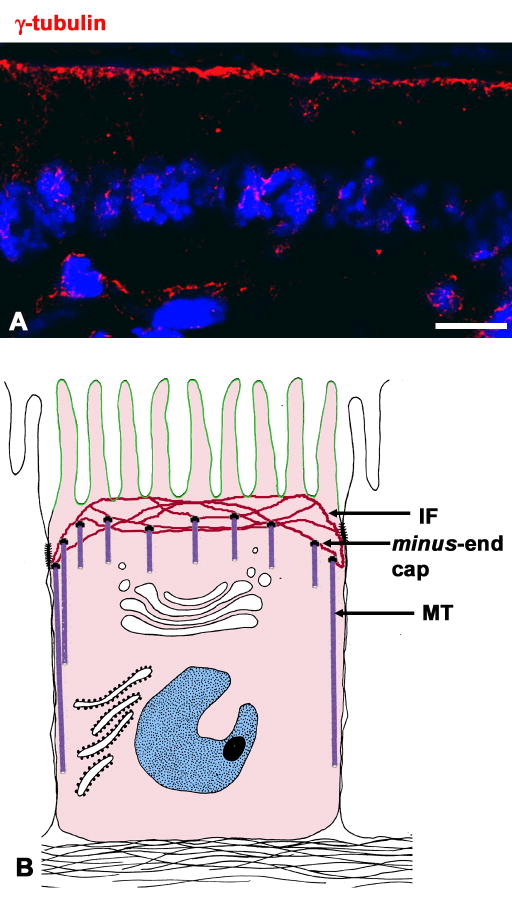
Without making any argument in favor of any particular model, it is clear that, in any case the localization of MTOCs will be a factor in the localization of the minus ends. In the first model, minus ends would be fully localized by the position of the γ–TurCs. In the second model, the minus-end generator, the centrosome, would be expected to spread newly nucleated MTs in its vicinity. Thus, the position of the putative minus-end protein cap would be of paramount importance. Centrosomes are known to be located under the apical domain in simple epithelial cells, instead of a perinuclear localization as in other cell types [43,70,71,72,73]. Equally important, apical layers of non-centrosomal γ–tubulin, presumably γ–TurCs, have been observed in vivo in kidney epithelia [17], in intestinal epithelia [28] and in epithelial cells in tissue culture [43] (Fig. 2A). This predominantly apical distribution of non-centrosomal γ–tubulin is also observed in the gut of C. elegans [74] indicating conservation in evolution. In the villus enterocytes, only 20% of the cells have centrosomes [75], yet all of them display an apical layer of non-centrosomal γ–tubulin (Fig. 2A). Only a few γ–tubulin puncta are visible in the basolateral domain (Fig. 2A, arrowheads) and have been shown to cap the minus-ends of a small subpopulation of basolateral MTs [18]. Therefore, the apical localization of the caps of the minus-ends, either isolated γ–TurCs, centrosomes, ninein, or, perhaps other proteins, is likely to be a major determinant of the positioning of the minus-ends (Fig. 2B) and, thus, an important determinant of polarized membrane traffic.
Figure 2.
Subcellular localization of γ-tubulin (a marker of MTOCs) in mouse small intestine. (A) Mouse intestine frozen sections were obtained, processed and imaged as described in Fig. 1, but using an anti-γ-tubulin antibody (red channel). Bar, 10 μm. (B) A model of MT distribution and minus-end cap localization modified from [63]. Depending on the still controversial interpretation by different groups the caps can be either γ-TurCs or a specific capping protein.
In general, a case has been made that IFs mediate cross-talk among other components of the cytoskeleton [76]. Several laboratories have shown that centrosomes colocalize with IFs in various cell types [43,77,78,79]. Likewise, overexpressed keratin accumulates around the centrosome [80]. Furthermore, this colocalization can be disrupted by phosphorylation mediated by recombinant active Cdk1(p34cdc2)/cyclin B in interphasic cells, and is naturally lost in mitotic cells. Cdk1 phosphorylates a 190 kDa protein that co-immunoprecipitates with γ–tubulin [39]. In addition, the apical localization of centrosomes and non-centrosomal γ–TurCs is abrogated by keratin knock-down in tissue culture cells [43] as well as in villus enterocytes in K8-null mice [28]. In both cases, the apico-basal orientation of MTs is severely disrupted. GCP6, a 190 kDa component of the γ–TurC in vertebrates [81], was found to interact directly with K8 and K19 in yeast two-hybrid assays and blot overlays. GCP6 knock-down, overexpression of the keratin-binding domain, and a point mutation in the Cdk1 phosphorylation site disrupt the localization of MTOCs as well as the architecture of MTs in epithelial cells in culture, making it a good candidate to mediate the attachment of MTOCs to IFs [44]. Other interactions between intermediate filaments may participate in the organization of the microtubular architecture. For example, molecular motors (kinesins and cytoplasmic dyneins) have been found associated to type III IFs [82]. Such an interaction has not been demonstrated for keratins yet, but remains an interesting possibility. Likewise, plectin is a well known cytolinker expressed in epithelia that can connect microtubules to IFs [83]. One can only speculate that the order and stability achieved by epithelial microtubules must include complex interactions with other proteins. This area of Cell Biology is still poorly understood and will require future investigations. What is clear is that IFs not only participate, but are strictly necessary for the apical distribution of MTOCs and the organization of MTs in simple epithelial cells. Keratin knock-downs in tissue culture cells [43] and the phenotype of the K8-null mice enterocytes [28] coincided to show loss of the apical γ–tubulin layer and disorganization (not depolymerization) of MTs with loss of the apico-basal arrangement. Yet, full mechanistic explanations for these phenotypes are still missing.
2. Role of keratin IFs in epithelial polarization
Various groups have shown that K8-null mice show decreased expression of apical proteins in the apical membrane. In some cases, mispolarization or intracellular localization was observed, suggesting that membrane traffic, rather than transcriptional/translational defects, was at culprit. In general, the effects were observed in epithelia where K7, a type II keratin redundant to K8, was not expressed. In villus enterocytes, we observed lack of apical syntaxin 3, and intracellular localization of CFTR, sucrase isomaltase, and alkaline phosphatase [28]. Partial loss of H+,K+-ATPase, and basolateral redistribution of AE1/2 and EnaC-γ was observed in the colon [84]. Reduction of apical membrane proteins was also found in the bile canaliculus [28,85]. Endothelial cells in a vimentin-null phenotype, show aberrant expression and distribution of surface molecules [86]. Once again, the phenotypes are striking, but the underlying mechanistic explanations remain obscure.
Several non-excluding mechanisms may account for the loss of apical membrane polarity in IF knockouts. One obvious possibility is that the changes in MT architecture and in the polarity of γ–TurCs, described in the previous section, may result in mis-targeted membrane traffic. Vimentin seems to be involved in the incorporation of proteins into lipid rafts [87,88], a mechanism deemed as an essential sorting pathway for apical membrane proteins [89]. Although an equivalent mechanism for keratin IFs has not been described, it remains a potential mechanism to be tested in future experiments. Another possibility is that membrane proteins may bind directly to IFs, which may be the case for polycystin-1 [90], a protein that, in addition to its well-known apical cilium localization, also concentrates around desmosomes [91]. Other examples are the interaction between aquaporin and filensin [92] and a 334-aa cancer-associated protein with the structure of a membrane protein that colocalizes with IFs [93]. It is also conceivable, that IFs may serve as scaffolds for extrinsic membrane proteins that, in turn interact with membrane proteins. An example of this possibility came from the observation of the phenotype of transgenic mice overexpressing K8. Strikingly, those animals presented, among other characteristics, an extensive atrophy of the intestinal brush border. An analysis of the subcellular localization of ezrin showed it bound to the abnormal cytoplasmic IFs while lacking under the apical membrane [34]. Ezrin is known to connect actin to the PDZ protein EBP50, and to membrane proteins [10], interactions that aid in the retention of apical membrane proteins such as CFTR [12]. More data will be necessary to determine the relevance of these and other possible mechanisms in the function of IFs.
Caenorhabditis elegans provided the original data that led to the discovery of Partitioning Deficient genes (PAR-1 to 5), now considered essential determinants of epithelial polarity [94]. Unlike arthropods, nematodes have IF genes encoding cytoplasmic products [95]. C. elegans has six known IF genes coexpressed in the intestine (B2, C1, C2,D1, and D2). Although single knock-downs lack phenotypes, possibly because of the usual keratin redundancy, double or triple knock-downs show intriguing phenotypes: the B2/D1/E1 RNAi phenotype strongly resembles the LET-413 (an ortholog of Scribble) knock-down. In addition, it also has common features with the pep-2 mutant (PEP-2 protein is an apical peptide transporter in the nematode intestine) [96]. Like in vertebrates, the phenotype strongly indicates a function in epithelial polarity, but work on the mechanistic details is also missing.
3. Is there a role of keratin IFs in vectorial exocytosis and secretion in epithelial cells?
Exocrine vectorial secretion is carried out by some epithelial cells and involves protein synthesis, packaging into vesicles, and vesicular transport / fusion to the apical domain, which is often regulated by hormonal signals [97]. It is broadly accepted that vectorial secretion requires the cytoskeleton. While this general statement would be understood by many as relating to MTs, IFs may also play a still poorly charaterized functional role in secretion. Similar considerations apply for vectorial exocytosis, that is the constitutive, apically-bound membrane traffic. IFs are necessary for Golgi localization [98], although this function may be an indirect consequence of the organization of MTs described in the first section. Keratin overexpression models in transgenic animals have shown unexpected secretory phenotypes. Ectopic expression of keratins in pancreatic endocrine cells critically affected the number of insulin secretory vesicles [99]. More importantly, transgenic mice overexpressing K8 displayed drastic decreases in trypsin secretion [100]. While the latter was accompanied by a strong inflammatory reaction with loss of acini, the origin of the pancreatitis remains unexplained, and the authors speculated that it may be related to the loss of polarized distribution of the zymogen granules. In the light of the loss of polarity described in previous paragraphs, it seems appropriate to ask whether loss of vectorial exocytosis may be part of the explanation of the pancreatitis in K8-overexpressing animals. As in many other aspects of the issues described in this review further investigation may be necessary. Keratin 8 and 18 knock-out mice, on the other hand do not seem to be more prone to pancreatitis than control animals, and stimulated enzymatic secretion was indistinguishable from controls [32]. Yet, the same authors found a significant 3-fold increase in basal amylase secretion that also remains unexplained. Interestingly, a report in adrenal tumor cells that express or not vimentin showed a similar effect: the cells that express vimentin do not secrete apoE protein, while the vimentin-null cells secrete. Because all the cells had similar levels of mRNA, the authors concluded that the difference was post-translational, perhaps due to rapid degradation of the apoE protein that fails to be secreted in the vimentin-containing cells [101].
The literature offers a few clues for potential molecular links between keratin IFs and apical exocytosis and secretion. One possibility is that the excessive and delocalized IFs retain essential proteins away from the apical domain, as in the case of ezrin described in the previous section. To support this notion, it is worth pointing out, for example, that vimentin filaments can be a reservoir for the SNARE protein SNAP 23 [102]. It is conceivable, although still unproven, that enlarging a reservoir for SNAREs necessary for membrane fusion may deplete the surface content of these proteins. Another intriguing interaction of vimentin filaments that still remains to be tested for keratin intermediate filaments, is their capability to bind yet another molecule involved in vesicular traffic, the adaptor complex AP-3 [103, 104, 105]. Whether IFs could be a reservoir of molecules involved in traffic, or an interaction between trafficking vesicles and IFs may be an as yet unsuspected step in their way to or from the apical domain of epithelial cells remains the matter of future investigations.
Conclusions and future directions
Barely ten years ago, intermediate filaments were considered inert cords holding the cells together because of their remarkable mechanical properties. Today, we know that they are dynamic, mobile, and, in the case of simple epithelia, also polarized. Although the lack of powerful genetic models has handicapped the field, the study of transgenic mice indicates that IFs play roles in microtubule organization, apical polarization and secretion in epithelia. We still lack detailed mechanistic models, but the data suggest that keratin IFs as well as other IFs in other cell types, are also potent scaffolds, capable of compartmentalizing proteins within the cytoplasm. For simple epithelia, such a compartment is highly concentrated under the apical domain. The scaffolding properties of keratins may be direct, as in the case of dormant ezrin [34] or the γ–TurC component GCP6 [44], or mediated by heat-shock proteins that bind keratin IFs [106]. Members of the 14-3-3 family also bind IFs [107,108], which, in turn, opens the possibility that IFs may localize signaling molecules. In fact, the latter has been already reported both in epithelia [109] and non-epithelial cell types [110]. Finally, RNA localization and compartmentalization within the cytoplasm is yet another possible way to generate subcellular asymmetry. IFs have been shown to play a role in RNA localization and polarization in neurons [111], monocytes [112], and keratin-expressing frog oocytes [113,114,115].
The field of intermediate filaments in polarity is still in its infancy, and, therefore, we do not know what combinations of these or other mechanisms will account for the polarity phenotypes observed so far. Accordingly, this review is opening more questions than it answers. However, it is hoped that it will serve to intrigue investigators, to induce them to include IFs in their hypotheses and test them in their experiments. And above all, that it will stop researchers from tossing IFs along with associated proteins in the insoluble pellet after every homogenization.
Acknowledgments
Supported by NIDDK grants RO1DK057805 and R01DK076652. Andrea S. Oriolo was a recipient of a scholarship from Department of Defense training grant 4-49497-LS-HSI, and Flavia A. Wald was a recipient of a Crohn and Colitis Foundation of America post-doctoral award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 4.Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- 5.La Carbona S, Le Goff C, Le Goff X. Fission yeast cytoskeletons and cell polarity factors: connecting at the cortex. Biol Cell. 2006;98:619–631. doi: 10.1042/BC20060048. [DOI] [PubMed] [Google Scholar]
- 6.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 8.Hofer D, Jons T, Kraemer J, Drenckhahn D. From cytoskeleton to polarity and chemoreception in the gut epithelium. Ann N Y Acad Sci. 1998;859:75–84. doi: 10.1111/j.1749-6632.1998.tb11112.x. [DOI] [PubMed] [Google Scholar]
- 9.Bartles JR. Parallel actin bundles and their multiple actin-bundling proteins. Curr Opin Cell Biol. 2000;12:72–78. doi: 10.1016/s0955-0674(99)00059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 11.Woroniecki R, Ferdinand JR, Morrow JS, Devarajan P. Dissociation of spectrin-ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia. Am J Physiol Renal Physiol. 2003;284:F358–F364. doi: 10.1152/ajprenal.00100.2002. [DOI] [PubMed] [Google Scholar]
- 12.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 13.Lopez C, Metral S, Eladari D, Drevensek S, Gane P, Chambrey R, Bennett V, Cartron JP, Le Van Kim C, Colin Y. The ammonium transporter RhBG: requirement of a tyrosine-based signal and ankyrin-G for basolateral targeting and membrane anchorage in polarized kidney epithelial cells. J Biol Chem. 2005;280:8221–8228. doi: 10.1074/jbc.M413351200. [DOI] [PubMed] [Google Scholar]
- 14.Wade RH, Hyman AA. Microtubule structure and dynamics. Curr Opin Cell Biol. 1997;9:12–17. doi: 10.1016/s0955-0674(97)80146-9. [DOI] [PubMed] [Google Scholar]
- 15.Bacallao R, Antony C, Dotti C, Karsenti E, Stelzer EH, Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989;109:2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mogensen MM, Tucker JB, Stebbins H. Microtubule polarities indicate that nucleation and capture of microtubules occurs at cell surfaces in Drosophila. J Cell Biol. 1989;108:1445–1452. doi: 10.1083/jcb.108.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wald FA, Figueroa Y, Oriolo AS, Salas PJI. Membrane repolarization is delayed in proximal tubules after ischemia/reperfusion: possible role of Microtubule-Organizing Centers. Am J Physiol Renal Physiol. 2003;285:F230–240. doi: 10.1152/ajprenal.00024.2003. [DOI] [PubMed] [Google Scholar]
- 18.Reilein A, Yamada S, Nelson WJ. Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. J Cell Biol. 2005;171:845–855. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilein A, Nelson WJ. APC is a component of an organizing template for cortical microtubule networks. Nat Cell Biol. 2005;7:463–473. doi: 10.1038/ncb1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musch A. Microtubule organization and function in epithelial cells. Traffic. 2004;5:1–9. doi: 10.1111/j.1600-0854.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 21.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 22.Franke WW, Appelhans B, Schmid E, Freudenstein C, Osborn M, Weber K. The organization of cytokeratin filaments in the intestinal epithelium. Eur J Cell Biol. 1979;19:225–268. [PubMed] [Google Scholar]
- 23.Fath KR, Mamajiwalla SN, Burgess DR. The cytoskeleton in development of epithelial cell polarity. J Cell Sci Suppl. 1993;17:65–73. doi: 10.1242/jcs.1993.supplement_17.10. [DOI] [PubMed] [Google Scholar]
- 24.Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez ML, Brignoni M, Salas PJI. A specifically apical sub-membrane intermediate filament cytoskeleton in non-brush-border epithelial cells. J Cell Sci. 1994;107:3145–3151. doi: 10.1242/jcs.107.11.3145. [DOI] [PubMed] [Google Scholar]
- 26.Salas PJI, Rodriguez ML, Viciana A, Vega-Salas DE, Hauri HP. The apical sub-membrane cytoskeleton participates in the organization of the apical pole in epithelial cells. J Cell Biol. 1997;137:359–375. doi: 10.1083/jcb.137.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quaroni A, Calnek D, Quaroni E, Chandler JS. Keratin expression in rat intestinal crypt and villus cells. Analysis with a panel of monoclonal antibodies. J Biol Chem. 1993;266:11923–11931. [PubMed] [Google Scholar]
- 28.Ameen NA, Figueroa Y, Salas PJI. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J Cell Sci. 2001;114:563–575. doi: 10.1242/jcs.114.3.563. [DOI] [PubMed] [Google Scholar]
- 29.Bachman S, Kriz W, Kuhn C, Franke WW. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry. 1983;77:365–394. doi: 10.1007/BF00490899. [DOI] [PubMed] [Google Scholar]
- 30.Olson GE, Winfrey VP, Blaeuer GL, Palisano JR, NagDas SK. Stage-specific expression of the intermediate filament protein cytokeratin 13 in luminal epithelial cells of secretory phase human endometrium and peri-implantation stage rabbit endometrium. Biol Reprod. 2002;66:1006–1015. doi: 10.1095/biolreprod66.4.1006. [DOI] [PubMed] [Google Scholar]
- 31.Regadera J, Espana G, Roias MA, Recio JA, Nistal M, Suarez-Quian CA. Morphometric and immunocytochemical study of the fetal, infant and adult human vas deferens. J Androl. 1997;18:623–636. [PubMed] [Google Scholar]
- 32.Toivola DM, Baribault H, Magin T, Michie SA, Omary MB. Simple epithelial keratins are dispensable for cytoprotection in two pancreatitis models. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1343–1354. doi: 10.1152/ajpgi.2000.279.6.G1343. [DOI] [PubMed] [Google Scholar]
- 33.Harada M, Strnad P, Resurreccion EZ, Ku NO, Omary MB. Keratin 18 overexpression but not phosphorylation or filament organization blocks mouse Mallory body formation. Hepatology. 2007;45:88–96. doi: 10.1002/hep.21471. [DOI] [PubMed] [Google Scholar]
- 34.Wald FA, Oriolo AS, Casanova ML, Salas PJI. Intermediate filaments interact with dormant ezrin in intestinal epithelial cells. Mol Biol Cell. 2005;16:4096–4107. doi: 10.1091/mbc.E05-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Furden D, Johnson K, Segbert C, Bossinger O. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodeling and tubulogenesis in the intestine. Dev Biol. 2004;272:262–276. doi: 10.1016/j.ydbio.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Woll S, Windoffer R, Leube RE. Dissection of keratin dynamics: different contributions of the actin and microtubule systems. Eur J Cell Biol. 2005;84:311–328. doi: 10.1016/j.ejcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Yan Y, Brown A. Neurofilament polymer transport in axons. J Neurosci. 2005;25:7014–7021. doi: 10.1523/JNEUROSCI.2001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueroa Y, Wald FA, Salas PJI. p34cdc2 mediated phosphorylation mobilizes microtubule organizing centers from the apical intermediate filament scaffold in Caco-2 epithelial cells. J Biol Chem. 2002;277:37848–37854. doi: 10.1074/jbc.M207037200. [DOI] [PubMed] [Google Scholar]
- 40.Fontao L, Dirrig S, Owaribe K, Kedinger M, Launay JF. Polarized expression of HD1: relationship with the cytoskeleton in cultured human colonic carcinoma cells. Exp Cell Res. 1997;231:319–327. doi: 10.1006/excr.1996.3465. [DOI] [PubMed] [Google Scholar]
- 41.Windoffer R, Kolsch A, Woll S, Leube RE. Focal adhesions are hotspots for keratin filament precursor formation. J Cell Biol. 2006;173:341–348. doi: 10.1083/jcb.200511124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 43.Salas PJI. Insoluble γ–tubulin-containing structures are anchored to the apical network of intermediate filaments in polarized CACO-2 epithelial cells. J Cell Biol. 1999;146:645–657. doi: 10.1083/jcb.146.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oriolo AS, Wald FA, Canessa G, Salas PJI. GCP6 Binds to Intermediate Filaments: a Novel Function of Keratins in the Organization of Microtubules in Epithelial Cells. Mol Biol Cell. 2007 doi: 10.1091/mbc.E06-03-0201. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinto M, Robine-Leon S, Appay D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 46.Grindstaff KK, Bacallao RL, Nelson WJ. Apiconuclear organization of microtubules does not specify protein delivery from the trans-Golgi network to different membrane domains in polarized epithelial cells. Mol Biol Cell. 1998;9:685–699. doi: 10.1091/mbc.9.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahuja V, Dieckgraefe BK, Anant S. Molecular biology of the small intestine. Curr Opin Gastroenterol. 2006;22:90–94. doi: 10.1097/01.mog.0000203865.25384.65. [DOI] [PubMed] [Google Scholar]
- 48.Dabike M, Koenig CS. Development of the actin and the cytokeratin cytoskeletons of parietal cells during differentiation of the rat gastric mucosa. Anat Rec. 1999;255:342–352. doi: 10.1002/(SICI)1097-0185(19990701)255:3<342::AID-AR10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 49.Windoffer R, Leube RE. Imaging of keratin dynamics during the cell cycle and in response to phosphatase inhibition. Meth Cell Biol. 2004;78:321–352. doi: 10.1016/s0091-679x(04)78012-7. [DOI] [PubMed] [Google Scholar]
- 50.Shabana AH, Oboeuf M, Forest N. Cytoplasmic desmosomes and intermediate filament disturbance following acrylamide treatment in cultured rat keratinocytes. Tissue Cell. 1994;26:43–55. doi: 10.1016/0040-8166(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 51.Burbank KS, Mitchison TJ. Microtubule dynamic instability. Curr Biol. 2006;16:516–517. doi: 10.1016/j.cub.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 52.Herget-Rosenthal S, Hosford M, Kribben A, Atkinson SJ, Sandoval RM, Molitoris BA. Characteristics of EYFP-actin and visualization of actin dynamics during ATP depletion and repletion. Am J Physiol Cell Physiol. 2001;281:C1858–C1870. doi: 10.1152/ajpcell.2001.281.6.C1858. [DOI] [PubMed] [Google Scholar]
- 53.Parry DA, Steinert PM. Intermediate filaments: molecular architecture, assembly, dynamics and polymorphism. Q Rev Biophys. 1999;32:99–187. doi: 10.1017/s0033583500003516. [DOI] [PubMed] [Google Scholar]
- 54.Erber A, Riemer D, Bovenschulte M, Weber K. Molecular phylogeny of metazoan intermediate filament proteins. J Mol Evol. 1998;47:751–762. doi: 10.1007/pl00006434. [DOI] [PubMed] [Google Scholar]
- 55.Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–122. doi: 10.1016/s0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- 56.Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 57.Lu H, Zimek A, Chen J, Hesse M, Bussow H, Weber K, Magin TM. Keratin 5 knockout mice reveal plasticity of keratin expression in the corneal epithelium. Eur J Cell Biol. 2006;85:803–811. doi: 10.1016/j.ejcb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp Cell Res. 2004;301:50–59. doi: 10.1016/j.yexcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Ueda N, Ohnishi H, Kanamaru C, Suzuki J, Tsuchida T, Mashima H, Yasuda H, Fujita T. Kinesin is involved in regulation of rat pancreatic amylase secretion. Gastroenterology. 2000;119:1123–1131. doi: 10.1053/gast.2000.18145. [DOI] [PubMed] [Google Scholar]
- 60.Wu K, Jerdeva GV, da Costa SR, Sou E, Schechter JE, Hamm-Alvarez SF. Molecular mechanisms of lacrimal acinar secretory vesicle exocytosis. Exp Eye Res. 2006;83:84–96. doi: 10.1016/j.exer.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Jerdeva G, Yarber FA, da Costa SR, Xie J, Qian L, Rose CM, Mazurek C, Kasahara N, Mircheff AK, Hamm-Alvarez SF. Cytoplasmic dynein participates in apically targeted stimulated secretory traffic in primary rabbit lacrimal acinar epithelial cells. J Cell Sci. 2003;116:2051–2065. doi: 10.1242/jcs.00398. [DOI] [PubMed] [Google Scholar]
- 62.Keating TJ, Borisy GG. Centrosomal and non-centrosomal microtubules. Biol Cell. 1999;91:321–329. [PubMed] [Google Scholar]
- 63.Bré MH, Pepperkok R, Hill AM, Levilliers N, Ansorge W, Stelzer EH, Karsenti E. Regulation of microtubule dynamics and nucleation during polarization in MDCK II cells. J Cell Biol. 1990;111:3013–3021. doi: 10.1083/jcb.111.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vorobjev IA, Svitikina TM, Borisy GG. Cytoplasmic assembly of microtubules in cultured cells. J Cell Sci. 1997;110:2635–2645. doi: 10.1242/jcs.110.21.2635. [DOI] [PubMed] [Google Scholar]
- 65.Yvon AMC, Wadsworth P. Non-centrosomal microtubule formation and measurement of minus end microtubule dynamics in A498 cells. J Cell Sci. 1997;110:2391–2401. doi: 10.1242/jcs.110.19.2391. [DOI] [PubMed] [Google Scholar]
- 66.Moritz M, Agard DA. Gamma-tubulin complexes and microtubule nucleation. Curr Opin Struct Biol. 2001;11:174–181. doi: 10.1016/s0959-440x(00)00187-1. [DOI] [PubMed] [Google Scholar]
- 67.Wiese C, Zheng Y. A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nat Cell Biol. 2000;2:358–364. doi: 10.1038/35014051. [DOI] [PubMed] [Google Scholar]
- 68.Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000;113:3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- 70.Buendia B, Bré MH, Griffiths G, Karsenti E. Cytoskeletal control of centrioles movement during the establishment of polarity in MDCK cells. J Cell Biol. 1990;110:1123–1135. doi: 10.1083/jcb.110.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rizzolo LJ, Joshi HC. Apical orientation of the microtubule organizing center and associated gamma-tubulin during the polarization of the retinal pigment epithelium in vivo. Dev Biol. 1993;157:147–156. doi: 10.1006/dbio.1993.1119. [DOI] [PubMed] [Google Scholar]
- 73.Meads T, Schroer TA. Polarity and nucleation of microtubules in polarized epithelial cells. Cell Motil & the Cytosk. 1995;32:273–288. doi: 10.1002/cm.970320404. [DOI] [PubMed] [Google Scholar]
- 74.Bobinnec Y, Fukuda M, Nishida E. Identification and characterization of Caenorhabditis elegans gamma-tubulin in dividing cells and differentiated tissues. J Cell Sci. 2000;113:3747–3759. doi: 10.1242/jcs.113.21.3747. [DOI] [PubMed] [Google Scholar]
- 75.Komarova YA, Vorobjev IA. Ultrastructural changes in the cell center during enterocyte differentiation in the mouse. Tsitologiia. 1993;35:36–43. [PubMed] [Google Scholar]
- 76.Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–613. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- 77.Trevor KT, McGuire JG, Leonova EV. Association of vimentin intermediate filaments with the centrosome. J Cell Sci. 1995;108:343–356. doi: 10.1242/jcs.108.1.343. [DOI] [PubMed] [Google Scholar]
- 78.Pockwinse SM, Krockmalnic G, Doxsey SJ, Nickerson J, Lian JB, van Wijnen AJ, Stein JL, Stein GS, Penman S. Cell cycle independent interaction of CDC2 with the centrosome, which is associated with the nuclear matrix-intermediate filament scaffold. Proc Natl Acad Sci U S A. 1997;94:3022–3027. doi: 10.1073/pnas.94.7.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mulari MT, Patrikainen L, Kaisto T, Metsikko K, Salo JJ, Vaananen HK. The architecture of microtubular network and Golgi orientation in osteoclasts--major differences between avian and mammalian species. Exp Cell Res. 2003;285:221–235. doi: 10.1016/s0014-4827(03)00033-8. [DOI] [PubMed] [Google Scholar]
- 80.Blouin R, Kawahara H, French SW, Marceau N. Selective accumulation of IF proteins at a focal juxtanuclear site in COS-1 cells transfected with mouse keratin 18 cDNA. Exp Cell Res. 1990;187:234–242. doi: 10.1016/0014-4827(90)90086-p. [DOI] [PubMed] [Google Scholar]
- 81.Murphy SM, Preble AM, Patel UK, O'Connell KL, Dias DP, Moritz M, Agard D, Stults JT, Stearns T. GCP5 and GCP6: Two New Members of the Human γ–Tubulin Complex. Mol Biol Cell. 2001;12:3340–3352. doi: 10.1091/mbc.12.11.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Helfand BT, Chang L, Goldman RD. Intermediate filaments are dynamic and motile elements of cellular architecture. J Cell Sci. 2004;117:133–141. doi: 10.1242/jcs.00936. [DOI] [PubMed] [Google Scholar]
- 83.Leung CL, Green KJ, Liem RK. Plakins: a family of versatile cytolinker proteins. Trends Cell Biol. 2002;12:37–45. doi: 10.1016/s0962-8924(01)02180-8. [DOI] [PubMed] [Google Scholar]
- 84.Toivola DM, Krishnan S, Binder HJ, Singh SK, Omary MB. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J Cell Biol. 2004;164:911–921. doi: 10.1083/jcb.200308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Satoh MI, Hovington H, Cadrin M. Reduction of cytochemical ecto-ATPase activities in keratin 8-deficient FVB/N mouse livers. Med Electron Microsc. 1999;32:209–212. doi: 10.1007/s007959900003. [DOI] [PubMed] [Google Scholar]
- 86.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 87.Friedlander G, Runembert I, Vrtovsnik F, Terzi F. Renal tubular cells cultured from genetically modified animals. Exp Nephrol. 1999;7:407–412. doi: 10.1159/000020638. [DOI] [PubMed] [Google Scholar]
- 88.Runembert I, Queffeulou G, Federici P, Vrtovsnik F, Colucci-Guyon E, Babinet C, Briand P, Trugnan G, Friedlander G, Terzi F. Vimentin affects localization and activity of sodium-glucose cotransporter SGLT1 in membrane rafts. J Cell Sci. 2002;115:713–724. doi: 10.1242/jcs.115.4.713. [DOI] [PubMed] [Google Scholar]
- 89.Delacour D, Jacob R. Apical protein transport. Cell Mol Life Sci. 2006;63:2491–505. doi: 10.1007/s00018-006-6210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu GM, Sikaneta T, Sullivan BM, Zhang Q, Andreucci M, Stehle T, Drummond I, Arnaout AA. Polycystin-1 interacts with intermediate filaments. J Biol Chem. 2001;276:46544–46552. doi: 10.1074/jbc.M107828200. [DOI] [PubMed] [Google Scholar]
- 91.Kottgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflugers Arch. 2005;451:286–293. doi: 10.1007/s00424-005-1417-3. [DOI] [PubMed] [Google Scholar]
- 92.Lindsey Rose KM, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK, Schey KL. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–1570. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- 93.Egland KA, Liu XF, Squires S, Nagata S, Man YG, Bera TK, Onda M, Vincent JJ, Strausberg RL, Lee B, Pastan I. High expression of a cytokeratin-associated protein in many cancers. Proc Natl Acad Sci USA. 2006;103:5929–34. doi: 10.1073/pnas.0601296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kemphues K. PARsing embryonic polarity. Cell. 2000;101:345–348. doi: 10.1016/s0092-8674(00)80844-2. [DOI] [PubMed] [Google Scholar]
- 95.Fridkin A, Karabinos A, Gruenbaum Y. Intermediate filaments in Caenorhabditis elegans. Methods Cell Biol. 2004;78:703–718. doi: 10.1016/s0091-679x(04)78024-3. [DOI] [PubMed] [Google Scholar]
- 96.Karabinos A, Schünemann J, Weber K. Most genes encoding cytoplasmic intermediate filament (IF) proteins of the nematode Caenorhabditis elegans are required in late embryogenesis. Eur J Cell Biol. 2004;83:457–468. doi: 10.1078/0171-9335-00407. [DOI] [PubMed] [Google Scholar]
- 97.Schrader M. Membrane targeting in secretion. Subcell Biochem. 2004;37:391–421. doi: 10.1007/978-1-4757-5806-1_12. [DOI] [PubMed] [Google Scholar]
- 98.Kumemura H, Harada M, Omary MB, Sakisaka S, Suganuma T, Namba M, Sata M. Aggregation and loss of cytokeratin filament networks inhibit Golgi organization in liver-derived epithelial cell lines. Cell Motil Cytoskeleton. 2004;57:37–52. doi: 10.1002/cm.10152. [DOI] [PubMed] [Google Scholar]
- 99.Blassing M, Ruther U, Franke WW. Ectopic synthesis of epidermal cytokeratins in pancreatic islet cells of transgenic mice interferes with cytoskeletal order and insulin production. J Cell Biol. 1993;120:743–755. doi: 10.1083/jcb.120.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casanova ML, Bravo A, Ramirez A, Morreale de Escobar G, Were F, Merlino G, Vidal M, Jorcano JL. Exocrine pancreatic disorders in transgenic mice expressing human keratin 8. J Clin Invest. 1999;103:1587–1595. doi: 10.1172/JCI5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holwell TA, Schweitzer SC, Reyland ME, Evans RM. Vimentin-dependent utilization of LDL-cholesterol in human adrenal tumor cells is not associated with the level of expression of apoE, sterol carrier protein-2, or caveolin. J Lipid Res. 1999;40:1440–1452. [PubMed] [Google Scholar]
- 102.Faigle W, Colucci-Guyon E, Louvard D, Amigorena S, Galli T. Vimentin filaments in fibroblasts are a reservoir for SNAP23, a component of the membrane fusion machinery. Mol Biol Cell. 2000;11:3485–3494. doi: 10.1091/mbc.11.10.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Styers ML, Salazar G, Love R, Peden AA, Kowalczyk AP, Faundez V. The endolysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol Biol Cell. 2004;15:5369–5382. doi: 10.1091/mbc.E04-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Styers ML, Kowalczyk AP, Faundez V. Intermediate filaments and vesicular membrane traffic: the odd couple's first dance? Traffic. 2005;6:359–365. doi: 10.1111/j.1600-0854.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- 105.Styers ML, Kowalczyk AP, Faundez V. Architecture of the vimentin cytoskeleton is modified by perturbation of the GTPase ARF1. J Cell Sci. 2006;119:3643–3654. doi: 10.1242/jcs.03147. [DOI] [PubMed] [Google Scholar]
- 106.Perng MD, Cairns L, van den Ijssel P, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alpha B-crystallin. J Cell Sci. 1999;112:2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- 107.Li H, Guo Y, Teng J, Ding M, Yu AC, Chen J. 14-3-3{gamma} affects dynamics and integrity of glial filaments by binding to phosphorylated GFAP. J Cell Sci. 2006;119:4452–4461. doi: 10.1242/jcs.03219. [DOI] [PubMed] [Google Scholar]
- 108.Ku NO, Liao J, Omary MB. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 1998;17:1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- 110.Roymans D, Willems R, Vissenberg K, De Jonghe C, Grobben B, Claes P, Lascu I, Van Bockstaele D, Verbelen JP, Van Broeckhoven C, Slegers H. Nucleoside diphosphate kinase beta (Nm23-R1/NDPKbeta) is associated with intermediate filaments and becomes upregulated upon cAMP-induced differentiation of rat C6 glioma. Exp Cell Res. 2000;261:127–138. doi: 10.1006/excr.2000.5037. [DOI] [PubMed] [Google Scholar]
- 111.Denis-Donini S, Branduardi P, Campiglio S, Carnevali MD. Localization of calcitonin gene-related peptide mRNA in developing olfactory axons. Cell Tissue Res. 1998;294:81–91. doi: 10.1007/s004410051158. [DOI] [PubMed] [Google Scholar]
- 112.Bocker U, Sirenko OI, Morris JS, Sartor RB, Singer MV, Haskill JS, Watson JM. Expression and localization of IL-1beta mRNA is interrelated with cytoskeletal rearrangement in monocytes stimulated by adherence: a light microscopy in situ hybridization study. Immunol Cell Biol. 2001;79:444–453. doi: 10.1046/j.1440-1711.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- 113.Pondel MD, King ML. Localized maternal mRNA related to transforming growth factor beta mRNA is concentrated in a cytokeratin-enriched fraction from Xenopus oocytes. Proc Natl Acad Sci U S A. 1988;85:7612–7616. doi: 10.1073/pnas.85.20.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bubunenko M, King ML. Biochemical characterization of a cellular structure retaining vegetally localized RNAs in Xenopus late stage oocytes. J Cell Biochem. 2001;80:560–570. doi: 10.1002/1097-4644(20010315)80:4<560::aid-jcb1010>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 115.Kloc M, Wilk K, Vargas D, Shirato Y, Bilinski S, Etkin LD. Potential structural role of non-coding and coding RNAs in the organization of the cytoskeleton at the vegetal cortex of Xenopus oocytes. Development. 2005;132:3445–3457. doi: 10.1242/dev.01919. [DOI] [PubMed] [Google Scholar]