Abstract
The first 15 years, or so, brought the realization that there existed a G protein coupled signal transduction mechanism by which hormone receptors regulate adenylyl cyclases and the light receptor rhodopsin activates visual phosphodiesterase. Three G proteins, Gs, Gi and transducin (T) had been characterized as αβγ heterotrimers, and Gsα-GTP and Tα-GTP had been identified as the sigaling arms of Gs and T. These discoveries were made using classical biochemical approaches, and culminated in the purification of these G proteins. The second 15 years, or so, are the subject of the present review. This time coincided with the advent of powerful recombinant DNA techniques. Combined with the classical approaches, the field expanded the repertoire of G proteins from 3 to 16, discovered the superfamily of seven transmembrane G protein coupled receptors (GPCRs) – which is not addressed in this article – and uncovered an amazing repertoire of effector functions regulated not only by αGTP complexes but also by βγ dimers. Emphasis is placed in presenting how the field developed with the hope of conveying why many of the new findings were made.
1. Introduction
I recently reviewed the step-by-step discovery of signal transduction by G proteins from the first experiments in 1969 in Martin Rodbell’s laboratory at the NIH, in which glucagon binding studies led to the discovery of a GTP dependent step in the activation of adenylyl cyclase, through the realization that there were two systems in which separate GTP binding proteins mediated effects of receptors, the positively and negatively regulated adenylyl cyclases and the light activated phospho-diesterase. The review also told how the three G proteins active in these two systems, Gs, Gi and T (transducin), were found to be αβγ trimers, of which the α subunits of Gs and transducin were GTPases that interacted with their target effectors as GTPα complexes, and that the β and γ subunits existed as dimers that dissociate from activated αGTP to allow for regulation of their effectors (Birnbaumer [1]). That Gi dissociated the same way as Gs, was also known, but how inhibition of adenylyl cyclase came about, still had to be shown. By 1983–1984, signal transduction by the Gs G protein was envisioned as a double regulatory cycle (Fig. 1), in which formation of the αGTP signalling arm was facilitated by the hormone receptor complex, the life-time of the αGTP complex was limited by its intrinsic GTPase activity, and the GDPα so formed reassociated with the βγ dimer, restoring the trimeric state. This positioned the G protein for a new round of activation, if the facilitating receptor was still being activated by ligand (or light).
Fig. 1.
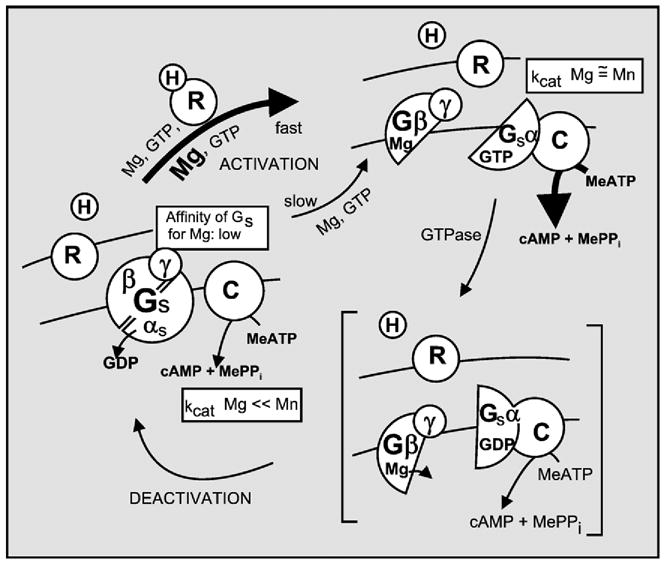
Signal transduction of hormone binding to receptor into activation of adenylyl cyclase by the Gs G protein as understood in 1983. Gs is shown to undergo a GTP–GDP driven subunit dissociation–reassociation cycle, in which the role of the receptor is to keep the system cycling by promoting the Mg2+-induced exchange of GTP for GDP and in which the role of GTPα is both the activation of the enzyme and its own time-limited inactivation that comes about when it hydrolyzes GTP to GDP. (Adapted from Hildebrandt et al., 1984a [119]).
Although by 1983 rhodopsin had been sequenced and deduced to span the plasma membrane seven times (Ovchinnikov, 1982 [2]), it was not yet known that this structure would be the founding member of a super family of receptors. The second member of this family, the β-adrenergic receptor (βAR), would not be cloned, independently by two research groups, until 1986 (Dixon et al., 1986 [3]; Yardeen et al., 1986 [4]). Much of the research on G proteins focused on the functional aspects of the adenylyl cyclase activation process revealing fascinating consequences at the level of the receptors, some of which still today have not been clarified from a molecular or mechanistic viewpoint. Receptors, primarily the β-adrenergic receptor, but others too, were found to change in affinity for ligand when guanine nucleotides (GTP, GMP-P(NH)P, GTPγS) or Mg2+ were added (Maguire et al., 1976 [5]; Lefkowitz et al., 1976 [6]; Iyengar et al., 1890 [7]). An effect of Mg2+ was also shown at the level of adenylyl cyclase and the activation of Gs, notably that high concentrations of Mg2+ mimicked the effect of hormone receptor to accelerate activation of the G protein by the guanine nucleotide. This effect of Mg2+ was first seen in intact membranes (Fig. 2A; Iyengar, 1981 [8]; Iyengar and Birnbaumer, 1981 [9]), then in reconstitution studies in which the activation of the G protein was separated from its interaction with the effector adenylyl cyclase (Fig. 2B; Iyengar and Birnbaumer, 1982 [10]) and, finally, was recapitulated with the purified Gs protein (Fig. 2C; Codina et al., 1984 [11]).
Fig. 2.

(A) Mg2+ mimics the effect of receptor to accelerate the activation of Gs by GMP-P(NH)P (from Iyengar and Birnbaumer, 1981 [9]). (B) Effect of the hormone–receptor complex to reduce the concentration at which Mg2+ facilitates activation of Gs by GMP-P(NH)P. Liver membranes were treated with N-ethyl maleimide to inactivate its adenylyl cyclase and then subjected to a 10-min incubation with GMP-P(NH)P in the absence and presence of glucagon at increasing concentrations of Mg2+ shown on the figure. Gs in the incubated membranes was extracted and analyzed for activity in a cyc− reconstitution assay. Note the hormone induced left shift in the Mg2+ concentration curve. Inset, expansion of lower range of the Mg2+ concentration axis (from Iyengar and Birnbaumer, 1982 [10]). (C) Mg2+ increases the rate at which GMP-P(NH)P activates purified Gs. Purified human erythrocyte Gs was preincubated for varying times with GMP-P(NH)P at varying concentrations of Mg2+. Progressive activation was then quantified in diluted aliquots using the cyc− reconstitution assay. Note that the rate of activation increased as Mg2+ concentration was increased. Top, data are shown with time as the x-axis variable. Bottom, the data are shown with Mg2+ concentration as the x-axis variable (adapted from Codina et al., 1984 [11]).
Taken together these studies showed that the effect of receptor to stimulate the GTP–GDP exchange on Gs is mediated by Mg2+ ion, the role of receptor being to lower the EC50 for the action of Mg2+ from ca 10–20 mM – well above the cytosolic concentration of ca. 0.5 mM – to around 10 μM, well below the cytosolic concentration of Mg2+. Thus while Gs by itself was essentially ‘blind’ to ambient Mg2+, it was saturated by ambient Mg2+ under the influence of an agonist-liganded receptor. This caused the release of GDP allowing GTP to enter its binding site on the α subunit and induce the conformational change that leads to subunit dissociation and activation of the adenylyl cyclase (Iyengar and Birnbaumer, 1982 [10]).
2. More trimeric G proteins than Gs, Gi and transducin
Three heterotrimeric G proteins were known by the end of 1983. Two regulated their effector functions by signalling through their α subunits. Whether the third, Gi, a pertussis toxin substrate, also regulated its effector through its α subunit would be a point of some discussion (see below).
That there were more than three G proteins came from studies by Sternweis and Robishaw in Dallas and Eva Neer’s group in Boston, who both set out to purify the PTX substrate (Gi) from brain. ADP-ribosylation reactions with PTX and [32P] NAD+ showed brain membranes to be extraordinarily rich in what appeared to be more than one substrate in the 40 kDa range. Biochemical resolution soon showed that brain had not one, but up to three PTX substrates (Neer et al., 1984 [12]). Of these, the one that migrated fastest in SDS-PAGE gels was by far the most abundant (Sternweis and Robishaw, 1984 [13]; Huff et al., 1985 [14]). Sternweis and Robishaw called the abundant and fast moving G protein Go, for the ‘other’ PTX substrate. This name is still used today.
A provocative article by Karen Halliday in 1984 drew the attention of many of us to the fact that two classes of regulatory GTPases, the ras oncogenes and protein synthesis elongation factors that regulate the stepwise entry of aminoacyl tRNA into the ribosome, were structurally related (Halliday, 1983–1984 [15]). This hinted at the existence of a family of structurally related GTPases with diverse regulatory functions. Heterotrimeric G protein α subunits – transducin, Gs, Gi and Go – might also belong to this family! This was confirmed in Mel Simon’s laboratory at CalTech, where partial amino acid sequences were obtained for α transducin, purified in Simon’s laboratory, and bovine brain Goα, purified by Janet Robishaw in Dallas. Some of the sequences obtained for the two proteins were highly homologous among them and to the ras family of GTPases. These sequences corresponded to the beginning of the 15-amino acid ‘identity box’, KLLLLGA-GESESGKS, of G protein α subunits, which in N-, K-, and H-ras proteins reads KLvvvGAGgvGKS (Fig. 3; Hurley et al., 1984a [16]).
Fig. 3.

Heterotrimeric G protein α subunits are discovered to be structural relatives of other regulatory GTPases. Tα, transducin α; Gα37, Goα: Sc ras1, ras1 of the baker’s yeast Saccharomyces cerevisiae; Ypt1, the Saccharomyces cerevisiae homologue of rab1 (rat brain ras like protein 1) (Adapted from Hurley et al., 1984a [16]).
The first G protein α subunit to be cloned was that of transducin. It was the result of classical cloning efforts of the 1980s: the purification of a protein was followed by sequencing of tryptic fragments by Edman degradation and synthesis of minimally redundant (degenerate) oligonucleotide probes (guessamers) that were used to screen cDNA libraries. Mel Simon at Caltech and Peter Seeburg at Genentech, the latter prodded no doubt by Henry Bourne, each cloned transducin α from retinal cDNa libraries. In February 1985, their papers were independently accepted for publication in different journals (Lochrie et al., 1985 [17]; Medynski et al., 1985 [18]). Once printed, instead of showing the same sequence, the papers showed sequences that were highly similar, but not identical. The Simon laboratory had cloned cone cell transducin and Seeburg’s group had cloned rod cell transducin.
Using guessamers based on the experimentally determined partial amino acid sequence of Goα, Al Gilman’s laboratory cloned a highly homologous cDNA that turned out to be, Oh surprise, Gsα (Robishaw et al., 1986 [19]), identical to the sequence of a cDNA cloned in Shosaku Numa’s laboratory in Kyoto (Nukada et al., 1986a [20]). The two sequences appeared in print at essentially the same time. In 1986 Numa’s group cloned a bovine brain Giα, and Marshal Nirenbeg’s group at the NIH cloned a human brain Giα cDNA (Nukada et al., 1986b [21]; Bray et al., 1987 [22]). The sequences differed slightly, a fact that was ascribed to a species difference. By this time, my laboratory had also established cloning techniques and we screened a human liver cDNA library made in Savio Woo’s laboratory at Baylor College of Medicine. We obtained a Giα-like cDNA, similar but not identical to Nirenberg’s. There were two Giα genes! While our paper on this discovery was in revision at FEBS Letters, Didsbury and Snyderman, at Duke University, published the sequence of a human Giα that was neither ours nor Nirenberg’s: there were three Gi genes!!. We thus changed the title of the paper from there being two non-allelic Giα genes to there being three such genes (Didsbury and Snyderman, 1987 [23]; Suki et al., 1987 [24]).
Goα was eventually also cloned, in not one, but several laboratories (Jones and Reed, 1987 [25]; VanMeurs et al., 1987 [26]; Itoh et al., 1986 [27]). It proved to have two splice variants: Go1 and Go2 (also GoA and GoB) (Hsu et al., 1990 [28]; Strathmann et al., 1990 [29]; Tsukamoto et al., 1991 [30]). A pertussis toxin insensitive homologue of the Giα’s, Gzα, was identified by homology cloning (Fong et al., 1988 [31]; Matsuoka et al., 1988 [32]). A third “sensory”, PTX-sensitive Gα subunit, gustducin, was cloned from taste buds and shown to be a close homologue of visual transducins (McLaughlin et al., 1992 [33]). From the olfactory neuroepithelium, Reed and colleagues cloned Golf-α, a structural and functional homologue of Gsα able to stimulate the olfactory type III adenylyl cyclase (Jones et al., 1988 [34]).
Upon cloning, the Gβ subunit of transducin was found to encode a protein with repetitive segments—now recognized as the WD40 motif also found in other proteins with unrelated functions (van der Voorn and Ploegh, 1992 [35]). Retinal rod transducin Gβ (Fong et al., 1986 [36]) was shown to be the same 340-amino acid protein as expressed in liver (Codina et al., 1986 [37]). One year later a second Gβ was discovered (Fong et al., 1987 [38]; Gao et al., 1987 [39]). The final count of Gβ subunits increased in time to 5, with Gβ1–4 being 82–92% identical among them, and Gβ5 diverging by approximately 50% from the others. Gβ5 is expressed in certain areas of the brain and in photoreceptor cells (Watson et al., 1996 [40]). In the retina, β5 interacts physiologically with the Gγ-like (‘giggle’) domain of RGS9, a multidomain protein with a GTPase activating RGS domain (Makino et al., 1999 [41]; Chen et al., 2000 [42]; vide infra).
As it had been with the α and β subunits, the first γ subunit to be cloned was that of transducin (Hurley et al., 1984b [43]; Yatsunami et al., 1985 [44]). There are now 12 non-allelic mammalian Gγ genes, encoding proteins between 68 and 75 amino acids in length which, in contrast to Gβ subunits, share only between 15 and 30% sequence identity. Gγ subunits form tight complexes with Gβs and confer structural diversity and, very likely, functional diversity that is not yet well understood.
By 1988, the count of G protein α subunit genes stood at 10: Gs, Golf, 3 Gi, 2 Gt, Ggust, 1Go and Gz. Sequence alignment allowed for the identification of invariant residues. These included a 15 amino acid stretch that we labeled “identity box” near the N-terminus and sequences homologous to similar sequence in ras and bacterial elongation factor Tu, involved in guanine nucleotide binding. One of these conserved sequences included a glutamine that in ras is Q61. ras[Q61L] is oncogenic. Gα[QtoL] mutants confer constitutive activity and were found to have reduced intrinsic GTPase activity (e.g., Graziano and Gilman, 1989 [45]) and to be transforming when transfected into cells. The other frequent oncogenic ras mutant is ras[G12V]. The homologous G is located in the identity box.
3. A G protein activates phospholipase Cβ (PLCβ)
3.1. Hydrolysis of phosphoinositides and release of Ca2+ from internal stores: IP3 as a second messenger
Towards the end of 1988, several G proteins had been purified and their functions, subunit structure and composition, had been elucidated. But, there still remained one unknown in the signal transduction by G proteins field: the mechanism by which Ca2+ is mobilized by a group of hormones, neurotrans-mitters, chemokines and secretagogues that also cause phosphoinositide hydrolysis in their respective target cells.
The original observation that eventually led to the discovery of phosphoinositides as targets of a secretagogue-activated phospholipase (phospholipases C), and of IP3 as the second messenger responsible for Ca2+ mobilization, came from a 1953 article by Mabel Hokin and Lowel Hokin (Hokin and Hokin, 1953 [46]). In it they reported that incubation of pancreatic slices with acetylcholine in the presence of 32Pi led to the rapid incorporation of inorganic phosphate into phospholipids. They identified phosphatidylinositols (PIs) as the newly synthesized phospholipids (Hokin and Hokin, 1955 [47]). Phosphatidylinositol (PI), phosphatidylinositol-4-phosphate (PIP) and phosphatidilylinositol-4,5-bisphosphate constitute only 7–8% of plasma membrane phospholipids and of this, 95–98% is PI. For the next 25 years many laboratories worked on the mechanism by which this phenomenon came about. It turned out that even though it was initially thought that acetylcholine stimulated the synthesis of PI, PIP and PIP2, it, in fact, stimulated the hydrolysis of phosphoinositides. What Hokin and Hokin had observed was the incorporation of Pi during re-synthesis of the phosphoinositides. Further, it was discovered that the consequence of this phospholipid-mobilizing effect is a rapid release of Ca2+ from internal stores into the cytosol and the extracellular milleu, followed by a passive uptake phase. This uptake phase was later termed capacitative calcium entry or CCE (Putney, 1986 [48]; Putney, 1990 [49]).
Definitive light on the mechanism by which receptors evoked phosphoinositidehydrolysis and Ca2+ mobilization was shed throughout 1981–1982. Only the tail end of these studies will be mentioned here by highlighting two contributions. One came from John Williamson’s laboratory at the University of Pennsylvania. He addressed the question whether changes in phosphoinositide levels occurred fast enough to account for the rate at which downstream metabolic responses were known to occur. Working with isolated hepatocytes he showed that vasopressin promoted rapid, but transient PIP and PIP2 breakdown. This was accompanied by a transient accumulation of diacylglycerol (DAG), which was indeed within the timeframe required for the known rapid vasopressin-induced activation of liver phosphorylase, its physiologic effect (Fig. 4). Phosphorylase activation in liver is the result of a reaction cascade that can be initiated by cAMP or by Ca2+, whose concentration increases in the cytosol in association with and/or in response to phosphoinositide hydrolysis. Cytosolic Ca2+ activates the PKA- and Ca2+/calmodulin-sensitive phosphorylase kinase, which in turn activates the liver phosphorylase measured in Williamson’s study (Thomas et al., 1983 [50]).
Fig. 4.
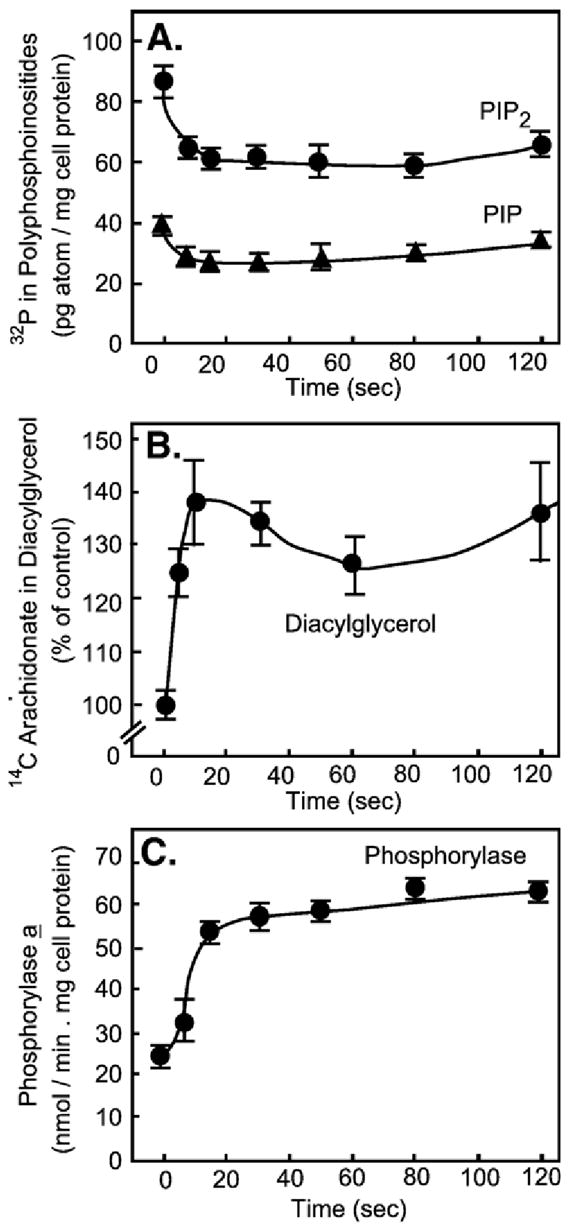
The rates of phosphoinositide mobilization and diacylglycerol formation are consistent with a primary effect of receptor on phospholipase C to promote phosphorylase activation. (Adapted from Thomas et al., 1983 [50]).
Williamson’s work, while pointing to phospholipase C as the likely effector of activated receptor, did not address whether diacylglycerol or one of the inositol phosphates formed concurrently were the second messengers intervening between phospholipid hydrolysis and the next step in the pathway leading to phosphorylase activation. This question was addressed by Michael Berridge in Cambridge (England), where the response of the blowfly’s salivary gland to serotonin was being studied. With Robin Irvine and others, Berridge discovered that the first step in phosphoinositide mobilization was the hydrolysis of PIP2 to 1,4,5-inositol trisphosphate (IP3) and diacylglycerol. Berridge proposed IP3 to be the second messenger responsible for the changes in intracellular Ca2+ concentration that ensued upon exposure of the salivary gland to secretagogues (Berridge et al., 1983 [51]). He went on to demonstrate that IP3 was indeed the intracellular second messenger and that it acted by promoting the release of Ca2+ from intracellular Ca2+ stores. The first of Berridge’s articles to show the second messenger character of IP3 was in collaboration with Irene Schulz in Cologne, Germany. Schulz had a permeabilized pancreatic acinar cell preparation with an active Ca2+ uptake system driven by the sarcoplasmic reticulum endoplasmic reticulum calcium ATPase, also SERCA pump. The permeabilized cells could load their Ca2+ stores upon addition of pyruvate and ATP to the incubation medium, and in so doing lowered ambient, extracellular, free Ca2+. Addition of the IP3 isolated in Berridge’s laboratory to the permeabilized cells resulted in Ca2+ release and an increase in ambient Ca2+, measured with a Ca2+ electrode (Fig. 5; Streb et al., 1983 [52]).
Fig. 5.
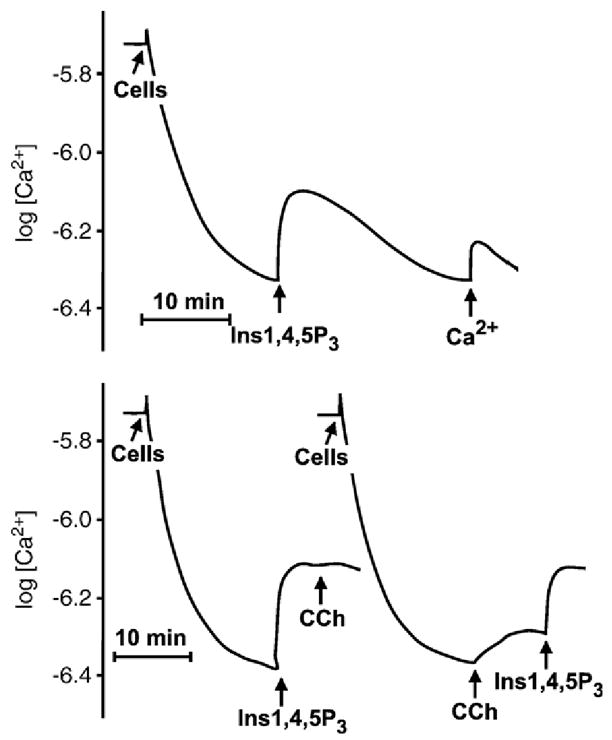
Inositol 1,4,5-tris phosphate (IP3) promotes rapid release of Ca2+ from the stores of permeabilized pancreatic acinar cells. Permeabilized cells were incubated in minimum medium containing physiologic concentration of Ca2+. At the indicated time, ATP was added as an energy source for the cells’ intracellular Ca2+ uptake system, causing ambient Ca2+ to fall. Addition of IP3 caused release of Ca2+ from internal stores — which equilibrated with extracellular Ca2+. As IP3 was hydrolyzed by phosphatases, Ca2+ was re-accumulated in stores. Ca2+ was monitored with a Ca2+ electrode. Traces in the bottom panel show that carbachol, an agonist of the pancreatic muscarinic receptor, mobilizes Ca2+ from the same pool affected by the externally added IP3. Other experiments had previously shown that pancreatic muscarinic receptors cause phosphoinositide hydrolysis and mobilize Ca2+ from internal stores (Adapted from Streb et al., 1983 [52]).
3.2. The G protein-PLC connection
By 1987 a significant amount of data had accumulated to support the idea that a GTP-dependent step was at the heart of the signal transduction mechanism by which secretagogue receptors activated a phospholipase (PLC). These data included stimulation of a low Km GTPase (e.g. Fig. 6; Grandt et al., 1986 [53]; Houslay et al., 1986 [54]), activation of membrane bound PLC by GTPγS (Harden et al., 1987 [55]), and, interestingly, the presence of a GTP shift in binding curves of hormone or chemoattractant to receptors that caused phosphoinositide breakdown and Ca2+ mobilization (e.g., Fig. 7; El-Refai et al., 1979 [56]; Geynet et al., 1980a [57]; Geynet et al., 1980b [58]; Koo et al., 1982 [59]; Evans et al., 1985 [60]). Cockcroft and Gomperts, based on the ability of GTPγS to stimulate conversion of PIP2 to IP3 in isolated neutrophil membranes, proposed the existence of a ‘Gp’, a PLC-activating G protein (Cockroft and Gomperts, 1985 [61]; reviewed in Deckmyn et al., 1990 [62]). Assays that showed a GTP-dependence in the activation of PLC by a receptor seemed doomed to failure and to stand in waiting for the discovery of a system where the biology and/or biochemistry was such that a GTP dependence became clear. This system turned out to be Berridge’s serotonin-activated blowfly salivary gland. The endocrinologist/physiologist John Fain, then at Brown University, became aware of this while spending a sabbatical in Michael Berridge’s laboratory in Cambridge. Upon his return to Brown University and with the help of Irene Litosch, a postdoctral fellow, he proved that indeed PLC activation by the 5-hydroxytryptamine (serotonin) receptor depends on GTP (Litosch et al., 1985 [63]).
Fig. 6.
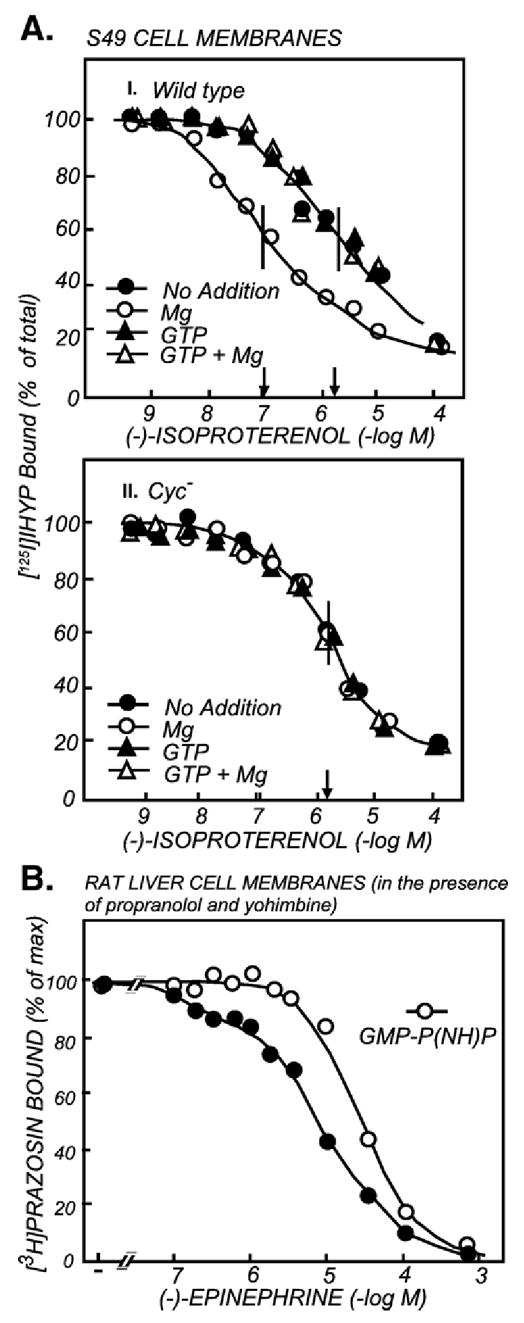
Receptors that activate the Gs-adenylyl cyclase signaling pathway share with receptors that activate the G protein-PLC signaling pathway the property of changing their affinity for agonist upon addition of guanine nucleotides. (A) GTP and Mg regulation of the β-adrenergic receptor (βAR) in membranes with (I) and without (II) the Gs G protein. The figure depicts the competitive inhibition by the βAR agonist (−)-isoproterenol of the binding of the βAR probe 125I-hydroxybenzylpindolol (125I-HYP) to βARs of wild type (I) and cyc− (II) S49 cell membranes. The incubations were carried out in the absence and presence of 5 mM MgCl2, 10 μM GTP and the combination of MgCl2 and GTP. Mg2+ and GTP do not affect the affinity of IHYP for the βAR (not shown). Note: Mg2+ increased the affinity of the βAR for the competing agonist, and GTP, which by itself had no effect, interfered with the effect of Mg2+. This phenomenon is mimicked by non-hydrolizable analogs of GTP and by GDP, and is commonly referred to as the “GTP-shift in agonist affinity”. There are receptors, such as the glucagon receptor (Rojas and Birnbaumer, 1985 [182]), that show high agonist affinity also in the absence of Mg2+ but shift to low agonist affinity when GTP is added. The high affinity state of the βAR is induced by its interaction with the Gs G protein; the shift is absent in cells lacking Gs (AII) (adapted from Hildebrandt et al., 1984b [118]; Hildebrandt et al., 1984a [119]). (B) The non-hydrolyzable GTP analogue GMP-P(NH)P causes a GTP affinity shift in the liver α1-adrenergic receptor known to trigger activation of phosphoinositide hydrolysis and promote Ca2+ mobilization, suggesting the existence of a G protein responsible for phosphoinositide and Ca2+ mobilization (adapted from Good-hardt et al., 1980 [58]).
Fig. 7.
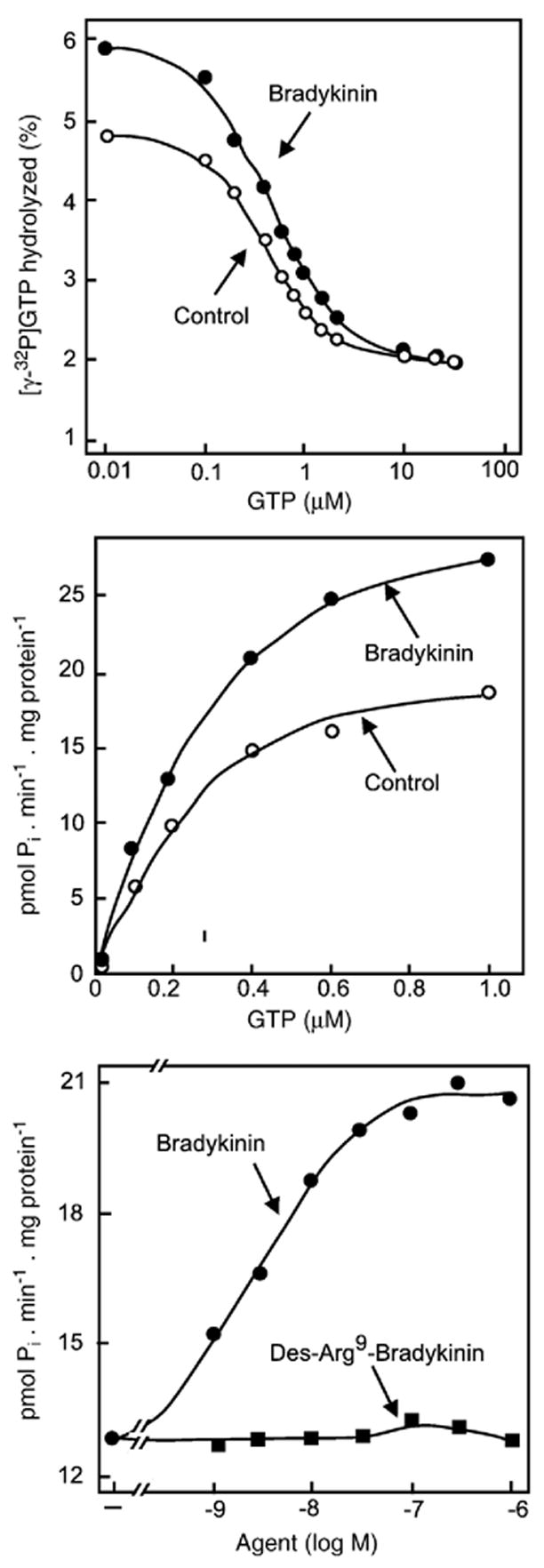
Bradykinin, an agonist that promotes phosphonositide hydrolysis and Ca2+ mobilization, activates a low Km GTPase in membranes from NG108-15 cells. (A and B) Hydrolysis of [γ-32P]GTP in the absence and presence of 1 μM bradykinin as a function of GTP concentration expressed as % [γ-32P]GTP hydrolyzed (A) or pmol of GTP hydrolyzed (B). (C) [γ-32P]GTP hydrolyzed in the presence of 0.3 μM unlabelled GTP as a function of varying the concentrations of the agonist bradykinin or the inactive des-Arg9-bradykinin analogue. (Adapted from Grandt et al., 1986 [53]).
Three research lines led to the identification of “Gp”, the phospholipase-activating G protein. One laboratory set out to purify from liver membranes additional G protein α subunits that would bind to Gβγ immobilized on Sepharose. This led to the purification of a 42 kDa GTP-binding protein of unknown function and unknown identity (Pang and Sternweis, 1990 [64]). The other laboratory, headed by John Exton at Vanderbilt University, developed an assay in which phospholipase C activity in cholate extracts of liver membranes could be activated by addition of GTPγS. He then successfully fractionated the extract by chromatography over heparin-Sepharose, into a GTPγS-insensitive PLC, recovered in a salt eluate, and an ‘activator’, recovered in the initial flow through. Standard protein purification techniques led to the isolation of a heterotrimeric G protein with an α subunit of 42–43 kDa (Taylor et al., 1990 [65]). Bringing the power of recombinant DNA techniques, specifically that of the polymerase chain reaction (PCR), to bear on G protein research, Mel Simon’s laboratory at CalTech identified several additional G protein α subunit candidates, referred to as αq, and α11 through α16 (Strathmann et al., 1989 [66]; Strathmann and Simon, 1990 [67]; Strathmann and Simon, 1991 [68]). The stage was set, with one laboratory, John Exton’s, having a G protein with an α subunit for which the function was known but not its identity; a second laboratory, Paul Sternweis’s in Dallas, having a G protein α subunit for which neither function nor identity was known, and a third laboratory, Mel Simon’s at in Pasadena (California), knowing the identity of novel cDNAs, whose functions were not known and their G protein nature as part of heterotrimers was a conjecture (Fig. 8A).
Fig. 8.

(A) Musings that may have happened during the “making” of the Gq/11 family of G proteins into signal transducers of PLCβ-activating receptors. (B) The Gq/11 signal transduction pathway.
Antibodies raised against unique sequences predicted by the Gαq and Gα11 cDNAs identified the bovine G proteins with 42–43 kDa α subunits purified in Sternwies’s and Exton’s laboratories as a mixture of Gqα and G11α (Taylor et al., 1990 [65]; Smrcka et al., 1991 [69]). Photoaffnity labeling of rat liver plasma membranes with azido-anilido-GTP in the absence and presence of vasopressin, followed by immuoprecipitation and immunoblotting identified the labeled proteins as Gqα and G11α. These experiments proved that these were the ‘Gp’ G proteins activated by the liver (V1a) vasopressin receptor (Wange et al., 1991 [70]). In reconstitution assays it was then shown that the purified PLC-activating proteins stimulated the activity of the class β PLC, but not the γ or δ PLCs, purified from bovine brain by Su Goo Rhee at the NIH (Taylor et al., 1991 [71]). In agreement with these in vitro biochemical reconstitutions of G-protein stimulated PLCβ systems, in cell reconstitutions by transient expression of the cDNAs encoding the interacting components, confirmed the existence of a receptor-G protein-PLCβ signal transduction pathway in which the active G proteins are of the Gq/11 class of G proteins (Fig. 8B; Wu et al., 1992 [72]).
Alignment of the amino acid sequences deduced from the mouse α14 and α15 cDNA’s showed them to be very similar to Gqα, G11α, and G16α, the latter cloned from a human cDNA library. The close structural relationships proved to be a predictors of function. Membrane bound Gq, G11, G14, and G15 α subunits expressed in COS-7 cells were able to stimulate the activity PLCβ that had been purified from bovine brain in Sue Goo Rhee’s laboratory, proving their “Gp” character (Lee et al., 1992 [73]). Recombinant G16α, purified from insect cells infected with recombinant baculovirus, was shown to activate purified bovine brain phospholipase Cβ (Kozasa et al., 1993 [74]) and purified recombinant proteins encoded in G14α (GL1α), G11α (GL2α) and Gqα cDNAs, likewise, activated purified bovine brain phospholipase Cβ (Nakamura et al., 1995 [75]). Independently, G14α and G11α had also been cloned under the names of GL1α and GL2α (Nakamura et al., 1991 [76]). Moreover, when reconstituted into phospholipid vesicles together with the M1 muscarinic receptor, trimeric G14, G11 and Gq bound GTPγS in response to carbachol, proving them to be true signal transducers of a receptor signal (Nakamura et al., 1995 [75]).
Although initially thought to be homologues, molecular cloning of the full length cDNAs showed mouse G15α and human G16α to be orthologues. Surprisingly, and in contrast to other G protein α subunits, G15 and G16 α subunits when expressed in COS7 cells were activated to stimulate phosphoi-nositide hydrolysis not only by receptors that activate the Gq family of G proteins (e.g. thromboxane, 5HT2c, thrombin, V1-vasopressin) but also by receptors that normally activate Gs (e.g. β2-adrenergic, A2-adenosine, D1-dopamine, V2-vasopressin) or Gi (e.g. M2-muscarinic, 5HT1A, μ-opioid, fMLP). G15/16 α subunits differed therefore from other G protein α subunits in that they are specific in terms of their effector system, PLCβ, but promiscuous in terms of the type of receptor by which they are activated (Offermanns and Simon, 1995 [77]).
4. Function of proteins encoded in G12α and G13α cDNAs
The nucleotide sequence of the full length G12α and G13α subunit cDNAs, reported in 1991 (Strathmann and Simon, 1991 [68]), showed them to be very similar and to constitute a fourth class of Gα subunits, joining the Gs/Golf, Gi/Go/Gt and Gq/11 classes of G protein α subunits. Similarities among the 16 cloned G protein α subunits, as revealed by amino acid sequence alignment, are shown in Fig. 9.
Fig. 9.
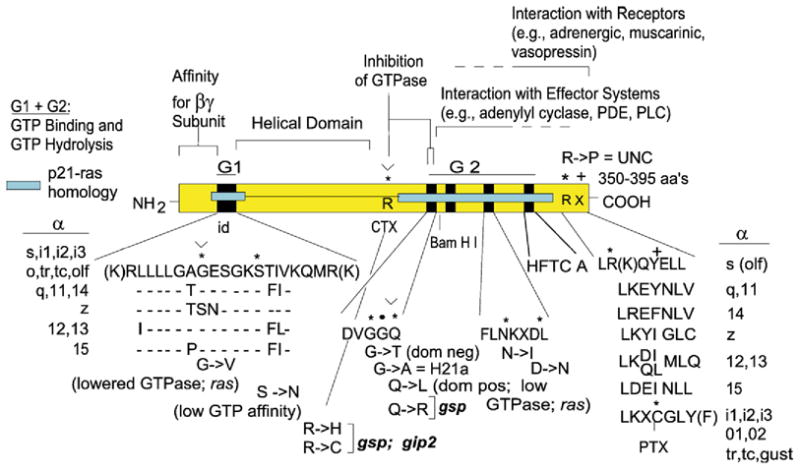
Linear diagram of a consensus open reading frame (yellow box) encoding α subunits of the family of heterotrimeric signal transducing G proteins. Cyan box, sequences of ras for which homologous sequences are present in Gα subunits. id, identity box sequence; its composition is expanded below; the id sequence is identical for αs, αi1-αi3, αt1, αt2, α-olf, αo. Deviant amino acids in αq, 11, 14, 12, 13, and z are shown. Black boxes, regions involved in guanine nucleotide binding. R*, location of the Arg ADP-ribosylated by cholera toxin in αs, α-olf and transducin α. The seven C-terminal amino acids of α subunits are expanded to include the Cys at position-4 which is ADP-ribosylated by pertussis toxin (C*). Several mutations that have been informative for the understanding of the G protein mediated signal transduction process are highlighted: G to V in the GAGES motif reduces GTPase activity; R* to C or H reduces GTPase activity, as does ADP-ribosylation, which allow for receptor independent activation by GTP; Q to L in the DVGGQ motif suppresses GTPase activity and confers transforming activity to mutant α subunits; G to T in the DVGGQ motif confers dominant negative properties; G to A in the DVGGQ motif (H21a mutant in αs, also reverse UNC) impedes activation by GTP or non-hydrolyzable GTPγS, but does not interfere with the G protein mediated GTP shift in receptor-agonist affinity; R to P at position-6 from the C terminus, UNC mutation, uncouples αs form receptor without interfering with activation by non-hydrolyzable GTP analogues. N to I and D to N in the FLNKXD motif change nucleotide association–dissociation dynamics. Mutants of Q in the DVGGQ motif, G in the GAGES motif and R* confer transforming activity in transfection assays and are referred to as gsp and gip when they reside in the αs and αi subunits, respectively.
In 1992, two groups at the NIH discovered independently that G12α has oncogenic (transforming) potential. One group, led by Stuart Aaronson, cloned from a soft tissue sarcoma expression cDNA library a transforming activity that turned out to be an overexpressed but un-mutated, wild type G12α (Chan et al., 1993 [78]). The other group, led by Silvio Gutkind, tested G12α for oncogenic potential in NIH-3T3 cells and found the wild type to be mildly transforming, but the constitutively activated Q229L mutant to be a potent oncogene (Xu et al., 1993 [79]). Expression of constitutively active G12α[Q229L] and G13α[Q226L] mutants were shown to lead to cell shape changes, to activation of the Na+/H+ exchanger, and to promote cell proliferation and transformation in several systems (Jiang et al., 1993 [80]; Dhanasekaran et al., 1994 [81]; Vara Prasad et al., 1994 [82]). A strong hint as to the mechanism by which G12 and G13 α subunits affected cell functions came from a study in which the stress fibers and focal adhesions induced by G12/13α in transfected cells was blocked by botulinum C3 exoenzyme (Buhl et al., 1995 [83]). The C3 exoenzyme is an ADP-ribosyltransferase that specifically ADP-ribosylates and inactivates Rho. Rho, for ras homologue, is a member of the family of small ras-like GTPases. When transfected into cells as a constitutively active Q63L mutant, Rho had the same effects as activated G12/13 α subunits.
It thus appeared that G12/13 activated Rho. Confirmation of this hypothesis came from an independent research line anchored in the study of new class of proteins, referred to as regulators of G protein signaling or RGSs.
4.1. The RGS proteins
RGS proteins constitute a family of about 20 members that were independently discovered in four different types of organisms: the budding yeast Saccharomyces cerevisiae, the nematode Caenorhabditis elegans, the fungus Aspergillus nidulans, and in mammals. In yeast, the SST2 gene product, for supersensitivity 2 (Chan and Otte, 1982b [84]; Chan and Otte, 1982a [85]; Dietzel and Kurjan, 1987a [86]), limits the lifetime of GPA1. Mutant sst2 cells are supersensitive to pheromone because they lack of adaptation, which is now known to be to lack of a GTPase activating function encoded in SST2 that affects GPA1 (Fig. 10A; Dohlman et al., 1995 [87]). In the nematode, egl-10 mutants fail to lay eggs. The frequency of egg laying is under the control of serotonergic motor neurons which activate the postsynaptic smooth muscle sertonin receptor, which in turn is coupled to effector function by a Go-like G protein α subunit, GAO-1. Over-expression of GOA-1 reduces frequency of egg laying. Mutant egl-10 worms have increased egg laying frequency and over-expression of EGL-10 reduces egg laying frequency. The egl-10 mutation is silent in goa-1 mutants (Koelle and Horvitz, 1996 [88]). Taken together these results indicate that EGL-10 down regulates GOA-1. In aspergillus, mutant flba cells proliferate, remain undifferentiated and in time lyse, under conditions in which they should arrest and sporulate. Over-expression of Flba, on the other hand, causes cells to sporulate when they should not. A suppressor mutation in gene fadA, encoding a G protein α subunit homolgue, changes a G to V in a site that in ras reduces GTPase activity and is transforming.
Fig. 10.
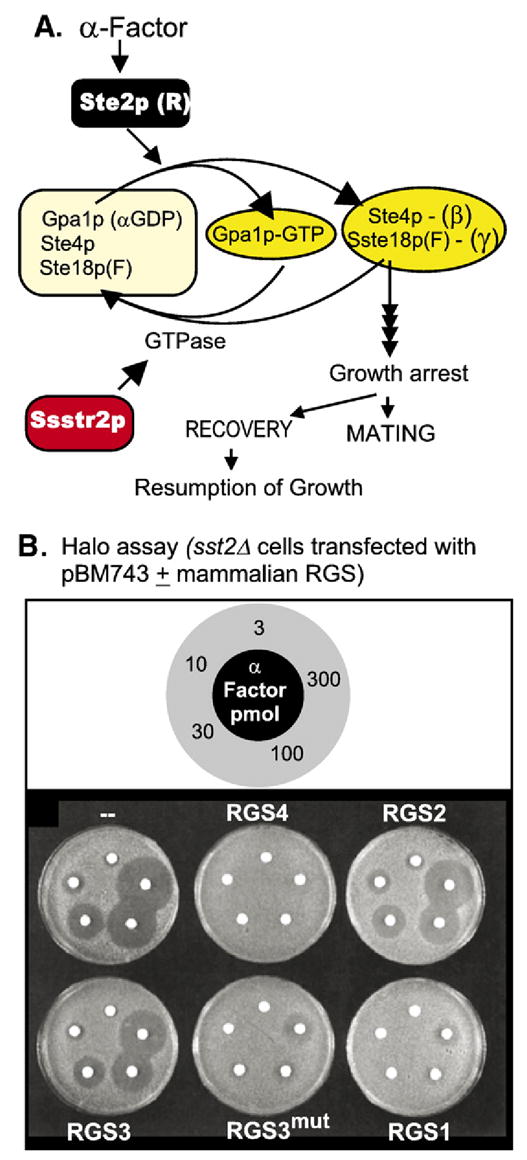
(A) Signaling by mating factor receptors Ste2p and Ste3p, activated by α and a factor, respectively, is transduced in haploid Saccharomyces cerevisiae cells by an αβγ signal transducing G protein encoded in genes GPA1, STE4 and STE18. α or a factor binding to their respective receptors leads to growth arrest. In this G protein coupled system the signaling arm is the βγ dimer (Step:Ste18p (F)) and not the Gpa1p.GTP (αGTP) complex (Jiang et al., 1993 [80]). If mating does not occur, cells resume growth, a phenomenon referred to as adaptation. The SSTE gene product, a GTPase activating protein for Gpa1p, participates in this adaptation process. Ste18p(F), denotes that Ste18p, the product of the STE18 gene, is farnesylated. (B) Mutant loss of function stte2Δ cells are supersensitive to mating factor, seen in the formation of large halos of growth arrested cells. Supersensitivity can be suppressed by expression of mammalian RGS proteins (adapted from Druey et al., 1996 [93]).
SST2, EGL-10 and Flba share sequence homology among them over an approximately 130–150 aa stretch at their C-termini. A similar sequence was also found to be encoded in several mammalian genes: in GAIP, a Gi3α interacting protein identified in a yeast two hybrid screen in Marilyn Farquhar’s laboratory in San Diego (De Vries et al., 1996 [89]); in BL34/1R20, transcribed in chronic lymphocytic leukemia, a B cell leukemia (Hong et al., 1993 [90]; Heximer et al., 1997 [91]); and in GOS8, a concanavalin A- and cycloheximide-induced gene in blood mononuclear cells (Siderovski et al., 1994 [92]). Current names for BL34/IR20, and GOS8 are RGS1 and RGS2. Working together, the molecular immunologist Joel Kehrl at the NIH, and the yeast geneticist Ken Blumer, introduced a brain cDNA expression library into mutant sst2 yeast cells and isolated sst2 suppressors. Among them they identified RGS1, RGS2 and RGS4, which all shared the RGS domain. Suppression of supersensitivity, as seem in α-factor-induced “halo” assays by expression of mammalian RGS proteins is illustrated in Fig. 10B. This proved the existence of a regulatory function conserved from yeast to mammals (Druey et al., 1996 [93]). Work in Dallas demonstrated that GAIP and RGS4 had the ability to accelerate the intrinsic GTPase activity of the Gi family of G protein α subunits (Berman et al., 1996 [94]), including transducin α (Nekrasova et al., 1997 [95]), and also that of Gqα (Hepler et al., 1997 [96]; Huang et al., 1997 [97]). Cloning of homologues and of full length cDNA versions of expressed sequence tags (ESTs) led to the gradual accumulation of the present 20 or so RGS proteins, which have been subclassified into several subfamilies.
4.2. RGS proteins improve temporal coupling of receptor signal to response
As GTPase activating proteins (GAPs), the RGS proteins were initially considered to act simply as negative regulators of G protein signalling. Indeed their discovery in yeast (SST2) and nematode (EGL-10) agree with this function. However they were also found to have a positive effect on the transduction mechanism, in that their action tightens the temporal coupling of receptor signaling to effector. This role was uncovered in two signaling systems, activation of the inwardly rectifying K+ channel (Fig. 11; (Doupnik et al., 1997 [98]; Saitoh et al., 1997 [99]) and visual signal transduction, in which loss of an RGS, RGS9-1, results in a dramatically slowed recovery of rod and cone photoresponses (Chen et al., 2000 [42]; Lyubarsky et al., 2001 [100]). The activation–deactivation of a G protein can mathematically be modeled as a bimolecular reaction by which G protein activation is directly proportional to ligand (hormone) occupancy of the receptor, and G protein deactivation can be collapsed into a first order decay reaction. Such a modeling exercise reveals that indeed accelerating the decay (sum of ligand dissociation and GTPase) results in faster approach to equilibrium and the associated tighter temporal coupling in a G protein mediated transduction process (Fig. 11).
Fig. 11.
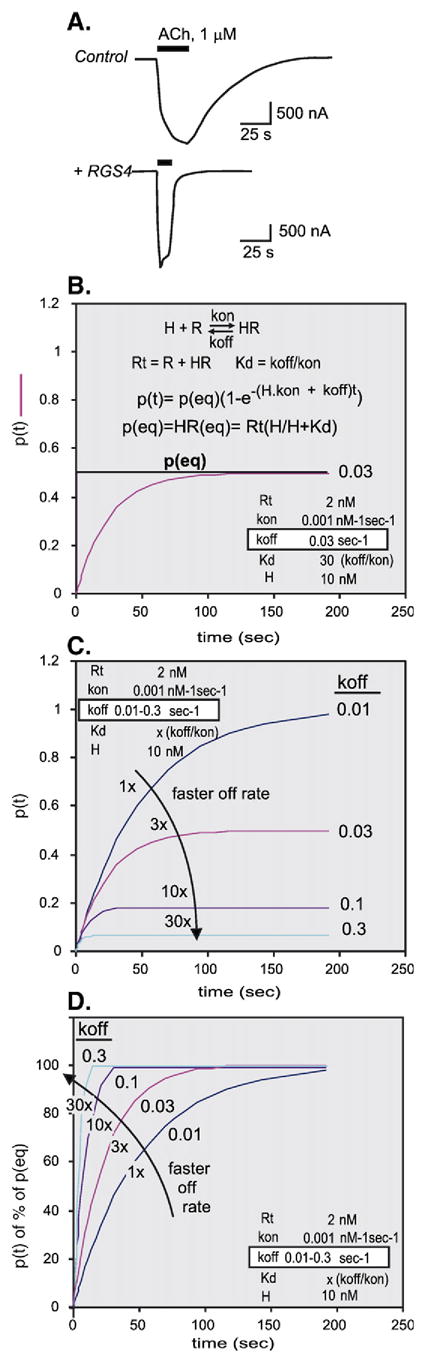
(A) Activation and de-activation of muscarinic inwardly rectifying K+ channels is accelerated by the GTPase activating protein RGS4. The effect of a pulse of acetylcholine on the development of K+ currents was measured by the two electrode voltage clamp method. Top trace, K+ currents evoked by addition of acetylcholine (ACh) during the time-span shown, were recorded in an oocyte that that had been injected with cRNAs encoding the M2 muscarinic receptor and the Kir3.1 and Kir3.2 K+ channel subunits. Bottom trace, K+ currents evoked by acetylcholine in an oocyte that had been injected in addition with cRNA encoding mammalian RGS4. Note faster response to ACh addition and faster deactivation upon ACh wash-out. (Adapted from Doupnik et al., 1997 [98]). (B) Simulation of HR complex formation [p=f(t)] from H and R as a function of time using the formulas and rate constants shown. (C) Same as (B) but calculated for koff values spanning from 0.01 to 30 s−1. Note that at increasing koff equilibrium is reached faster and also, that the p(eq) values decrease. (D) Same as (C) but normalizing by expressing p(t) as a function of its value at equilibrium.
4.3. Mutlifunctionality of RGS proteins: RhoGEFs
Many of the RGS proteins are multifunctional having two or more domains mediating protein:protein interactions, which confer specialized properties to these RGSs. The GGL domain, Gγ-like (“giggle”), is found in RGS 6, 7, 9 and 11, and confers the ability of RGS9 to form a functional dimer with Gβ5 (Makino et al., 1999 [41]). The GoLoco motif found in RGS 12 and 14, interacts with Gi/Go α subunits and acts as a guanine nucleotide dissociation inhibitor towards Giα, trapping the protein in its inactive GDP-liganded state (Kimple et al., 2001 [101]; reviewed in Dohlman and Thorner, 1997 [102] and in Neubig and Siderovski, 2002 [103]). A PDZ domain at the N-terminus of PDZ-RGS3 mediates the bridging between cytosolic domain of EphrinB1 (PDZ ligand) and the Gi protein (RGS target) mediating the chemoattractant response of CXCR4 to extracellular SDF1 (stroma derived factor 1). ‘Reverse’ signaling of the ephrin receptor EhpB, downregulates CXCR4 signalling and releases the cells from control by SDF1, allowing them to respond to a different migrational cue (Lu et al., 2001 [104]). Of interest in the context of this review, is that a search for Rho binding partners in Gideon Bollag’s laboratory at ONYX Pharmaceuticals led to the identification of a candidate having a Dbl domain next to a PH domain (DHPH) (Hart et al., 1996 [105]). This configuration is typical of Rac and Cdc42 guanine nucleotide exchange factors. The protein, p115-RhoGEF was cloned and subsequently shown to facilitate release of GDP from RhoA and to promote its activation by GTP, thus confirming its GEF activity. Analysis of the sequence for known domains identified the presence of an RSG motif.
Tests for a GTPase activating effect of purified recombinant p115-RhoGEF (Hart et al., 1996 [105]) towards recombinant G12α (Kozasa and Gilman, 1995 [106]) or recombinant G13α (Singer et al., 1994 [107]) subunits, carried out in Dallas, showed the p115-RhoGEF RGS domain to be functional, i.e., to activate the GTPase activity of both G12α and G13α (Kozasa et al., 1998 [108]). Possibly more interesting than the discovery of an RGS acting on G12 and G13 α subunits, was that incubation of RhoGEF with G13α, activated with either aluminum fluoride or GTPγS, stimulated its Rho GEF activity. GEF activity was measured by assaying for release of GDP from RhoA. p115-RhoGEF was therefore the missing link between G13 and activation of Rho (Hart et al., 1998 [109]). In contrast to G13α, G12α did not activate p115-GEF’s GEF activity even though p155-RhoGEF was active as a GAP towards G12α (Hart et al., 1998 [109]) Subsequent studies showed that the G12α also fails to activate the GEF activity of as second RhoGEF, PDZ-RhoGEF, which like p115-RhoGEF, is activated by G13α (Tanabe et al., 2004 [110]).
The puzzle as to how G12α promotes activation of Rho in stress fiber and focal adhesion formation assays, in which G12α and G13α are indistinguishable, was solved by Tohru Kozasa in his new laboratory in Chicago. He completed the cloning of a third RGS motif-bearing candidate RhoGEF, the leukemia-associated RhoGEF or LARG, and was able to show that it is activated by G12α, provided that LARG is primed by tyrosine phosphorylation. This phosphorylation is mediated by a tyrosine kinase of the Tec family of tyrosine kinases (Fig. 12; Suzuki et al., 2003 [111]). In this context, the earlier somewhat perplexing findings that both Gqα (Bence et al., 1997 [112]) and G12α (Jiang et al., 1998 [113]), aided by Gβγ interacting with the PH domain of the Btk kinase (Tsukada et al., 1994 [114]) stimulate Btk, also a Tec family kinase, acquired new meaning. It suggests the formation of a multiprotein signaling complex having the specific function of insuring that upon activation of Gq, there is an associated activation of the Rho signaling pathway. So far, whenever tested, receptors that activate Gq, also activate G12/13 with concomitant activation of the Rho signaling pathway (reviewed in Wettschureck and Offermanns, 2005 [115]). Signal transduction through the G12/13 G proteins, incorporating the roles of Gqα, Gβγ and Tec kinases, is summarized in Fig. 13.
Fig. 12.
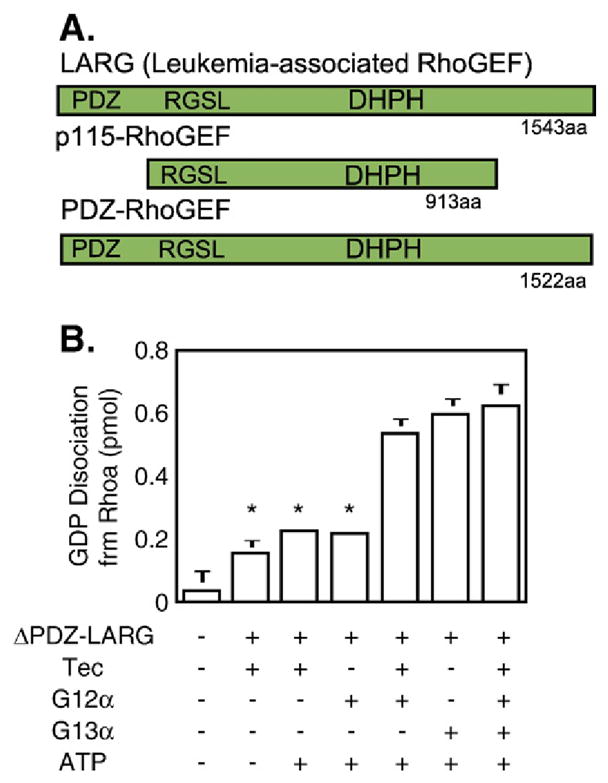
Stimulation of LARG’s guanine nucleotide exchange activity by G12α depends on Tec kinase, but that of G13α does not. Top: linear diagram of RhoGEF proiesn with relative location of the DHPH GEF domains, the RGSL domain (for extended RGS domain) and, when present, the PDZ domain. Bottom, in vitro stimulation of GDP release from RhoA by recombinant G12α and G13α in the absence and presence of Tec kinase and ATP. Tec was prepared by expressing a myc-tagged Tec cDNA in COS cells and concentrating the expressed protein by immunoprecipitation. G12α, G13α and LARG (without its N-terminal PDZ domain) were purified from Sf9 insect cells infected with the corresponding recombinant baculovirus. For further details see (Suzuki et al., 2003 [111]).
Fig. 13.
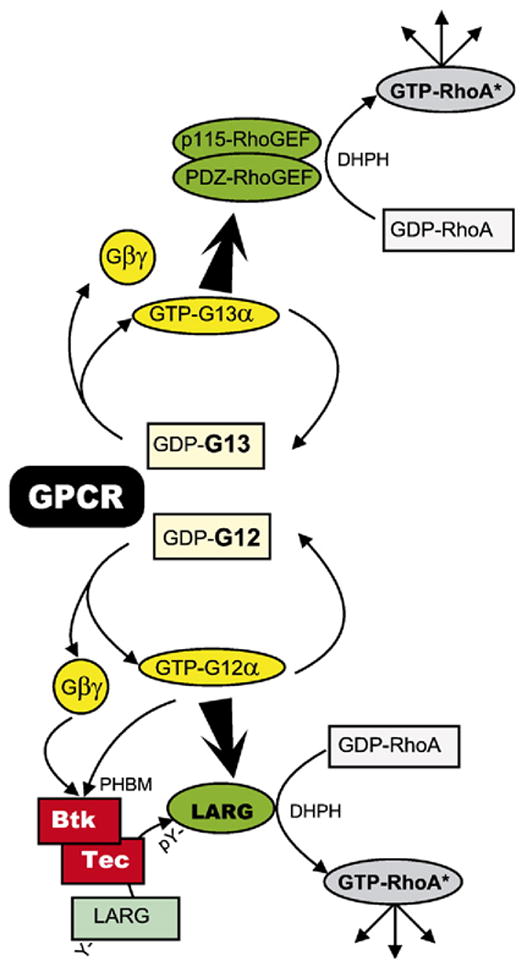
The G12/13 signaling pathway. Rho-GEF activities involve their DHPH domains. DH, Dbl-domain; PH, PH domain; BM, Btk domain; Y, Tyrpsine; pY, phosphotyrosine. G12α and Gβγ are shown as activators of the Tec kinases Tec and Btk. The interaction has been shown to involve the PHBM domain of the Tec kinase (Tsukada et al., 1994 [114]; Langhans-Rajasekaran et al., 1995 [183]; Jiang et al., 1998 [113]).
5. Roles for Gβγ dimers in transmembrane signalling
The acceptance that, as a signaling molecule, the Gβγ dimer is in fact an equal partner with the Gα subunit was slow in coming and, at the beginning, marred by controversies generated both by differences in data interpretation and by reports with conflicting results. Up to the time when Gi was purified, the effects of activated G proteins – transducin and Gs – had been mediated by α subunits. Unexpectedly, Gilman and collaborators proposed Gβγ as mediator of hormonal inhibition of adenylyl cyclase activity. This proposal was based on titration studies that evaluated the effect of adding exogenous Gβγ to wild type S49 cell membranes (Katada et al., 1984a [116]; Katada et al., 1984b [117]). Since added Gβγ inhibited activity stimulated by GTP–Gsα, it seemed that Gi-derived Gβγ might be acting by mere reversal of the Gs activation reaction:
This was coupled, no doubt, to an initial inability to reconstitute inhibition of adenylyl cyclase with activated Gi α subunits. In contrast, experiments by John Hildebrandt in my laboratory, showed that the irreversibly activated Gsα* reconstituted (activated) cyc− membrane adenylyl cyclase with the same potency when their Gi was unactivated or irreversibly activated (Hildebrandt et al., 1984b [118]). We proposed that AC was likely to have independent binding sites for Giα and Gsα (Hildebrandt et al., 1984a [119]). Eventual reconstitution of inhibition by activated Giα (Taussig et al., 1993 [120]) and co-crystallization of the two-lobed catalytic domain of adenylyl cylase with Giα (Dessauer et al., 1998 [121]) fully supported John Hildenbrandt’s conclusions. Thus, regulation by α subunit prevailed.
Studies on the mechanism of action of pheromones in baker’s yeast, Saccharomyces cerevisiae, uncovered a completely different signaling architecture from the one that was being uncovered studying signal transduction by G proteins in mammalian systems. In haploid yeast cells, type α and type a pheromones trigger a developmental program that leads to cell cycle arrest and preparation for mating. Pheromones α and a bind to pheromone receptors Ste2p and Ste3p on a and α cells, respectively. The STE2 and STE3 gene products, Ste2p and Ste3p, activate a heterotrimeric G protein of subunit composition Gpa1p:Ste4p:Ste18Fp (α:β: γ). Activation of the G protein then triggers cell cycle arrest. Genetic inactivation of GPA1 was found to be lethal, as cells fail to traverse the cell cycle, suggesting that pheromone receptor signaling is mediated by the Gβγ and not the Gpa1p α subunit (Dietzel and Kurjan, 1987b [122]). This idea received support from further genetic studies. The lethality of mutant gpa1 was relieved by mutation of either STE4 or STE18 (Fig. 10A) and over-expression of Ste4p mimicked loss of Gpa1p, causing cell cycle arrest and promotion of mating behavior (Whiteway et al., 1990 [123]).
Signalling in yeast was viewed by some, including myself, as peculiar to lower eukaryotic life. But, it turned out not to be so. A second, this time successful attempt for Gβγ dimers at penetrating the signalling field of the animal kingdom happened as a consequence of results obtained by Eva Neer, David Clapham and collaborators, studying the activation of the cardiac inwardly rectifying K+ channel (Kir channel) by muscarinic receptors (Logothetis et al., 1987 [124]). Previous studies on the regulation of the muscarinic K+ channel (KirACh) had shown that it proceeded by a membrane delimited mechanism without involvement of a soluble second messenger (Soejima and Noma, 1984 [125]), and that the transduction mechanism involved a PTX-sensitive G protein that could be activated with a non-hydrolyzable analogue of GTP (Pfaffinger et al., 1985 [126]; Breitwieser and Szabo, 1985 [127]). The experiments by Neer, Clapham and collaborators showed activation of the K+ channel by purified brain Gβγ added to inside-out membrane patches, under conditions in which GTPγS-activated Giα (Giα*) failed to activate the channel.
As a result of a collaboration between my laboratory and that of Arthur M. Brown at Baylor College of Medicine, we contested the validity of these results. While in our hands the inwardly rectifying K+ channel was stimulated by activated human erythrocyte Gi, i.e. a mixture of Gβγ and GTPγS-Giα (that had been extensively dialyzed to remove free nucleotide; Yatani et al., 1987 [128]), addition of the resolved components –GTPγS-Giα* or Gβγ – showed no effect of Gβγ dimers and robust activation by the GTPγS-Giα* complex (Codina et al., 1987 [129]). In fact, the K+ channel was not only activated by the natural, purified human erythrocyte mix of Giα subunits (mostly Gi2α and Gi3α), but also by GTPγS-activated recombinant Giα1, Giα2 and Gi3α (Yatani et al., 1988 [130]). Moreover, not only did our Gβγ not activate the channel, but it inhibited channel activity in patches with spontaneously active channels and in patches with channels that had been activated with carbachol plus GTP. The reason for this discrepancy has not been clarified.
Experiments from other laboratories confirmed the data of Neer, Clapham and collaborators, and failed to confirm ours (e.g., Ito et al., 1992 [131]; Kofuji et al., 1995 [132]; Nair et al., 1995 [133]). This gave the ‘upper hand’ to Gβγ dimers as executing signalling molecules in higher eukaryotes. It should be noted that: 1. the cardiac ATP-inhibited inwardly rectifying K+ channel (KirATP), which we also reported to be activated by our Giα* preparations (Kirsch et al., 1990 [134]), is indeed activated by Giα under conditions where the muscarinic channel is not (Ito et al., 1992 [131]; Terzic et al., 1994 [135]); 2. β5-containing Gβγ dimers have been found to differ from β1 through β4 containing Gβγ dimers in that instead of activating, they inhibit KirACh channel activity (Lei et al., 2000 [136]) 3. βγ dimers can be inactivated by an endogenous mono-ADP-ribosyltransferase that derivatizes Gβ at Arg-129 (Lupi et al., 2000 [137]; Lupi et al., 2002 [138]). We know neither the subunit composition nor the ADP-ribosylation status of the human erythrocyte Gβγ dimers used in our 1987–1989 studies. With respect to the KirATP it is currently thought that both Gi/oα and Gβγ stimulate the channel, composition Kir6: :SUR2, by releaving inhibition by ATP, and that they do so by interacting directly with the regulatory SUR subunit (Terzic et al., 1994 [135]; Wada et al., 2000 [139]).
The third, this time uncontested case in which Gβγ dimers were shown to be a bonafide signalling arm of mammalian activated heterotrimeric G proteins was stimulation of phospholipase Cβ by the chemoattractant peptide formyl-Met–Leu–Phe (fMLP). Evidence had accumulated throughout the mid and late 1980s for the existence in human, rabbit and guinea pig neutrophils and in human leukemic HL60 cells, of both a PTX-sensitive and a PTX-insensitive G protein-coupled pathway leading from receptor to phosphoinositide breakdown and Ca2+ mobilization. The PTX-insensitive pathway was mediated by the Gq/11 class of G proteins described above. The data for a role of a G protein in the action of fMLP included presence of a GTP shift in fMLP binding curves to human neutrophil membranes (Koo et al., 1982 [59]), rapid mobilization of intracellular Ca2+ in neutrophils in response to fMLP (Yano et al., 1983 [140]), rapid breakdown of neutrophil PIP and PIP2 in response to fMPL (Volpi et al., 1983 [141]), requirement of GTP for activation of phosphoinositide hydrolysis by fMLP in isolated human polymorphonuclear membranes (Smith et al., 1985 [142]), and inhibition of the effects of fMLP by PTX (Brandt et al., 1985 [143]). The identity of the PTX-sensitive ‘Gp’ was then predicted by Michio Ui’s laboratory to be either Gi or Go, as either of these proteins purified from bovine brain was able to reconstitute fMLP-stimulated IP3 formation in PTX-treated HL60 cell membranes (Kikuchi et al., 1986 [144]).
That the activator of the neutrophil and HL60 cell phospholipase C was Gi-derived βγ dimer – HL60 cells do not express Goα – came from experiments in Peter Gierschik’s laboratory, then in Heidelberg, Germany. As a follow up to his novel observation that cytosolic PLC activity could be activated by addition of GTPγS (Camps et al., 1990 [145]), GTPγS was added together with Gβγ. HL60 cell cytosol had previously been shown to contain a limited amount of soluble PTX-sensitive α subunits, detectable when ADP-ribosylation reactions were fortified by addition of Gβγ dimers (Bokoch et al., 1988 [146]). Expecting that the kinetics with which GTPγS activated cytosolic PLC might be improved by addition of Gβγ, Gierschik and colleagues added Gβγ purified from bovine retinas, and indeed saw an enhanced PLC activity over that obtained with GTPγS alone. But, surprisingly, the transducin βγ dimers also stimulated PLC activity in the absence of GTPγS. Moreover, addition of GDP-loaded transducin α prevented the βγ dimer from stimulating PLC activity (Fig. 14). Gβγ purified from bovine brain had the same effect. The cytosolic PLC activity was fractionated by Q-Sepharose chromatography into two fractions of which one was and the other was not activated by Gβγ (Camps et al., 1992 [147]).
Fig. 14.
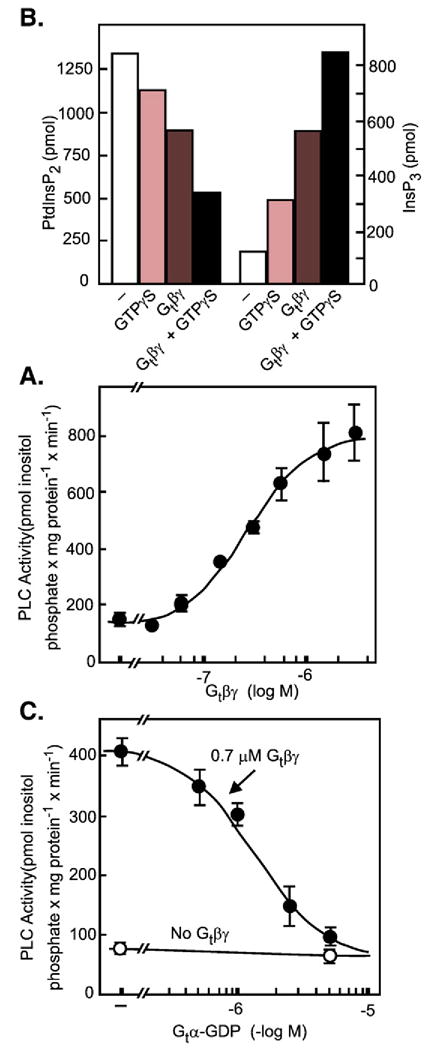
Stimulation of PLC activity by G protein βγ dimers. (A) Either GTPγS or transducin βγ (Gtβγ) stimulate phosphinositidase activity in a high speed cytosol supernatant from differentiated HL60 cells. Formation of inositol 1,4,5-trisphosphate (InsP3) correlates with hydrolysis of labeled phosphatidyl-4,5-bisphosphate (PtdInsP2) added to the incubations. (B) Dose–response relation for the stimulatory effect of transducin βγ. (C) The effect of transducin βγ is prevented by increasing concentrations of GDP-liganded transducin α (Gtα-GDP). (Adapted from Camps et al., 1992 [147]).
Subsequent work identified the cytosolic Gβγ-responsive PLC as PLCβ, the same enzyme that is also activated by Gαq/11, and revealed that the original observation that the cytosolic PLC activity is stimulated by GTPγS (Wada et al., 2000 [139]), was unrelated to heterotrimeric G protein regulation. The effect of GTPγS had its root in the fact that PLCβ is also activated by the Rac/Cdc42 members of the Rho family of low molecular weight GTPases (Fig. 15; Illenberger et al., 1998 [148]). This makes PLCβ a point of convergence of three separate signaling mechanisms: activation by Gq/11 acting through their α subunits, activation by Gi G proteins acting through their βγ dimers, and activation by the Rho family of GTPases, which are in turn activated by yet a third set of regulatory factors, including downstream effects of integrin ligation that alter cytoskeletal structures. It should be noted that different PLCβ isoforms respond differently to different Gβγ isoforms. For example, β1γ1/2 stimulates PLCβ3 better than PLCβ2, while β5γ2 stimulates PLCβ2 very well but barely affects the activity of PLCβ3 (Illenberger et al., 2003 [149]).
Fig. 15.
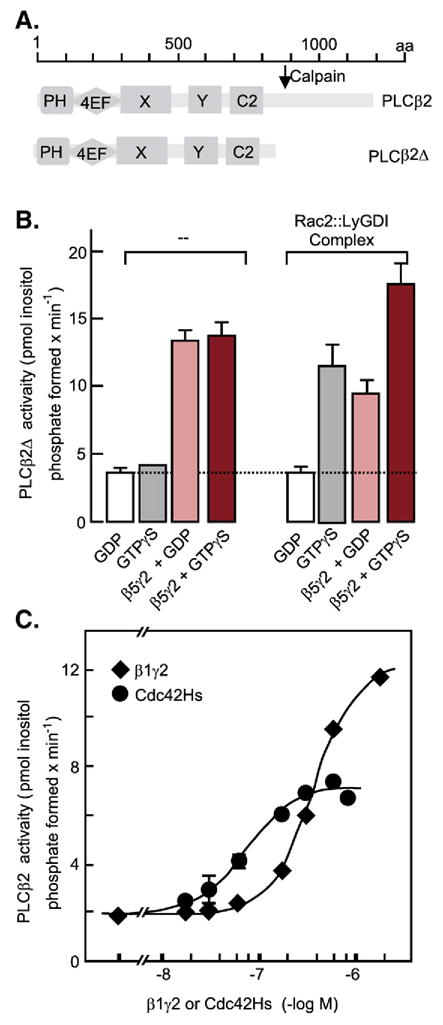
Stimulation of recombinant PLCβ activity by the Rac2 and Cdc42Hs members of the Rho family of GTPases. (A) Diagram of domain distribution along the PLCβ polypeptide chain. PH, plekstrin homology domain; 4EF, four EF hand folds, X and Y catalytic domains; C2, Ca2+-binding C2 domain. (B) Recombinant PLCβ2Δ, lacking the C-terminal portion that confers responsiveness to Gq/11α subunits, was incubated with substrate and recombinant Rac2: :LyGDI dimers in the presence of GDP, GDP+ recombinant β5γ2 dimers, GTPγS, or GTPγS+ recombinant β5γ2 dimers. (C) Recombinant PLCB2Δ was incubated with substrate and increasing concentrations of either recombinant β1γ2, or recombinant Cdc42Hs. Recombinant proteins were synthesized in SF9 cells infected with the corresponding recombinant baculoviruses, extracted, and purified to homogeneity by conventional techniques. For details see Illenberger et al., 2003 [149]. (Adapted from Figs. 9A, 8B and 7B) in Illenberger et al., 2003 [149]).
Given that GPCRs “proofread” G proteins for not only the nature of their α subunit, but also those of the β and γ subunits (Kleuss et al., 1991 [150]; Kleuss et al., 1992 [151]; Kleuss et al., 1993 [152]), it is likely that different GPCRs exhibit differing potencies regarding phosphoinositide mobilization and Ca2+ signaling. These differences would be set by the cellular complement of subunit isoforms that make up the G proteins interacting with the receptors, and the PLCβ subtypes that are expressed by the target cell.
With the ice broken, signalling through Gβγ dimers was then looked for and discovered at a fast pace. The newly cloned adenylyl cyclases, which was a focus of Gilman’s laboratory at the beginning of the 1990s, were shown all to be activated by Gsα, but to fall into one of three categories regarding regulation by Gβγs (Fig. 16). One was found to be inhibited by βγ dimers (ACI), another to be stimulated by βγ dimers (ACII and ACIV) and a third was unaffected by βγ dimers (ACV and ACVI) (Tang and Gilman, 1991 [153]). These categories of adenylyl cyclases differed in more respects than regulation by Gβγ dimers, notably their response to Ca2+ and Ca2+/CaM. ACI, ACII and ACVIII are stimulated by Ca2+/CaM, ACVand ACVI are inhibited by Ca2+, while AC II, IVand VII are unaffected by Ca2+. Adenylyl cyclases also differed in their responses to Giα subunits (Fig. 16A; Taussig et al., 1994 [154]).
Fig. 16.
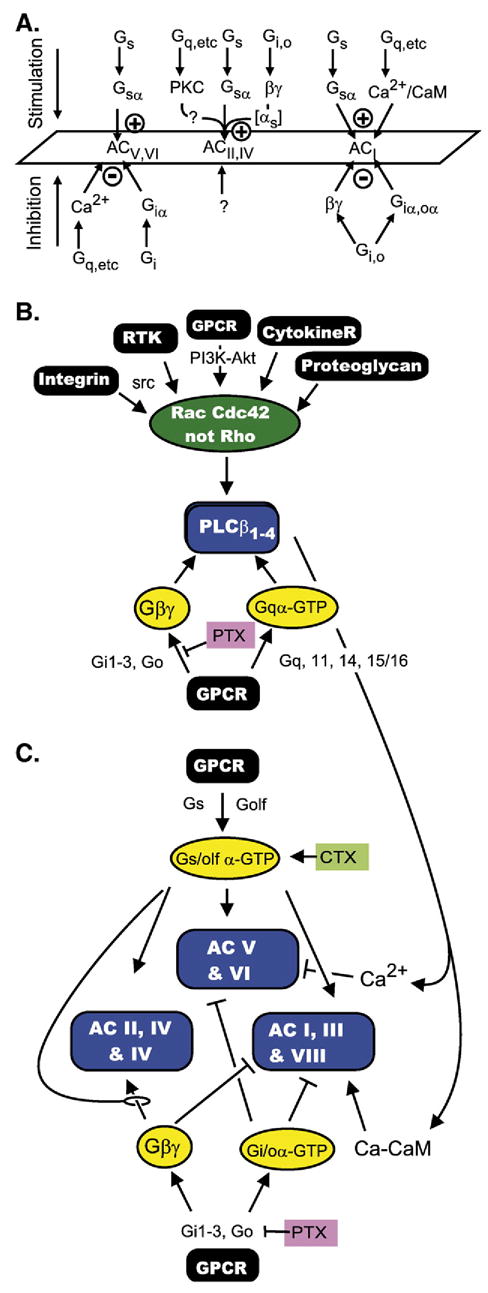
(A) Adenylyl cyclases are targets of multiple regulatory signaling pathways and respond differently, depending on which group they belong to. The figure shows these differences as summarized in 1994 (adapted from Taussig et al., 1994 [154]. (B) The β-type phosphoinositide-specific phospholipases Cβ are targets of three different signaling pathways (for G protein triggered signals see text; for signals impinging on the Rac-Cdc42 GTPases see Cerione (2004 [184]) and references therein), the Gqα family of α subunits and Gβγ dimers. (C) Example of cross talk from the Gq (Gqα), Gi (Gβγ) and Cdc42-Rac signalling pathways to the Gs signaling pathway may generate increases of decreases in cAMP levels depending on the subset of adenylyl cyclases expressed in the target cell.
Two forms of phosphatidylinositol-3-kinase were shown to be activated by βγ dimers. One, PI3 kinase γ (subunit composition: p101c: :p110r; c, catalytic and r, regulatory subunits) is activated by βγ interacting with p101c, without requiring co-activator(s) (Stephens et al., 1994 [155]; Stoyanov et al., 1995 [156]; Tang and Downes, 1997 [157]). Another, PI3 kinase β (subunit composition: p110c: :p85r) is activated only weakly by βγ alone (interacting with p110c), but responds synergistically to βγ in combination with phosphotyrosine containing peptides – or, in real life – in combination with a tyrosine phosphorylated substrate of receptor or non-receptor tyrosine kinases—interacting with the regulatory p85 subunit (Tang and Downes, 1997 [157]; Okada et al., 1996 [158]; Kurosu et al., 1997 [159]).
In 1996 and at the beginning of 1997, Gβγ dimers were shown to inhibit N-, P/Q- and R-type voltage gated Ca2+ channels, encoded in the CaV2.2 (α1B), CaV2.1 (α1A) and CaV2.3 (α1E) subunit genes (Herlitze et al., 1996 [160]; Ikeda, 1996 [161]; De Waard et al., 1997 [162]; Qin et al., 1997 [163]). Gβγ dimers were also shown to activate type-2 and-3 GPCR kinases (GRK-2 and GRK-3, also called bARK1 and bARK-2) giving Gβγ dimers a role in the negative feedback regulation of GPCR signalling (Pitcher et al., 1992 [164]; Haga and Haga, 1992 [165]; Koch et al., 1993 [166]). As first shown for rhodopsin and confirmed for many other GPCRs, phosphoryla-tion of activated GPCRs by GPCR kinases is an integral part of the desensitization process initiated at the same time as G proteins are activated. Among the biochemical consequences of GPCR phosphorylation by GRKs is the binding of arrestin to the newly phosphorylated receptors. Receptor-associated arrest-ins then serve as scaffolds for the initiation new signaling cascades (reviewed in Lefkowitz and Shenoy, 2005 [167]). In addition, by interacting with the SH3 domain of c-src, GPCR-bound arrestins complete the activation cascade by which GPCRs stimulate c-src activity. c-Src activation by GPCRs is yet another effect mediated by Gβγ dimers acting as plasma membrane-attached scaffolding proteins to nucleate adaptor proteins such as Shc and Grb2 in association with c-src to facilitate c-src activation by the ras guanine nucleotide exchange factor SOS. Gβγ dimers are therefore central to GPCR-initiated activation of the Erk1/2 MAP kinase signalling pathway via activation of c-src, as well as to other c-src mediated events (Luttrell et al., 1997 [168]).
Gβγ dimers have also been implicated in regulation of endocytosis as they inhibit dynamin’s intrinsic GTPase activity (Lin and Gilman, 1996 [169]), and in regulation of intracellular vesicle budding events (Lin et al., 1998 [170]), possibly through a direct stimulatory action on protein kinase D (Jamora et al., 1999 [171]). More recently, the diverse activities of Gβγ dimers received an additional boost from the finding that they interact with the glucocorticoid receptor (GR), a member of the nuclear receptor family of transcription factors, and that, in so doing, interfere in the nucleus with GR’s activity as a transcriptional regulator (Kino et al., 2005 [172]). Tables 1 and 2 summarize the different effector system affected by G protein α subunits and βγ dimers.
Table 1.
G protein α subunit effector systems identified by functional and direct interactiona
| α-Subunits | Effector | Effect-Comment |
|---|---|---|
| Gsα | AC I through VII, also AC IX | Stimulation |
| Caveolin | Subcellular localization | |
| Golfα | Most likely all ACs | |
| Gi1α through | All Acs except | Inhibition |
| Gi3α | AC II and IV | |
| AC IX not tested | ||
| αi2 | Kir 6: :SUR | Stimulation by reducing inhibition by ATP |
| αI1 | PI3 kinase γ | Stimulation |
| Goα | ||
| αo | AC I (not AC V or VI) | Inhibition |
| αi/o | rap1-GAPII (GoLoco domain) | Promotes degradation of rap1-GAPII |
| αo | PKAcat α and β | Sequestration in extranuclear compartment |
| Gtα rod, cone | PDE6γ subunit | Releases inhibition of cGMP-specific PDEαβ by PDE |
| Ggustα | cAMP-specific Ca/CaM-stimulated PDE1Ab | Stimulation (mechanism: ?) |
| Gqα | ||
| αq, α11, α14 and α16 | Phospholipase Cβ1 through β4 | Stimulation |
| αq | Btk | Stimulation |
| G13α | ||
| α13 | p115-RhoGEF | Stimulation |
| PDZ-RhoGEF | Stimulation | |
| Leukemia-associated RhoGEF (LARG) | Stimulation | |
| α12 | Tec kinase tyrosine- phosphorylated LARG | Stimulation |
| Btk | Stimulation | |
| ras-GAP1m | Stimulation | |
For details see text.
Robert Margolskee, personal communication.
Table 2.
G protein βγ dimer effector systems identified by functional and direct interaction a
| Effector/biding partner | Effect-Comment |
|---|---|
| AC I | Inhibition |
| AC II and IV | Stimulation; dependent of GTP-αs |
| PLCβ1–3, not PLCβ4 | Stimulation |
| Kir3 (GIRKs) | Stimulation, dependent on PIP2 |
| Kir3.1:Kir3.2 | |
| Kir3.2a:Kir3.2c | |
| Kir3.1:Kir3.4 (CIR) | |
| [Kir3.4]2 | |
| Kir6 (Kir.ATP) | Stimulation [reduction of inhibition by ATP]; the interaction appears to be with SUR |
| Kir6.2 (BIR):SUR2A | |
| GRK 2 and 3 (βARK1 and 2) Ca channels | Gβγ acts as scaffold and as an activator Inhibition—revealed by depolarization(e.g. prepulse) |
| CaV2.1–2–3 (α1C–B–E) | |
| PKD (PKC3) | Stimulation—affects vesicle budding processes |
| Dynamin | Inhibits GTPase—affects dynamin-dependent vesicle budding |
| Shc | βγ acts as scaffold to nucleate Shc:Grb2:SOS:src |
| PI3Kγ (p101:p110) | Stimulation |
| Pi3Kβ (p110:p85) | Stimulation—depends on pY interacting with p85 |
| Glucocorticoid receptor | Inhibits activation of transcription by GR in the nucleus |
| Bruton’s kinase (Btk) | Interaction with PH domain—facilitates activation by Gqα and G12α |
For details see text.
6. Integrated views of signalling through G proteins
What appeared rather simple at the beginning of the G protein signal transduction era, – i.e., several hormone receptors activate a single adenylyl cyclase [system] (Birnbaumer and Rodbell, 1969 [173]; Rodbell et al., 1970 [174]) – became immensely complex when the molecular diversity of the individual effector systems was unraveled. Instead of one adenylyl cyclase there are nine—all activated by Gsα, with some, but not all, being inhibited by Gi/oα subunits (Fig. 16A, Table 1). Instead of one PLCβ there are four, all activated by the Gq-class of α subunits, but varying in their responses to Gβγ dimers and Rho GTPases (Illenberger et al., 2003 [149]). Likewise, instead of one voltage gated Ca2+ channel there is a family of which some (CaV3.1–3.3, former α1A, α1B and α1E)) are, and others (CaV1, CaV3) are not affected by Gβγ dimers (Table 2). The same applies to PI3 kinases, inwardly rectifying K+ channels, G protein coupled receptor kinases (GRKs), and cyclic nucleotide phosphodiesterases. To a greater or lesser extent, each of these effector systems receives inputs from more than one signaling pathway, so that cellular responses are tuned by the isozymes they express. For example, while Ca2+/CaM activate ACI, Ca2+ inhibits ACV and ACVI; while ACV and ACVI are inhibited by Giα, ACII and ACIV appear to be unresponsive to Giα.
Cellular cAMP responses also depend on the G protein selectivity/specificity of the receptors being stimulated. Ca2+ affects different adenylyl cyclase in opposite ways. ACI is stimulated by Ca2+/CaM, ACV and ACVI are inhibited by Ca2+ and various forms of phosphorylation by PKC and PKA, and ACs of the ACII, IV and VII appear insensitive to changes in intracellular Ca2+. In the intact cell, the effects of Ca2+ depend not only on the isozyme that is expressed but also on the site at which Ca2+ increases take place. Dermott Cooper made the interesting observation that in C6 glioma cells which express predominantly ACVI, inhibition due to rises in intracellular Ca2+, occurred primarily by Ca2+ entering the cell via the store depletion activated or capacitative entry pathway (Cooper et al., 1994 [175]; Chiono et al., 1995 [176]; Fagan et al., 1996 [177]). In other cells expressing ACII, ACIV or ACVII, increases in Ca2+ and diacylglycerol may lead to an increase in cAMP mediated by Ca2+ and DAG stimulated phosphorylation of the cAMP forming enzymes. PKA-mediated phosphorylation, on the other hand, may act as a negative feedback regulator of ACV and ACVI (see for example discussion in Lin et al., 2002 [178]). Fig. 16B illustrates the multiple inputs affecting PLCβ activity and the effect this may have on cAMP levels depending on the subtype of adenylyl cyclase expressed in the target cell.
7. Final remarks
By the end of the 1990s “signal transduction by G proteins” had become a household term and was part of college text books. Regulatory networks activated or attenuated by the receptor-mediated activation of G proteins were recognized to vary immensely from cell type to cell type, and became impossible to generalize requiring clear definition of the cells and organs that were being addressed.
With the knowledge of the players (receptors, G proteins, effectors) and their biochemical properties, the next questions to address became the delineation of which receptors, which G proteins and which effectors were active under which conditions. Signal transduction by G proteins became part of research questions that addressed homeostatic and developmental mechanisms. Manipulation of the mouse genome had been developed in the early 1990s and it was applied to G proteins, primarily G protein α subunits. Roles of G proteins in platelet aggregation, in axon guidance, in taste, in cardiac hypertrophy, and many other physiological and pathophysiological processes are now being addressed.
Along a completely different train of thoughts, X-ray crystallography also had an impact on our knowledge and the way we visualize signal transduction by G proteins. The first to be crystallized, just as it had been the first to be identified as a GTPase, first to be purified, and the first to be cloned, was transducin α and came out of a collaboration between Heidi Hamm, then at the University of Illinois, Chicago, and the crystallographer Paul Sigler, at Yale (Noel et al., 1993 [179]). The crystal revealed that its sequence homology with respect to ras carries over into the three dimensional space with α of an αβγ heterotrimeric G protein being a “ras”-like GTPase that has received a insert at the level of ras’ “effector” domain, This insert is formed of seven α helices (A through G). The crystal of αt, was followed by that of a trimer, Gi1 (αi1.β1. γ2), this time generated by the competing group in Dallas, headed by Steven Sprang (Wall et al., 1995 [180]). The trimer showed the βγ dimer as a seven-bladed propeller, a fold now referred to as the Gβ fold. The trimer also showed how the three subunits are oriented with respect to each other. Rhodopsin was also crystallized (Palczewski et al., 2001 [181]), solidifying many assumptions as to the relationship among transmembrane helices and the role of 11-trans retinal in stabilizing these relationships.
Yet, in spite of all these advances, the answer to one fundamental question, that of the molecular mechanism by which a G protein coupled receptor promotes the GDP–GTP exchange on Gα, and the role that Gβγ plays in this process, if any, is still unknown. The answer should tell us why and how GPCRs acquire their high affinity form and why GTP and GDP cannot be distinguished by ligand binding—either one causes receptors to shift into their low affinity states.
Acknowledgments
Supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1.Birnbaumer L. The Discovery of Signal Transduction by G Proteins. A personal account and an overview of the initial findings and contributions that led to our present understanding. Biochim Biophys Acta Biomembranes. 2007;1768:756–771. doi: 10.1016/j.bbamem. 2006.09.027. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovchinnikov YA. Rhodopsin and bacteriorhodopsin: Strucnture-Function Relationships. FEBS Letters. 1982;148:179–191. doi: 10.1016/0014-5793(82)80805-3. [DOI] [PubMed] [Google Scholar]
- 3.Dixon RAF, Kobilka BK, Strader DJ, Benovic JL, Dohlman HC, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE, Mumford RA, Slater EE, Sigal IS, Caron MG, Lefkowitz RJ, Strader CD. Cloning of the Gene and cDNA for Mammalian Beta-adrenergic Receptor and Homology with Rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. (March 11; published, May issue). [DOI] [PubMed] [Google Scholar]
- 4.Yardeen Y, Rodriguez H, Wong SKF, Brandt DR, May DC, Burnier J, Harkins RN, Chen EY, Ramachandran J, Ullrich A, Ross EM. The Avian beta-Adrenergic Receptor: Primary Structure and Topology. Proc Natl Acad Sci USA. 1986;83:6795–6799. doi: 10.1073/pnas.83.18.6795. Acceptd June 9, published Sept Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire ME, Van Arsdale PM, Gilman AG. An Agonist-Specific Effect of Guanine Nucleotides on Binding to the Beta Adrenergic Receptor. Mol Pharmacol. 1976;12:335–339. [PubMed] [Google Scholar]
- 6.Lefkowitz RJ, Mullikan D, Caron MG. Regulation of B-adrenergic Receptors by Guanyl-5′-yl Imidodiphosphate and other Purine Nucleotides. J Biol Chem. 1976;251:4686–4692. [PubMed] [Google Scholar]
- 7.Iyengar R, Abramowitz J, Bordelon-Riser ME, Blume AJ, Birnbaumer L. Regulation of Hormone-Receptor Coupling to Adenylyl Cyclases: Effects of GTP and GDP. J Biol Chem. 1980;255:10312–10321. [PubMed] [Google Scholar]
- 8.Iyengar R. Hysteretic Activation of Adenylyl Cyclases: II. Mg Ion Regulation of the Activation of the Regulatory Component as Analyzed by Reconstitution. J Biol Chem. 1981;256:11042–11050. [PubMed] [Google Scholar]
- 9.Iyengar R, Birnbaumer L. Hysteretic Activation of Adenylyl Cyclases. I Effect of Mg Ion on the Rate of Activation by Guanine Nucleotides and Fluoride. J Biol Chem. 1981;256:11036–11041. [PubMed] [Google Scholar]
- 10.Iyengar R, Birnbaumer L. Hormone Receptor Modulates the Regulatory Component of Adenylyl Cyclases by Reducing its Requirement for Mg Ion and Enhancing its Extent of Activation by Guanine Nucleotides. Proc Natl Acad Sci USA. 1982;79:5179–5183. doi: 10.1073/pnas.79.17.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Codina J, Hildebrandt JD, Birnbaumer L, DSekura R. Effects of Guanine Nucleotides and Mg on Human Erythrocyte Ni and Ns, the Regulatory Components of Adenylyl Cyclase. J Biol Chem. 1984;259:11408–11418. [PubMed] [Google Scholar]
- 12.Neer EJ, Lok JM, Wolf LG. Purification and Properties of the Inhibitory Guanine Nucleotide Regulatory Unit of Brain Adenylate Cyclase. J Biol Chem. 1984;259:14222–14229. [PubMed] [Google Scholar]
- 13.Sternweis PC, DRobishaw J. Isolation of Two Proteins with High Affinity for Guanine Nucleotides from Membranes of Bovine Brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 14.Huff RM, Axton JM, Neer EJ. Physical and Immunological Characterization of a Guanine Nucleotide-binding Protein Purified from Bovine Cerebral Cortex. J Biol Chem. 1985;260:10864–10871. [PubMed] [Google Scholar]
- 15.Halliday KR. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983–1984;9:435–448. [PubMed] [Google Scholar]
- 16.Hurley JB, Simon MI, Teplow DB, Robishaw JD, Gilman AG. Homologies Between Signal Transducing G Proteins and ras Gene Products. Science. 1984;226:860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- 17.Lochrie MA, Hurley JB, Simon MI. Sequence of the alpha subunit of Photoreceptor G Protein: Homologies Between Transducin, Ras and Elongation Factors. Science. 1985;228:96–99. doi: 10.1126/science.3856323. [DOI] [PubMed] [Google Scholar]
- 18.Medynski DC, Sullivan K, Smith D, Van Dop C, Chang F-H, Fung BK-K, Seeburg PH, RBourne H. Amino Acid Sequence of the Alpha Subunit of Transducin Deduced from the cDNA Sequence. Proc Natl Acad Sci USA. 1985;82:4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robishaw JD, Russell DW, Harris BA, Smigel MD, Gilman AG. Deduced Primary Structure of the alpha Subunit of the GTP-binding Stimulatory Protein of Adenylate Cyclase. Proc Natl Acad Sci USA. 1986;83:1251–1255. doi: 10.1073/pnas.83.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nukada T, Tanabe T, Takahashi H, Noda M, Hirose T, Inayama S, Numa S. Primary Structure of the alpha-Subunit of Bovine Adenylate Cyclase-stimulating G-protein Deduced from the cDNA Sequence. FEBS Letters. 1986a;195:220–224. doi: 10.1016/0014-5793(86)80164-8. [DOI] [PubMed] [Google Scholar]
- 21.Nukada T, Tanabe T, Takahashi H, Noda M, Haga K, Haga T, Ichiyama A, Kangawa K, Hiranaga M, Matsuo H, Numa S. Primary Structure of the alpha-subunit of Bovine Adenylate Cyclase Inhibiting G-protein Deduced from the cDNA sequence. FEBS Letters. 1986b;197:305–310. doi: 10.1016/0014-5793(86)80347-7. [DOI] [PubMed] [Google Scholar]
- 22.Bray P, Carter A, Guo V, Puckett C, Kamholz J, Spiegel A, Nirenberg M. Human cDNA Clones for an α Subunit of Gi Signal-transduction Protein. Proc Natl Acad Sci USA. 1987;84:5115–5119. doi: 10.1073/pnas.84.15.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Didsbury JR, Snyderman R. Molecular Cloning of a New Human G Protein. Evidence for Two G1α-like Protein Families. FEBS Letters. 1987;219:259–263. doi: 10.1016/0014-5793(87)81228-0. [DOI] [PubMed] [Google Scholar]
- 24.Suki W, Abramowitz J, Mattera R, Codina J, Birnbaumer L. The Human Genome Encodes at Least Three Non-Allelic G Proteins with alphai-type Subunits. FEBS Letters. 1987;220:187–192. doi: 10.1016/0014-5793(87)80900-6. [DOI] [PubMed] [Google Scholar]
- 25.Jones DT, RReed R. Molecular Cloning of Five GTP-Binding Protein cDNA Species from Rat Olfactory Neuroepithelium. J Biol Chem. 1987;262:14241–14249. [PubMed] [Google Scholar]
- 26.VanMeurs KP, Angus W, Lavu S, Kung HF, Czarnecki SK, Moss J, Vaughan M. Deduced Amino Acid Sequence of Bovine Retinal Goα: Similarities to Other Guanine Nucleotide-Binding Proteins. Proc Natl Acad Sci USA. 1987;84:3107–3111. doi: 10.1073/pnas.84.10.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh H, Kozasa T, Nagata S, Nakamura S, Katada T, Ui M, Iwai S, Ohtsuka E, Kawasaki H, Suzuki K, Kaziro Y. Molecular Cloning and Sequence Determination of cDNAs for the Alpha Subunits of the Guanine Nucleotide-binding Proteins Gs, Gi, and Go from Rat Brain. Proc Natl Acad Sci US. 1986;83:3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu WH, Rudolph U, Sanford J, Bertrand P, Olate J, Nelson C, Moss LG, Boyd AE, III, Codina J, Birnbaumer L. Molecular Cloning of a Novel Splice Variant of the α Subunit of the Mammalian Go Protein. J Biol Chem. 1990;265:11220–11226. [PubMed] [Google Scholar]
- 29.Strathmann M, Wilkie T, Simon MI. Alternative Splicing Procedures Transcripts Encoding Two Forms of the α Subunit of GTP-binding Protein Go. Proc Natl Acad Sci USA. 1990;87:6477–6481. doi: 10.1073/pnas.87.17.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukamoto T, Toyama R, Itoh H, Kozasa T, Matsuoka M, Kaziro Y. Structure of the Human Gene and Two Rat cDNAs Encoding the α Chain of GTP-binding Regulatory Protein Go: Two Different mRNAs Are Generated by Alternative Splicing. Proc Natl Acad Sci USA. 1991;88:2974–2978. doi: 10.1073/pnas.88.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong HK, Yoshimoto KK, Eversole-Cire P, ISimon M. Identification of a GTP-binding Protein α Subunit that Lacks an Apparent ADP-ribosylation Site for Pertussis Toxin. Proc Natl Acad Sci. 1988;85:3066–3070. doi: 10.1073/pnas.85.9.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuoka M, Itoh H, Kozasa T, Kaziro Y. Sequence Analysis of cDNA and Genomic DNA for a Putative Pertussis Toxin Insensitive Gunaine Nucleotide Binding Regulatory Protein α Subunit. Proc Natl Acad Sci USA . l988;85:5384–5388. doi: 10.1073/pnas.85.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a Taste-Cell-Specific G Protein Closely Related to the Transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 34.Jones DT, Barbosa E, Reed RR. Expression of G-protein α Subunits in Rat Olfactory Neuroepithelium; Candidates for Olfactory Signal Transduction. Cold Spring Harbor Symp Quant Biology. l988;53:349–353. doi: 10.1101/sqb.1988.053.01.042. [DOI] [PubMed] [Google Scholar]
- 35.van der Voorn L, Ploegh HL. The WD40 Repeat. FEBS Lett. 1992;307:131–134. doi: 10.1016/0014-5793(92)80751-2. [DOI] [PubMed] [Google Scholar]
- 36.Fong H, K W, Hurley JB, Hopkins RS, Miake-Lye R, Johnson MS, Doolittle RF, ISimon M. Repetitive Segmental Structure of the Transducin Beta Subunit: Homology with the CDC4 Gene and Identification of Related mRNAs. Proc Natl Acad Sci USA. 1986;83:2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Codina J, Stengel D, Woo SLC, Birnbaumer L. Beta Subunits of the Human Liver Gs/Gi Signal Transducing Proteins and Those of Bovine Retinal Rod Cell Transducin are Identical. FEBS Letters. 1986;207:187–192. doi: 10.1016/0014-5793(86)81486-7. [DOI] [PubMed] [Google Scholar]
- 38.Fong HKW, Amatruda TT, III, Birren BW, Simon MI. Distinct Forms of the β Subunit of GTP-binding Regulatory Proteins Identified by Molecular Cloning. Proc Natl Acad Sci USA. 1987;84:3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao B, Gilman AG, Robishaw JD. A Second Form of the β Subunit of Signal-transducing G Proteins. Proc Natl Acad Sci USA. 1987;84:6122–6125. doi: 10.1073/pnas.84.17.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson AJ, Aragay AM, Slepak VZ, ISimon M. A novel form of the G protein β Subunit, Gβ5, is specifically expressed in the vertebrate retina. J Biol Chem. 1996;271:28154–28160. doi: 10.1074/jbc.271.45.28154. [DOI] [PubMed] [Google Scholar]
- 41.Makino ER, Handy JW, Li T, YArshavsky V. The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein beta subunit. Proc Natl Acad Sci USA. 1999;96:1947–1952. doi: 10.1073/pnas.96.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CK, Burns ME, He W, Wensel TG, Baylor DA, ISimon M. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- 43.Hurley JB, Fong HKW, Teplow DB, Dreyer WJ, Simon MI. Isolation and Characterization of a cDNA Clone for the Gamma Subunit of Bovine Retinal Transducin. Proc Natl Acad Sci USA. 1984a;81:6948–6952. doi: 10.1073/pnas.81.22.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yatsunami K, Pandya BV, Oprian DD, GKhorana H. cDNA-Derived Amino Acid Sequence of the Gamma Subunit of GTPase from Bovine Rod Outer Segments. Proc Natl Acad Sci USA. 1985;82:1936–1940. doi: 10.1073/pnas.82.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graziano MP, GGilman A. Synthesis in Escherichia coli of GTPase-ydeficient mutants of Gs alpha. J Biol Chem. 1989;264:15475–15482. [PubMed] [Google Scholar]
- 46.Hokin MR, EHokin L. Enzyme secretion and the incorporation of P32 into phospholipids of pancreas slices. J Biol Chem. 1953;203:967–977. [PubMed] [Google Scholar]
- 47.Hokin LE, RHokin M. Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim Biophys Acta. 1955;18:102–110. doi: 10.1016/0006-3002(55)90013-5. [DOI] [PubMed] [Google Scholar]
- 48.Putney JW., Jr A Model for Receptor-Regulated Calcium Entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 49.Putney JW., Jr Capacitative Calcium Entry Revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 50.Thomas AP, Marks JS, Coll KE, RWilliamson J. Quantitation and Early Kinetics of Inositol Lipid Changes Induced by Vasopressin in Isolated, I. Cultured Hepatocytes. J Biol Chem. 1983;258:5716–5725. [PubMed] [Google Scholar]
- 51.Berridge MJ, Dawson RMC, Downes CP, Heslop JP, Irvine RF. Changes in the Levels of Inositol Phosphates after Agonist-dependent Hydrolysis of 482. J des Membrane Phosphoinositi Biochem. 1983;212:473. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a Nonmitochondrial Intracellular Store in Pancreatic Acinar Cells by Inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 53.Grandt R, Greiner C, Zubin P, HJakobs K. Bradykinin stimulates GTP hydrolysis in NG108-15 membranes by a high-affinity, pertussis toxin-insensitive GTPase. FEBS Lett. 1986;196:279–283. doi: 10.1016/0014-5793(86)80263-0. [DOI] [PubMed] [Google Scholar]
- 54.Houslay MD, Bojanic D, Wilson A. Platelet activating factor and U44069 stimulate a GTPase activity in human platelets which is distinct from the guanine nucleotide regulatory proteins, Ns and Ni. Biochem J. 1986;234:737–740. doi: 10.1042/bj2340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harden TK, Stephens L, Hawkins PT, PDownes C. Turkey Erythrocyte Membranes as a Model for Regulation of Phospholipase C by Guanine Nucleotides. J Biol Chem. 1987;262:9057–9061. [PubMed] [Google Scholar]
- 56.El-Refai MF, Blackmore PF, Exton JH. Evidence for two α-adrenergic binding sites. in liver plasma membranes Studies with [3H]epinephrine and [3H]dihydroergocryptine. J Biol Chem. 1979;254:4375–4386. [PubMed] [Google Scholar]
- 57.Geynet P, Borsodi A, Ferry N, Hanoune J. Proteolysis of rat liver plasma membranes cancels the guanine nucleotide sensitivity of agonist binding to the alpha-adrenoceptor. Biochem Biophys Res Commun. 1980;97:947–954. doi: 10.1016/0006-291x(80)91468-0. [DOI] [PubMed] [Google Scholar]
- 58.Geynet P, Borsodi A, Ferry N, Hanoune J. Proteolysis of rat liver plasma membranes cancels the guanine nucleotide sensitivity of agonist binding to the alpha-adrenoceptor. Biochem Biophys Res Commun. 1980;97:947–954. doi: 10.1016/0006-291x(80)91468-0. [DOI] [PubMed] [Google Scholar]
- 59.Koo C, Lefkowitz RJ, Snyderman R. The oligopeptide chemotactic factor receptor on human polymorphonuclear leukocyte membranes exists in two affinity states. Biochem Biophys Res Commun. 1982;106:442–449. doi: 10.1016/0006-291x(82)91130-5. [DOI] [PubMed] [Google Scholar]
- 60.Evans T, Martin MW, Hughes AR, KHarden T. Guanine Nucleotide-Sensitive, High Affinity Binding of Carbachol to Muscarinic Cholinergic Receptors of 1321N1 Astrocytoma Cells Is Insensitive to Pertussis Toxin. Mol Pharmacol. 1985;27:32–37. [PubMed] [Google Scholar]
- 61.Cockroft S, DGomperts B. Role of Guanine Nucleotide Binding Protein in the Activation of Polyphosphoinositide Phosphodiesterase. Nature. 1985;314:534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- 62.Deckmyn H, Whiteley BJ, Majaerus PW. Phosphatidylinositol phospholipase C. In: Iyengar R, Birnbaumer L, editors. G Proteins. Academic Press; San Diego, CA: 1990. pp. 429–452. [Google Scholar]
- 63.Litosch I, Wallis C, Fain JN. 5-Hydroxytryptamine Stimulates Inositol Phosphate Production in a Cell-free System from Blowfly Salivary Glands. Evidence for a Role of GTP in Coupling Receptor Activation to Phosphoinositide Breakdown. J Biol Chem. 1985;260:5464–5471. [PubMed] [Google Scholar]
- 64.Pang IH, CSternweis P. Purification of Unique α Subunits of GTP-binding Regulatory Proteins (G Proteins) by Affinity Chromatography with Immobilized βγ Subunits. J Biol Chem. 1990;265:18707–18712. [PubMed] [Google Scholar]
- 65.TaylorJ M, Smith JA, HExton J. Purification from bovine liver membranes of a guannine nucleotide-dependent activator of phosphoinositide-sepcific phopsholipase C. Immunologic identification of as a novel G protein α subunit. J Biol Chem. 1990;265:17150–17156. [PubMed] [Google Scholar]
- 66.Strathmann MP, Wilkie TM, Simon MI. Diversity of the G-protein Family: Sequences from Five Additional α Subunits in the Mouse. Proc Natl Acad Sci USA. 1989;86:7407–7409. doi: 10.1073/pnas.86.19.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strathmann MP, Simon MI. G Protein Diversity: A Distinct Class of α Subunits is Present in Vertebrates and Invertebrates. Proc Natl Sci USA. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. Gq G11 α subunits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strathmann MP, Simon MI. Gα12 and Gα13 Subunits Define a Fourth Class of G Protein α Subunits. Proc Natl Acad Sci USA. 1991;88:5582–5586. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smrcka AV, Helper JR, Brown KO, CSternweis P. Regulation of Polyphosphoinositide-specific Phospholipase C Activity by Purified Gq. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 70.Wange RL, Smrcka AV, Sternweis PC, ExtonH J. Photoaffinity labelling of two rat liver plasma memebrane proteins with [γ-32P]azidoanilido-GTP in response to vasopresin. Immunologic identifiction as α subunits of the Gq class of G protiens. J Biol Chem. 1991;266:11409–11412. [PubMed] [Google Scholar]
- 71.Taylor SJ, Chae HZ, Rhee SG, HExton J. Activation of the β1 Isozyme of Phospholipase C by α Subunits of the Gq Class of G Proteins. Nature. 1991b;350:516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- 72.Wu D, Lee CH, Rhee SG, ISimon M. Activation of Phospholipase C by the α Subunit of the Gq and G11 Protein in Transfected Cos-7 Cells. J Biol Chem. 1992;267:1811–1817. [PubMed] [Google Scholar]
- 73.Lee CH, Park D, Wu D, Rhee SG, Simon MI. Members of the Gq Alpha Subunit Gene Family Activate Phospholipase C-beta Isozymes. J Biol Chem. 1992;267:16044–16047. αq, 11, 14, 16 in cell membranes +purified PLCβ. [PubMed] [Google Scholar]
- 74.Kozasa T, Hepler JR, Smrcka AV, Simon MI, Rhee SG, Sternweis PC, Gilman AG. Purification and characterization of recombinant G16α from Sf9 cells: activation of purified phospholipase C isozymes by G-protein α subunits. Proc Natl Acad Sci USA. 1993;90:9176–9180. doi: 10.1073/pnas.90.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakamura F, Kato M, Kameyama K, Nukada T, Haga T, Kato H, Takenawa T, Kikkawa U. Characterization of Gq family G proteins GL1α (G14α), GL2α (G11α), and Gqα expressed in the baculovirus-insect cell system. J Biol Chem. 1995;270:6246–6253. doi: 10.1074/jbc.270.11.6246. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura F, Ogata K, Shiozaki K, Kameyama K, Ohara, Haga KT, Nukada T. Identification of two novel GTP-binding protein alpha-subunits that lack apparent ADP-ribosylation sites for pertussis toxin. J Biol Chem. 1991;266:12676–12681. cloning of GL1 and GL2=G14 and G11α. [PubMed] [Google Scholar]
- 77.Offermanns S, ISimon M. G15α and G16α couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- 78.Chan AM, Fleming TP, McGovern ES, Chedid M, Miki T, AAaronson S. Expression cDNA cloning of a transforming gene encoding the wild-type G12α gene product. Mol Cell Biol. 1993;13:762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu N, Bradley L, Ambdukar I, SGutkind J. A mutant α subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc Natl Acad Sci USA. 1993;90:6741–6745. doi: 10.1073/pnas.90.14.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang H, Wu D, ISimon M. The transforming activity of activated G12α. FEBS Lett. 1993;330:319–322. doi: 10.1016/0014-5793(93)80896-3. [DOI] [PubMed] [Google Scholar]
- 81.Dhanasekaran N, Vara Prasad M, Wadsworth SJ, Dermott JM, van Rossum G. Protein kinase C-dependent and-independent activation of Na+/H+exchanger by G12α class of G proteins. J Biol Chem. 269:11802–11806. [PubMed] [Google Scholar]
- 82.Vara Prasad M, Shore SK, Dhanasekaran N. Activated mutant of Gα13 induces Egr-1, c-fos, and transformation in NIH 3T3 cells. Oncogene. 1994;9:2425–2429. [PubMed] [Google Scholar]
- 83.Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL. Gα12 and Gα13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. ExoC3. [DOI] [PubMed] [Google Scholar]
- 84.Chan RK, AOtte C. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982b;2:21–29. doi: 10.1128/mcb.2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan RK, AOtte C. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982a;2:11–20. doi: 10.1128/mcb.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dietzel C, Kurjan J. Pheromonal regulation and sequence of the Saccharomyces cerevisiae SST2 gene: a model for desensitization to pheromone. Mol Cell Biol. 1987;7:4169–4177. doi: 10.1128/mcb.7.12.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dohlman HG, Apaniesk D, Chen Y, Song J, Nusskern D. Inhibition of G-protein signaling by dominant gain-of-function mutations in Sst2p, a pheromone desensitization factor in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:3635–3643. doi: 10.1128/mcb.15.7.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koelle MR, RHorvitz H. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell. 1996;84:115–125. doi: 10.1016/s0092-8674(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 89.De Vries L, Elenko E, Hubler L, Jones TLZ, Farquhar MG. GAIP is membrane-anchored by palmitoylation and interacts with the activated (GTP-bound) form of Giα subunits. Proc Natl Acad Sci USA. 1996;93:15203–15208. doi: 10.1073/pnas.93.26.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong JX, Wilson GL, Fox CH, HKehrl J. Isolation and characterization of a novel B cell activation gene. J Immunol. 1993;150:3895–3904. [PubMed] [Google Scholar]
- 91.Heximer SP, Cristillo AD, Forsdyke DR. Comparison of mRNA expression of two regulators of G-protein signaling, RGS1/BL34/1R20 and RGS2/G0S8, in cultured human blood mononuclear cells. DNA Cell Biol. 1997;16:589–598. doi: 10.1089/dna.1997.16.589. [DOI] [PubMed] [Google Scholar]
- 92.Siderovski DP, Heximer SP, Forsdyke DR. A human gene encoding a putative basic helix-loop-helix phosphoprotein whose mRNA increases rapidly in cycloheximide-treated blood mononuclear cells. DNA Cell Biol. 1994;13:125–147. doi: 10.1089/dna.1994.13.125. GOS8 RGS2 RGS2. [DOI] [PubMed] [Google Scholar]
- 93.Druey KM, Blumer KJ, Kang VH, HKehrl J. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature. 1996;379:742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- 94.Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 95.Nekrasova ER, Berman DM, Rustandi RR, Hamm HE, Gilman AG, Arshavsky VY. Activation of transducin guanosine triphosphatase by two proteins of the RGS family. Biochemistry. 1997;36:7638–7643. doi: 10.1021/bi970427r. RGS4 and GAIP. [DOI] [PubMed] [Google Scholar]
- 96.Hepler JR, Berman DM, Gilman AG, Kozasa T. RGS4 and GAIP are GTPase-activating proteins for Gqα, S.M. block activation of phospholipase Cβ by γ-thio-GTP-Gqα. Proc Natl Acad Sci USA. 1997;94:428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang C, Hepler JR, Gilman AG, Mumby SM. Attenuation of Gi-and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci USA. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doupnik CA, Davidsonm N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- 100.Lyubarsky AL, Naarendorp F, Zhang X, Wensel T, Simon MI, Pugh EN., Jr RGS9-1 is required for normal inactivation of mouse cone phototransduction. Mol Vis. 2001;7:71–78. [PubMed] [Google Scholar]
- 101.Kimple RJ, De Vries L, Tronchere H, Behe CI, Morris RA, Farquhar MG, Siderovski DP. RGS12 and RGS14 GoLoco motifs are Giα interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- 102.Dohlman HG, Thorner J. RGS proteins and signaling by heterotrimeric G proteins. J Biol Chem. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- 103.Neubig RR, Siderovski PD. Regulators of G protein signalling as new central nervous system drug targets. Nature Rev Drug Dis. 200;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- 104.Lu Q, Sun EE, Klein RS, GFlanagan J. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 105.Hart MJ, Sharma S, el Masry N, Qiu RG, McCabe P, Polakis P, Bollag G. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem. 1996;271:25452–25458. doi: 10.1074/jbc.271.41.25452. p115-RhoGEF. [DOI] [PubMed] [Google Scholar]
- 106.Kozasa T, GGilman A. Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of α12 and inhibition of adenylyl cyclase by αz. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 107.Singer WD, Miller RT, Sternweis PC. Purification and characterization of the α subunit of G13. J Biol Chem. 1994;269:19796–19802. [PubMed] [Google Scholar]
- 108.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, CSternweis P. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 109.Hart MJ, Jiang X, Kozasa T, Roscoe W, D Singer W, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by G13α. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 110.Tanabe S, Kreutz B, Suzuki N, Kozasa T. Regulation of RGS-RhoGEFs by Gα12, T. Gα13 proteins. Methods Enzymol. 2004;390:285–294. doi: 10.1016/S0076-6879(04)90018-3. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki N, Nakamura S, Mano H, Kozasa T. Gα12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF (LARG) Proc Natl Acad Sci USA. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bence K, Ma W, Kozasa T, Huang XY. Direct stimulation of Bruton’s tyrosine kinase by Gq-protein α-subunit. Nature. 1997;389:296–299. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 113.Jiang Y, Ma W, Wan Y, Kozasa T, Hattori S, YHuang X. The G protein G12α stimulates Bruton’s tyrosine kinase and a rasGAP through a conserved PH/BM domain. Nature. 1998;395:808–813. doi: 10.1038/27454. [DOI] [PubMed] [Google Scholar]
- 114.Tsukada S, Simon MI, Witte ON, Katz A. Binding of βγ subunits of heterotrimeric G proteins to the PH domain of Bruton tyrosine kinase. Proc Natl Acad Sci USA. 1994;91:11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wettschureck N, Offermanns S. Mammalian G Proteins and Their Cell Type Specific Functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 116.Katada T, Northup JK, Bokoch GM, Ui M, Gilman AG. The Inhibitory Guanine Nucleotide-binding Regulatory Component of Adenylate Cyclase. Subunit Dissociation and Guanine Nucleotide-Dependent Hormonal Inhibition. J Biol Chem. 1984a;259:3578–3885. [PubMed] [Google Scholar]
- 117.Katada T, Bokoch GM, Smigel MD, Ui M, Gilman AG. The Inhibitory Guanine Nucleotide-binding Regulatory Component of Adenylate Cyclase. Subunit Dissociation and the Inhibition of Adenylate Cyclase in S49 Lymphoma cyc− and Wild Type Membranes. J Biol Chem. 1984b;259:3586–3595. [PubMed] [Google Scholar]
- 118.Hildebrandt JD, Codina J, Birnbaumer L. Interaction of the Stimulatory and Inhibitory Regulatory Proteins of the Adenylyl Cyclase System with the Catalytic Component of cyc− S49 Cell Membranes. J Biol Chem. 1984b;259:13178–13185. [PubMed] [Google Scholar]
- 119.Hildebrandt JD, Codina J, Iyengar R, Rojas FJ, Kirchick HJ, Abramowitz J, Hunzicker-Dunn M, Birnbaumer L. Transmem-brane Regulations of Adenylyl Cyclases. In: Saxena BB, Catt KJ, Birnbaumer L, Martini L, editors. Hormone Receptors in Growth and Reproduction. Raven Press; New York: 1984a. pp. 111–140. [Google Scholar]
- 120.Taussig R, Iniguez-Lluhi JA, Gilman AG. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 121.Dessauer CW, Tesmer JJ, Sprang SR, Gilman AG. Identification of a Giα binding site on type V adenylyl cyclase. J biol Chem. 1998;273:25831–25839. doi: 10.1074/jbc.273.40.25831. [DOI] [PubMed] [Google Scholar]
- 122.Dietzel C, Kurjan J. The Yeast SCG1 Gene: A Gα-like Protein Implicated in the a-and α-Factor Response Pathway. Cell. 1987;50:1001–1010. doi: 10.1016/0092-8674(87)90166-8. [DOI] [PubMed] [Google Scholar]
- 123.Whiteway M, Hougan L, Thomas DY. Overexpression of the STE4 gene leads to mating response in haploid Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:217–222. doi: 10.1128/mcb.10.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ Subunits of GTP-binding Proteins Activate the Muscarinic K+ Channel in Heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 125.Soejima M, Noma A. Mode of Regulation of the ACh-sensitive K-channel by the Muscarinic Receptor in Rabbit Atrial Cells. Pfluegers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- 126.Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding Proteins Couple Cardiac Muscarinic Receptors to a K Channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 127.Breitwieser GE, Szabo G. Uncoupling of Cardiac Muscarinic and beta-Adrenergic Receptors from Ion Channels by a Guanine Nucleotide Analogue. Nature. 1985;317:538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- 128.Yatani A, Codina J, Brown AM, Birnbaumer L. Direct Activation of Mammalian Atrial Muscarinic Potassium Channels by GTP Regulatory Protein Gk. Science. 1987;235:207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- 129.Codina J, Yatani A, Grenet D, Brown AM, Birnbaumer L. The alpha Subunit of the GTP Binding Protein Gk Opens Atrial Potassium Channels. Science. 1987;236:442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- 130.Yatani A, Mattera R, Codina J, Graf R, Okabe K, Padrell E, Iyengar R, Brown AM, Birnbaumer L. The G Protein-gated Atrial K+ Channel is Stimulated by Three Distinct Giα-subunits. Nature. 1988;336:680–682. doi: 10.1038/336680a0. [DOI] [PubMed] [Google Scholar]
- 131.Ito H, Tung RT, Sugimoto T, Kobayashi I, Takahashi K, Katada T, Ui M, Kurachi Y. On the mechanism of G protein βγ subunit activation of the muscarinic K+ channel in guinea pig atrial cell membrane. Comparison with the ATP-sensitive K+ channel. J Gen Physiol. 1992;99:961–983. doi: 10.1085/jgp.99.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by Gβγ subunits and function as heteromultimers. Proc Natl Acad Sci USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nair LA, Inglese J, Stoffel R, Koch WJ, Lefkowitz RJ, Kwatra MM, Grant AO. Cardiac muscarinic potassium channel activity is attenuated by inhibitors of Gβγ. Circ Res. 1995;76:832–838. doi: 10.1161/01.res.76.5.832. [DOI] [PubMed] [Google Scholar]
- 134.Kirsch G, Codina J, Birnbaumer L, MBrown A. Coupling of ATP-sensitive K+ Channels to Purinergic Receptors by G-Proteins in Rat Ventricular Myocytes. AmJ Physiol. 1990;259:H820–H826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- 135.Terzic A, Tung RT, Inanobe A, Katada T, Kurachi Y. G proteins activate ATP-sensitive K+ channels by antagonizing ATP-dependent gating. Neuron. 1994;12:885–893. doi: 10.1016/0896-6273(94)90340-9. Gi1α Gi2α Goα. [DOI] [PubMed] [Google Scholar]
- 136.Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, Bayliss DA. Activation and inhibition of G protein-coupled inwardly rectifying potassium 776. (Kir3) channels by G protein βγ subunits. Proc Natl Acad Sci USA. 2000;97:9771–9. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lupi R, Corda D, Di Girolamo M. Endogenous ADP-ribosylation of the G protein β subunit prevents the inhibition of type 1 adenylyl cyclase. J Biol Chem. 2000;275:9418–9424. doi: 10.1074/jbc.275.13.9418. [DOI] [PubMed] [Google Scholar]
- 138.Lupi R, Dani N, Dietrich A, Marchegiani A, Turacchio S, Berrie CP, Moss J, Gierschik P, Corda D, DiGirolamo M. Endogenous mono-ADP-ribosylation of the free Gβγ prevents stimulation of phosphoinositide 3-kinase-γ and phospholipase C-β2 and is activated by G-protein-coupled receptors. BiochemJ. 2002;367:825–832. doi: 10.1042/BJ20020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wada Y, Yamashita T, Imai K, Miura R, Takao K, Nishi M, Takeshima H, Asano T, Morishita R, Nishizawa K, Kokubun S, Nukada T. A region of the sulfonylurea receptor critical for a modulation of ATP-sensitive K+ channels by G-protein βγ subunits. EMBO J. 2000;19:4915–4925. doi: 10.1093/emboj/19.18.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yano K, Nakashima S, Nozawa Y. Coupling of polyphosphoinositide breakdown with calcium efflux in formyl-methionyl-leucyl-phenylalanine-stimulated rabbit neutrophils. FEBS Lett. 1983;161:296–300. doi: 10.1016/0014-5793(83)81028-x. [DOI] [PubMed] [Google Scholar]
- 141.Volpi M, Yassin R, Naccache PH, ISha’afi R. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983;112:957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]
- 142.Smith CD, Lane BC, Kusaka I, Verghese MW, Snyderman R. Chemoattractant Receptor-induced Hydrolysis of Phosphatidylinositol 4,5-Bisphosphate in Human Polymorphonuclear Leukocyte Membranes. Requirement for a Guanine Nucleotide Regulatory Protein. J Biol Chem. 1985;260:5875–5878. [PubMed] [Google Scholar]
- 143.Brandt SJ, Dougherty RW, Lapetina EG, ENiedel J. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci USA. 1985;82:3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kikuchi A, Kozawa O, Kaibuchi K, Katada T, Ui M, Takai Y. Direct Evidence for Involvement of a Guanine Nucleotide-binding Protein in Chemotactic peptide-stimulated Formation of Inositol Bisphosphate and Trisphosphate in Differentiated Human Leukemic (HL-60) Cells. Reconstitution with Gi or Go of the Plasma Membranes ADP-Ribosylated by Pertussis Toxin. J Biol Chem. 1986;261:11558–11562. [PubMed] [Google Scholar]
- 145.Camps M, Hou CF, Jakobs KH, Gierschik P. Guanosine 5′-[gamma-thio]triphosphate-stimulated hydrolysis of phosphatidylinositol 4,5-bisphosphate in HL-60 granulocytes. Evidence that the guanine nucleotide acts by relieving phospholipase C from an inhibitory constraint. Biochem J. 1990;271:743–748. doi: 10.1042/bj2710743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bokoch GM, Bickford K, Bohl BP. Subcellular localization and quantitation of the major neutrophil pertussis toxin substrate, Gn. J Cell Biol. 1988;106:1927–1936. doi: 10.1083/jcb.106.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Camps M, Hou C, Sidiropoulos D, Stock JB, Jakobs KH, Gierschik P. Stimulation of Phospholipase C by Guanine-Nucleotide-Binding Protein βγ Subunits. EurJ Biochem. 1992;206:821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- 148.Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, Gierschik P. Stimulation of phospholipase C-β2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J. 1998;17:6241–6249. doi: 10.1093/emboj/17.21.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Illenberger D, Walliser C, Nuernberg B, Diaz Lorente M, Gierschik P. Specificity and G., B. structural requirements of phospholipase Cβ stimulation by Rho GTPases versus G protein βγ dimers. J Biol Chem. 2003;278:3006–3014. doi: 10.1074/jbc.M208282200. [DOI] [PubMed] [Google Scholar]
- 150.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of G-protein Subtypes to Specific Receptors Inducing Inhibition of Calcium Currents. Nature. 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 151.Kleuss C, Scherübl H, Hescheler J, Schultz G, Wittig B. Different β-Subunits Determine G Protein Interaction with Transmem-brane Receptors. Nature. 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 152.Kleuss C, Scherübl H, Hescheler J, Schultz G, Wittig B. Selectivity in Signal Transduction Determined by γ Subunits of Heterotrimeric G Proteins. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 153.Tang WJ, GGilman A. Type Specific Regulation of Adenylyl Cyclase by G Protein βγ Subunits. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- 154.Taussig R, Tang WJ, Hepler JR, GGilman A. Distinct patterns of bidirectional regulation of mammalian adenylyl cyclases. J Biol Chem. 1994;269:6093–6100. [PubMed] [Google Scholar]
- 155.Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. (P3Kβ) [DOI] [PubMed] [Google Scholar]
- 156.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nuernberg B, Gierschk P, Seedorf K, Hsuan JJ, Waterfield MD, Wetzger R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. (γ) Science. 1995;269:690–693. doi: 10.1126/science.7624799. (Gαi and Gβγ) [DOI] [PubMed] [Google Scholar]
- 157.Tang X, PDownes C. Purification and characterization of Gβγ-responsive phposphoinositide 3-kinase from pig platelet cytosol. J Biol Chem. 1997;272:14193–14199. doi: 10.1074/jbc.272.22.14193. [DOI] [PubMed] [Google Scholar]
- 158.Okada T, Hazeki O, Ui M, Katada T. Synergistic activation of PtdIns 3-kinase by tyrosine-phosphorylated peptide and βγ-subunits of GTP-binding proteins. Biochem J. 1996;317:475–480. doi: 10.1042/bj3170475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 160.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 161.Ikeda SR. Voltage-dependent modulation of N-type Calcium Channels by G Protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 162.De Waard M, Liu H, Walker D, Scott VES, Gurnett CA, Campbell K. Direct Binding of G-Protein βγ Complex to Voltage-dependent Calcium Channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 163.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct Interaction of Gβγ with a Novel C-terminal Gβγ Binding Domain of the Ca2+Channel α1 Subunit is Responsible for Inhibition by G Protein-Coupled Receptors. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of βγ Subunits of G Proteins in Targeting of the β-Adrenergic Receptor Kinase to Membrane-Bound Receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 165.Haga K, Haga T. Activation by G Protein βγ Subunits of Agonist-or Light-dependent Phosphorylation of Muscarinic Acetylcholine Receptors and Rhosopsin. J Biol Chem. 1992;267:2222–2227. [PubMed] [Google Scholar]
- 166.Koch WJ, Inglese J, Stone WC, JLefkowitz R. The Binding Site for the βγ Subunits of Heterotrimeric G Proteins on the β-Adrenergic Receptor Kinase. JBiolChem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- 167.Lefkowitz RJ, KShenoy S. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 168.Luttrell LM, Della Rocca GJ, van Biesen T, Luttrell DK, Lefkowitz RJ. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 169.Lin HC, Gilman AG. Regulation of dynamin I GTPase activity by G protein betagamma subunits and phosphatidylinositol 4,5-bispho-sphate. J Biol Chem. 1996;271:27979–27982. doi: 10.1074/jbc.271.45.27979. [DOI] [PubMed] [Google Scholar]
- 170.Lin HC, Duncan JA, Kozasa T, Gilman AG. Sequestration of the G protein beta-gamma subunit complex inhibits receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1998;95:5057–5060. doi: 10.1073/pnas.95.9.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. 1999 doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 172.Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP. G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–896. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Birnbaumer L, Rodbell M. Adenyl Cyclase in Fat Cells. II Hormone Receptors. J Biol Chem. 1969;244:3477–3482. [PubMed] [Google Scholar]
- 174.Rodbell M, Birnbaumer L, Pohl SL, Krans HMJ. Hormone Receptors and Adenyl Cyclase Activity in Mammalian Cells. In: Rodbell M, Condliffe P, editors. Colloquium on the Role of Adenyl Cyclase and Cyclic AMP. Fogarty International Center, U.S. Government Printing Office; Washington, D.C.: 1970. pp. 59–76. [Google Scholar]
- 175.Cooper DMF, Yoshimura M, Zhang Y, Chiono M, Mahey R. Capacitative Ca2+entry regulates Ca2+-sensitive adenylyl cyclases. BiochemJ. 1994;297:437–440. doi: 10.1042/bj2970437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chiono M, Mahey R, Tate G, Cooper DMF. Capacitative Ca2+ Entry Exclusively Inhibits cAMP Synthesis in C6-2B Glioma Cells. Evidence That Physiologically Evoked Ca2+ Entry Regulateds Ca2+-inhibitable Adenylyl Cyclclase in Non-excitable Cells. J Biol Chem. 1995;270:1149–1155. doi: 10.1074/jbc.270.3.1149. [DOI] [PubMed] [Google Scholar]
- 177.Fagan KA, Mahey R, Cooper DMF. Functional co-localization of transfected Ca2+- stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem. 1996;271:12438–12444. doi: 10.1074/jbc.271.21.12438. [DOI] [PubMed] [Google Scholar]
- 178.Lin TH, Lai HL, Kao YY, Sun CN, Hwang MJ, Chern Y. Protein kinase C inhibits type VI adenylyl cyclase by phosphorylating the regulatory N domain and two catalytic C1 and C2 domains. J Biol Chem. 2002;277:15721–1578. doi: 10.1074/jbc.M111537200. [DOI] [PubMed] [Google Scholar]
- 179.Noel JP, Hamm HE, Sigler PB. The 2.2 Å Crystal Structure of Transducin α-GTPγS. Nature. 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 180.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Gi1α β1 γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 181.Palczewski KT, Kumasaka T, Hori CA, Behnke H, Motoshima BA, Fox I, Le Trong DC, Teller T, Okada RE, Stenkamp M, Yamamoto M, Miyano Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2001;289:739–745. doi: 10.1126/science.289.5480.739. 1F88. [DOI] [PubMed] [Google Scholar]
- 182.Rojas FJ, Birnbaumer L. Regulation of Glucagon Receptor Binding. Lack of Effect of Mg and Preferential Role for GDP. J Biol Chem. 1985;260:7829–7835. [PubMed] [Google Scholar]
- 183.Langhans-Rajasekaran SA, Wan Y, Huang XY. Activation of Tsk and Btk tyrosine kinases by G protein βγ subunits. Proc Natl Acad Sci USA. 1995;92:8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cerione RA. Cdc42: new roads to travel. Trends Cell Biol. 2004;14:127–132. doi: 10.1016/j.tcb.2004.01.008. [DOI] [PubMed] [Google Scholar]


