Abstract
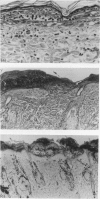
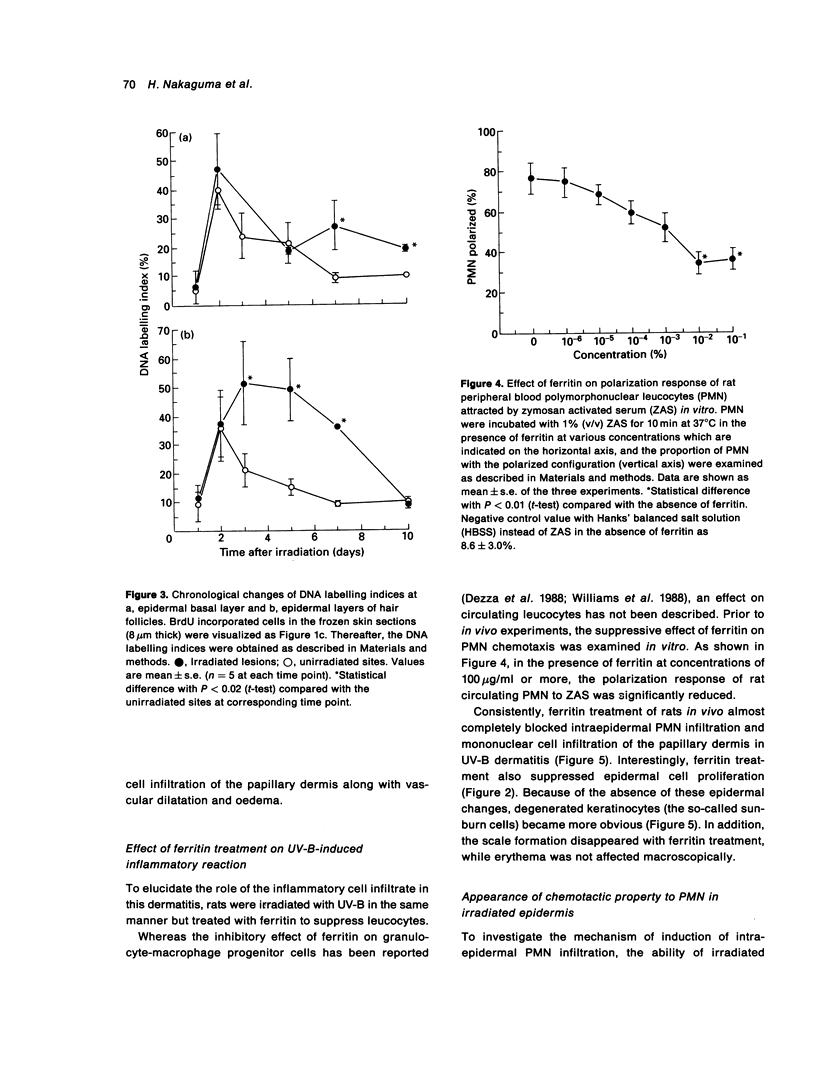
Ultraviolet ray B (UV-B) induced dermatitis in the rat may be a model for human psoriasis vulgaris. Detailed studies of this model are reported. Rat skin responded to UV-B irradiation quite differently from human, guinea-pig, or mouse skin. Rat UV-B dermatitis was characterized by a sharply demarcated brownish-red lesion with scale formation lasting for 10 days. Histologically, microvascular dilatation, intraepidermal accumulation of polymorphonuclear leucocytes with microabscess, mononuclear cell infiltration at the papillary dermis and hyperproliferation of epidermal cells were observed. These features were similar to those of clinical psoriasis vulgaris in man. Leucocyte suppression, induced by systemic ferritin administration to the irradiated rats, resulted in loss of the epidermal hyperproliferation and inhibition of the tissue leucocytosis. This leucocyte suppression remodelled the picture of the rat UV-B dermatitis into that seen in other mammalian species, where microvascular dilatation and degeneration of keratinocytes (so-called sunburn cells) are characteristic. The irradiated epidermis of the rats treated with ferritin possessed an in vitro PMN chemotactic property. Rat UV-B dermatitis seems to be a useful model to investigate aetiopathogenic mechanisms in psoriasis vulgaris. However, the former heals after injury and does not relapse as does psoriasis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzoni F., Cassatella M. A., Rossi F., Ceska M., Dewald B., Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991 Mar 1;173(3):771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers E., Schröder J. M., Mrowietz U. Identification of two endogenous neutrophil-activating peptides in psoriatic skin and inflammatory cells: C5ades arg and NAP. Dermatologica. 1989;179 (Suppl 1):9–15. doi: 10.1159/000248440. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezza L., Cazzola M., Bergamaschi G., Stella C. C., Pedrazzoli P., Recalde H. R. Effects of recombinant human H-subunit and L-subunit ferritins on in vitro growth of human granulocyte-monocyte progenitors. Br J Haematol. 1988 Mar;68(3):367–372. doi: 10.1111/j.1365-2141.1988.tb04216.x. [DOI] [PubMed] [Google Scholar]
- Fogh K., Herlin T., Kragballe K. Eicosanoids in acute and chronic psoriatic lesions: leukotriene B4, but not 12-hydroxy-eicosatetraenoic acid, is present in biologically active amounts in acute guttate lesions. J Invest Dermatol. 1989 Jun;92(6):837–841. doi: 10.1111/1523-1747.ep12696858. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Kato T., Terui T., Takematsu H., Tagami H. Effects of psoriatic scale extracts on oxidative metabolic responses in granulocytes assessed by chemiluminescence. Inflammation. 1989 Feb;13(1):59–66. doi: 10.1007/BF00918963. [DOI] [PubMed] [Google Scholar]
- Kukita I., Yamamoto T., Kawaguchi T., Kambara T. Fifth component of complement (C5)-derived high-molecular-weight macrophage chemotactic factor in normal guinea pig serum. Inflammation. 1987 Dec;11(4):459–479. doi: 10.1007/BF00915989. [DOI] [PubMed] [Google Scholar]
- Matsubara S., Yamamoto T., Tsuruta T., Takagi K., Kambara T. Complement C4-derived monocyte-directed chemotaxis-inhibitory factor. A molecular mechanism to cause polymorphonuclear leukocyte-predominant infiltration in rheumatoid arthritis synovial cavities. Am J Pathol. 1991 May;138(5):1279–1291. [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y., Saga K., Bando M., Takahashi M. In-vitro DNA synthesis of keratinocytes in normal human skin, psoriasis, seborrhoeic keratosis, Bowen's disease and basal cell carcinoma. Br J Dermatol. 1991 Jul;125(1):9–13. doi: 10.1111/j.1365-2133.1991.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Nakaguma H., Takahashi H. Remarkable elevation of leukotriene B4 in rat skin after induction of UV photodermatitis. Inflammation. 1990 Apr;14(2):195–203. doi: 10.1007/BF00917458. [DOI] [PubMed] [Google Scholar]
- Pinkus H., Mehregan A. H. The primary histologic lesion of seborrheic dermatitis and psoriasis. J Invest Dermatol. 1966 Jan;46(1):109–116. doi: 10.1038/jid.1966.16. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Kasahara K., Allen R., Showell H. J., Standiford T. J., Kunkel S. L. Human neutrophils exhibit disparate chemotactic factor gene expression. Biochem Biophys Res Commun. 1990 Dec 14;173(2):725–730. doi: 10.1016/s0006-291x(05)80095-6. [DOI] [PubMed] [Google Scholar]
- Takematsu H., Tagami H. Quantification of chemotactic peptides (C5a anaphylatoxin and IL-8) in psoriatic lesional skin. Arch Dermatol. 1993 Jan;129(1):74–80. [PubMed] [Google Scholar]
- Takeya M., Tsuchiya T., Shimokawa Y., Takahashi K. A new monoclonal antibody, PM-2K, specifically recognizes tissue macrophages but not blood monocytes. J Pathol. 1991 Apr;163(4):315–321. doi: 10.1002/path.1711630408. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Cooper S., Broxmeyer H. E. Effects of hematopoietic suppressor molecules on the in vitro proliferation of purified murine granulocyte-macrophage progenitor cells. Cancer Res. 1988 Mar 15;48(6):1548–1550. [PubMed] [Google Scholar]