Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Malcolm L., Chosich N., Miller J. F. Inflammation but not autoimmunity occurs in transgenic mice expressing constitutive levels of interleukin-2 in islet beta cells. Eur J Immunol. 1992 May;22(5):1115–1121. doi: 10.1002/eji.1830220503. [DOI] [PubMed] [Google Scholar]
- Asano M., Hayashi M., Yoshida E., Kawade Y., Iwakura Y. Induction of interferon-alpha by interferon-beta, but not of interferon-beta by interferon-alpha, in the mouse. Virology. 1990 May;176(1):30–38. doi: 10.1016/0042-6822(90)90227-i. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Boulay J. L., Paul W. E. The interleukin-4 family of lymphokines. Curr Opin Immunol. 1992 Jun;4(3):294–298. doi: 10.1016/0952-7915(92)90079-t. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Bodine D. M., Dunbar C. E., Nienhuis A. W. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990 Aug;86(2):592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. M., Maini R. N., Feldmann M. TNF alpha--a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992 May;31(5):293–298. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Tepper R. I., Leder P., Abbas A. K. Humoral immune functions in IL-4 transgenic mice. J Immunol. 1991 Nov 1;147(9):2950–2956. [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chambers C. A., Kang J., Hozumi N. Long term expression of IL-4 in vivo using retroviral-mediated gene transfer. J Immunol. 1992 Nov 1;149(9):2899–2905. [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Gonda T. J., Johnson G. R. Long-term exposure to retrovirally expressed granulocyte-colony-stimulating factor induces a nonneoplastic granulocytic and progenitor cell hyperplasia without tissue damage in mice. J Clin Invest. 1989 Nov;84(5):1488–1496. doi: 10.1172/JCI114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Lang R. A., Gonda T. J., Johnson G. R. Nonneoplastic hematopoietic myeloproliferative syndrome induced by dysregulated multi-CSF (IL-3) expression. Blood. 1989 May 1;73(6):1487–1497. [PubMed] [Google Scholar]
- Chen X. Z., Yun J. S., Wagner T. E. Enhanced viral resistance in transgenic mice expressing the human beta 1 interferon. J Virol. 1988 Oct;62(10):3883–3887. doi: 10.1128/jvi.62.10.3883-3887.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Turksen K., Yu Q. C., Schreiber H., Teng M., Fuchs E. Cachexia and graft-vs.-host-disease-type skin changes in keratin promoter-driven TNF alpha transgenic mice. Genes Dev. 1992 Aug;6(8):1444–1456. doi: 10.1101/gad.6.8.1444. [DOI] [PubMed] [Google Scholar]
- Colombo M. P., Ferrari G., Stoppacciaro A., Parenza M., Rodolfo M., Mavilio F., Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991 Apr 1;173(4):889–897. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Metcalf D., Edwards S. J., Handman E. GM-CSF produced by recombinant vaccinia virus or in GM-CSF transgenic mice has no effect in vivo on murine cutaneous leishmaniasis. J Parasitol. 1988 Oct;74(5):763–767. [PubMed] [Google Scholar]
- Cuthbertson R. A., Lang R. A., Coghlan J. P. Macrophage products IL-1 alpha, TNF alpha and bFGF may mediate multiple cytopathic effects in the developing eyes of GM-CSF transgenic mice. Exp Eye Res. 1990 Sep;51(3):335–344. doi: 10.1016/0014-4835(90)90030-x. [DOI] [PubMed] [Google Scholar]
- Cuthbertson R. A., Lang R. A. Developmental ocular disease in GM-CSF transgenic mice is mediated by autostimulated macrophages. Dev Biol. 1989 Jul;134(1):119–129. doi: 10.1016/0012-1606(89)90083-3. [DOI] [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Interferon-gamma. Curr Opin Immunol. 1992 Jun;4(3):321–326. doi: 10.1016/0952-7915(92)90083-q. [DOI] [PubMed] [Google Scholar]
- Dent L. A., Strath M., Mellor A. L., Sanderson C. J. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990 Nov 1;172(5):1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Faulkner-Jones B. E., Stanton H., Hamilton J. A., Metcalf D. Plasminogen activator in granulocyte-macrophage-CSF transgenic mice. J Immunol. 1992 Dec 1;149(11):3678–3681. [PubMed] [Google Scholar]
- Elliott M. J., Strasser A., Metcalf D. Selective up-regulation of macrophage function in granulocyte-macrophage colony-stimulating factor transgenic mice. J Immunol. 1991 Nov 1;147(9):2957–2963. [PubMed] [Google Scholar]
- Escary J. L., Perreau J., Duménil D., Ezine S., Brûlet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993 May 27;363(6427):361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- Fausto N. Growth factors in liver development, regeneration and carcinogenesis. Prog Growth Factor Res. 1991;3(3):219–234. doi: 10.1016/0955-2235(91)90008-r. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Martin G. R. Cut, paste, and save: new approaches to altering specific genes in mice. Cell. 1989 Jan 27;56(2):145–147. doi: 10.1016/0092-8674(89)90887-8. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Metcalf D., Moore J. G., Nicola N. A. Elevated levels of GM-CSF and IL-1 in the serum, peritoneal and pleural cavities of GM-CSF transgenic mice. Immunology. 1989 Jun;67(2):216–220. [PMC free article] [PubMed] [Google Scholar]
- Giroir B. P., Brown T., Beutler B. Constitutive synthesis of tumor necrosis factor in the thymus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4864–4868. doi: 10.1073/pnas.89.11.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroir B. P., Johnson J. H., Brown T., Allen G. L., Beutler B. The tissue distribution of tumor necrosis factor biosynthesis during endotoxemia. J Clin Invest. 1992 Sep;90(3):693–698. doi: 10.1172/JCI115939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroir B. P., Peppel K., Silva M., Beutler B. The biosynthesis of tumor necrosis factor during pregnancy: studies with a CAT reporter transgene and TNF inhibitors. Eur Cytokine Netw. 1992 Nov-Dec;3(6):533–538. [PubMed] [Google Scholar]
- Gordon J. W. Transgenic animals. Int Rev Cytol. 1989;115:171–229. doi: 10.1016/s0074-7696(08)60630-0. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Waldschmidt T. J., Finkelman F. D., Hess B. W., Alpert A. R., Boiani N. E., Namen A. E., Morrissey P. J. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993 Jul 1;178(1):257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ramos J. C., Martínez C., Köhler G., Iglesias A. Analysis of T-cell subpopulations in human IL-2R alpha transgenic mice: expansion of Thy1.2- thymocytes and depletion of double-positive T-cell precursors. Res Immunol. 1989 Sep;140(7):661–674. doi: 10.1016/0923-2494(89)90020-5. [DOI] [PubMed] [Google Scholar]
- Halter S. A., Dempsey P., Matsui Y., Stokes M. K., Graves-Deal R., Hogan B. L., Coffey R. J. Distinctive patterns of hyperplasia in transgenic mice with mouse mammary tumor virus transforming growth factor-alpha. Characterization of mammary gland and skin proliferations. Am J Pathol. 1992 May;140(5):1131–1146. [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Okazaki H., Ishida Y., Onuma M., Kano S., Honjo T., Minato N. Expression of murine IL-2 receptor beta-chain on thymic and splenic lymphocyte subpopulations as revealed by the IL-2-induced proliferative response in human IL-2 receptor alpha-chain transgenic mice. J Immunol. 1990 May 15;144(10):3809–3815. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- Hekman A. C., Trapman J., Mulder A. H., van Gaalen J. L., Zwarthoff E. C. Interferon expression in the testes of transgenic mice leads to sterility. J Biol Chem. 1988 Aug 25;263(24):12151–12155. [PubMed] [Google Scholar]
- Higuchi Y., Herrera P., Muniesa P., Huarte J., Belin D., Ohashi P., Aichele P., Orci L., Vassalli J. D., Vassalli P. Expression of a tumor necrosis factor alpha transgene in murine pancreatic beta cells results in severe and permanent insulitis without evolution towards diabetes. J Exp Med. 1992 Dec 1;176(6):1719–1731. doi: 10.1084/jem.176.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi Y., Yamaguchi N., Korenaga M., Mita S., Tominaga A., Takatsu K. In vivo administration of antibody to murine IL-5 receptor inhibits eosinophilia of IL-5 transgenic mice. Int Immunol. 1991 Feb;3(2):135–139. doi: 10.1093/intimm/3.2.135. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Nishi M., Taguchi O., Inaba K., Hattori M., Minato N., Kawaichi M., Honjo T. Expansion of natural killer cells but not T cells in human interleukin 2/interleukin 2 receptor (Tac) transgenic mice. J Exp Med. 1989 Oct 1;170(4):1103–1115. doi: 10.1084/jem.170.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Nishi M., Taguchi O., Inaba K., Minato N., Kawaichi M., Honjo T. Effects of the deregulated expression of human interleukin-2 in transgenic mice. Int Immunol. 1989;1(2):113–120. doi: 10.1093/intimm/1.2.113. [DOI] [PubMed] [Google Scholar]
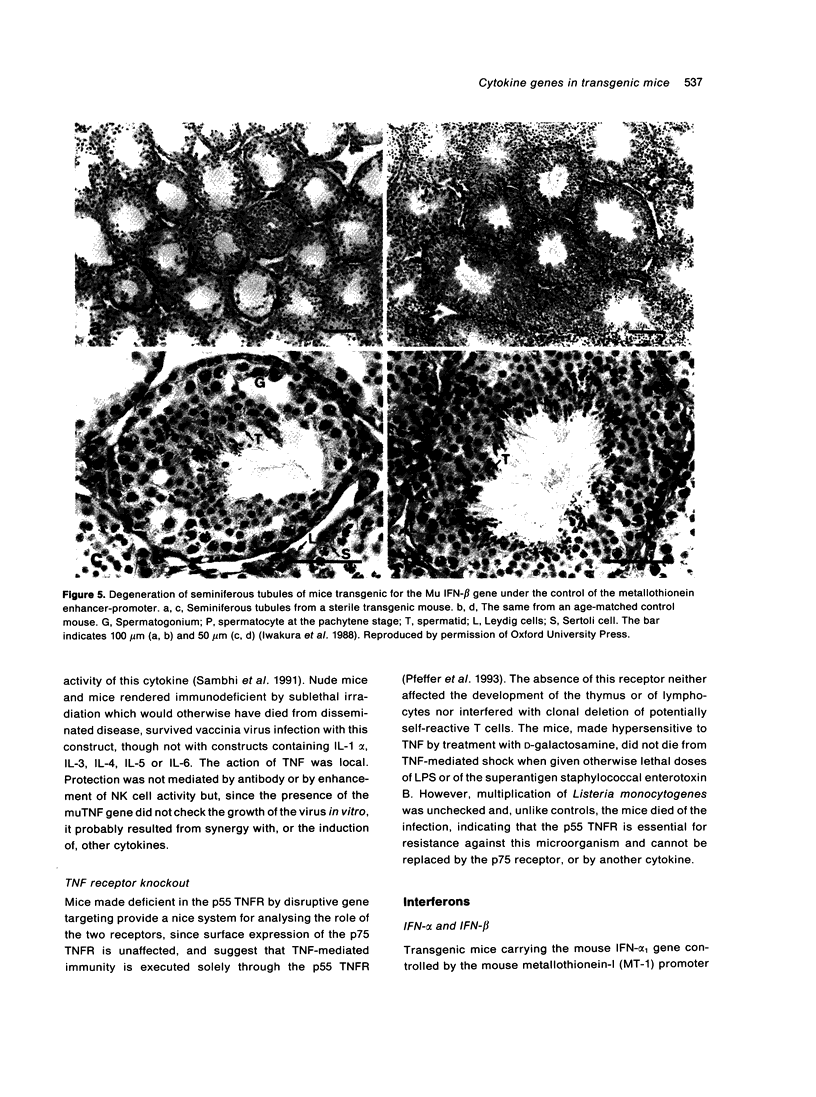
- Iwakura Y., Asano M., Nishimune Y., Kawade Y. Male sterility of transgenic mice carrying exogenous mouse interferon-beta gene under the control of the metallothionein enhancer-promoter. EMBO J. 1988 Dec 1;7(12):3757–3762. doi: 10.1002/j.1460-2075.1988.tb03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S., Bendig M. M., Kanai Y., Shultz L. D., Hitoshi Y., Takatsu K., Tominaga A. Maintenance of CD5+ B cells at an early developmental stage by interleukin-5: evidence from immunoglobulin gene usage in interleukin-5 transgenic mice. DNA Cell Biol. 1993 Jul-Aug;12(6):481–491. doi: 10.1089/dna.1993.12.481. [DOI] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohonen-Corish M. R., King N. J., Woodhams C. E., Ramshaw I. A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-gamma. Eur J Immunol. 1990 Jan;20(1):157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M. C., Bluethmann H., Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993 Mar 18;362(6417):245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Koury S. T., Bondurant M. C., Koury M. J., Semenza G. L. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood. 1991 Jun 1;77(11):2497–2503. [PubMed] [Google Scholar]
- Kroemer G., Wick G. The role of interleukin 2 in autoimmunity. Immunol Today. 1989 Jul;10(7):246–251. doi: 10.1016/0167-5699(89)90262-4. [DOI] [PubMed] [Google Scholar]
- Kroemer G., de Cid R., Moreno de Alborán I., Gonzalo J. A., Iglesias A., Martínez C., Gutierrez-Ramos J. C. Immunological self-tolerance: an analysis employing cytokines or cytokine receptors encoded by transgenes or a recombinant vaccinia virus. Immunol Rev. 1991 Aug;122:173–204. doi: 10.1111/j.1600-065x.1991.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Endogenous tumor necrosis factor alpha is required for enhanced antimicrobial activity against Toxoplasma gondii and Listeria monocytogenes in recombinant gamma interferon-treated mice. Infect Immun. 1992 Dec;60(12):5107–5112. doi: 10.1128/iai.60.12.5107-5112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal L. M., Moss D. W., Kuhn R., Müller W., Liew F. Y. Interleukin-4 transgenic mice of resistant background are susceptible to Leishmania major infection. Eur J Immunol. 1993 Feb;23(2):566–569. doi: 10.1002/eji.1830230241. [DOI] [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Forbush K. A., Carpenter J., Sato T. A., Grossman A., Liggitt D. H., Perlmutter R. M. Interleukin 4 expressed in situ selectively alters thymocyte development. J Exp Med. 1991 Jan 1;173(1):89–100. doi: 10.1084/jem.173.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye A. P., Abrams J. S., Silver J. E., Ottesen E. A., Nutman T. B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990 Jul 1;172(1):399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- Maliszewski C. R., Morrissey P. J., Fanslow W. C., Sato T. A., Willis C., Davison B. Delayed allograft rejection in mice transgenic for a soluble form of the IL-4 receptor. Cell Immunol. 1992 Sep;143(2):434–448. doi: 10.1016/0008-8749(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Halter S. A., Holt J. T., Hogan B. L., Coffey R. J. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990 Jun 15;61(6):1147–1155. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Elliott M. J., Nicola N. A. The excess numbers of peritoneal macrophages in granulocyte-macrophage colony-stimulating factor transgenic mice are generated by local proliferation. J Exp Med. 1992 Apr 1;175(4):877–884. doi: 10.1084/jem.175.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Gearing D. P. Fatal syndrome in mice engrafted with cells producing high levels of the leukemia inhibitory factor. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5948–5952. doi: 10.1073/pnas.86.15.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Leukemia inhibitory factor--a puzzling polyfunctional regulator. Growth Factors. 1992;7(3):169–173. doi: 10.3109/08977199209046921. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Mechanisms contributing to the sex difference in levels of granulocyte-macrophage colony-stimulating factor in the urine of GM-CSF transgenic mice. Exp Hematol. 1988 Oct;16(9):794–800. [PubMed] [Google Scholar]
- Metcalf D., Moore J. G. Divergent disease patterns in granulocyte-macrophage colony-stimulating factor transgenic mice associated with different transgene insertion sites. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7767–7771. doi: 10.1073/pnas.85.20.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Transgenic mice as models of hemopoiesis. Cancer. 1991 May 15;67(10 Suppl):2695–2699. doi: 10.1002/1097-0142(19910515)67:10+<2695::aid-cncr2820671704>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Minasi L. E., Kamogawa Y., Carding S., Bottomly K., Flavell R. A. The selective ablation of interleukin 2-producing cells isolated from transgenic mice. J Exp Med. 1993 May 1;177(5):1451–1459. doi: 10.1084/jem.177.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz J. D., Zhou T., Johnson L. Production of transgenic mice and application to immunology and autoimmunity. Am J Med Sci. 1990 Nov;300(5):322–329. doi: 10.1097/00000441-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Müller W., Kühn R., Rajewsky K. Major histocompatibility complex class II hyperexpression on B cells in interleukin 4-transgenic mice does not lead to B cell proliferation and hypergammaglobulinemia. Eur J Immunol. 1991 Apr;21(4):921–925. doi: 10.1002/eji.1830210410. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Williams D. E., Goodwin R. G. Interleukin-7: a new hematopoietic growth factor. Prog Clin Biol Res. 1990;338:65–73. [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M., Ishida Y., Honjo T. Expression of functional interleukin-2 receptors in human light chain/Tac transgenic mice. Nature. 1988 Jan 21;331(6153):267–269. doi: 10.1038/331267a0. [DOI] [PubMed] [Google Scholar]
- Owen W. F., Rothenberg M. E., Petersen J., Weller P. F., Silberstein D., Sheffer A. L., Stevens R. L., Soberman R. J., Austen K. F. Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med. 1989 Jul 1;170(1):343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K., Matsuyama T., Kündig T. M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P. S., Krönke M., Mak T. W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993 May 7;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Picarella D. E., Kratz A., Li C. B., Ruddle N. H., Flavell R. A. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10036–10040. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Widera G., Cowing C., Heber-Katz E., Palmiter R. D., Flavell R. A., Brinster R. L. Tissue-specific, inducible and functional expression of the E alpha d MHC class II gene in transgenic mice. EMBO J. 1985 Sep;4(9):2225–2230. doi: 10.1002/j.1460-2075.1985.tb03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer C., Richter G., Uberla K., Müller W., Blöcker H., Diamantstein T., Blankenstein T. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur J Immunol. 1992 May;22(5):1179–1184. doi: 10.1002/eji.1830220511. [DOI] [PubMed] [Google Scholar]
- Porter D. L., Goldberg M. A. Regulation of erythropoietin production. Exp Hematol. 1993 Mar;21(3):399–404. [PubMed] [Google Scholar]
- Probert L., Keffer J., Corbella P., Cazlaris H., Patsavoudi E., Stephens S., Kaslaris E., Kioussis D., Kollias G. Wasting, ischemia, and lymphoid abnormalities in mice expressing T cell-targeted human tumor necrosis factor transgenes. J Immunol. 1993 Aug 15;151(4):1894–1906. [PubMed] [Google Scholar]
- Ramsay A. J., Ruby J., Ramshaw I. A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993 Apr;14(4):155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Ruby J., Ramsay A. Cytokine expression by recombinant viruses--a new vaccine strategy. Trends Biotechnol. 1992 Dec;10(12):424–426. doi: 10.1016/0167-7799(92)90291-3. [DOI] [PubMed] [Google Scholar]
- Rich B. E., Campos-Torres J., Tepper R. I., Moreadith R. W., Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med. 1993 Feb 1;177(2):305–316. doi: 10.1084/jem.177.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle N. H. Tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta). Curr Opin Immunol. 1992 Jun;4(3):327–332. doi: 10.1016/0952-7915(92)90084-r. [DOI] [PubMed] [Google Scholar]
- Sambhi S. K., Kohonen-Corish M. R., Ramshaw I. A. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4025–4029. doi: 10.1073/pnas.88.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren E. P., Luetteke N. C., Palmiter R. D., Brinster R. L., Lee D. C. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990 Jun 15;61(6):1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Liggitt D., Pitts S. L., Hansen S. E., Stewart T. A. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell. 1988 Mar 11;52(5):773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvetnick N., Shizuru J., Liggitt D., Martin L., McIntyre B., Gregory A., Parslow T., Stewart T. Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature. 1990 Aug 30;346(6287):844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Pollard R. B., Schmitt D., Suzuki F. Transforming growth factor-beta in the regulation of the immune response. Clin Immunol Immunopathol. 1992 Oct;65(1):1–9. doi: 10.1016/0090-1229(92)90241-f. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hünig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991 Aug 15;352(6336):621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Semenza G. L., Koury S. T., Nejfelt M. K., Gearhart J. D., Antonarakis S. E. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8725–8729. doi: 10.1073/pnas.88.19.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Traystman M. D., Gearhart J. D., Antonarakis S. E. Polycythemia in transgenic mice expressing the human erythropoietin gene. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2301–2305. doi: 10.1073/pnas.86.7.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Cheever A. W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990 Dec 1;145(11):3911–3916. [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2. Curr Opin Immunol. 1992 Jun;4(3):271–276. doi: 10.1016/0952-7915(92)90076-q. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Kay A. B., Gleich G. J. Eosinophils 1992. Immunol Today. 1992 Oct;13(10):384–387. doi: 10.1016/0167-5699(92)90085-L. [DOI] [PubMed] [Google Scholar]
- Stoppacciaro A., Melani C., Parenza M., Mastracchio A., Bassi C., Baroni C., Parmiani G., Colombo M. P. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J Exp Med. 1993 Jul 1;178(1):151–161. doi: 10.1084/jem.178.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strath M., Dent L., Sanderson C. Infection of IL5 transgenic mice with Mesocestoides corti induces very high levels of IL5 but depressed production of eosinophils. Exp Hematol. 1992 Feb;20(2):229–234. [PubMed] [Google Scholar]
- Suematsu S., Matsuda T., Aozasa K., Akira S., Nakano N., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu S., Matsusaka T., Matsuda T., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington F. W. Lymphotoxin, tumor necrosis factor, and gamma interferon are cytostatic for normal human keratinocytes. J Invest Dermatol. 1989 Jun;92(6):798–805. doi: 10.1111/1523-1747.ep12696816. [DOI] [PubMed] [Google Scholar]
- Takatsu K. Interleukin-5. Curr Opin Immunol. 1992 Jun;4(3):299–306. doi: 10.1016/0952-7915(92)90080-x. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Levinson D. A., Stanger B. Z., Campos-Torres J., Abbas A. K., Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990 Aug 10;62(3):457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Tominaga A., Takaki S., Koyama N., Katoh S., Matsumoto R., Migita M., Hitoshi Y., Hosoya Y., Yamauchi S., Kanai Y. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp Med. 1991 Feb 1;173(2):429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H. T., Metcalf D., Cheers C. Anti-bacterial activity of peritoneal cells from transgenic mice producing high levels of GM-CSF. Immunology. 1990 Nov;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
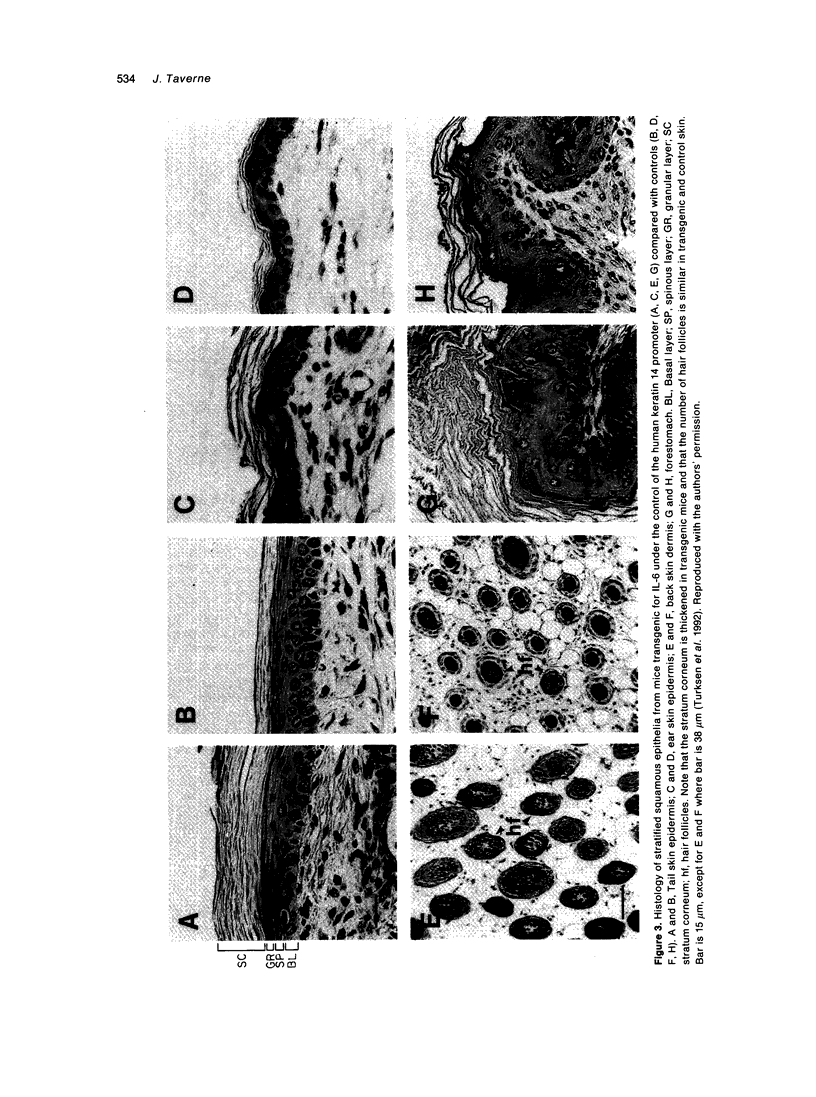
- Turksen K., Kupper T., Degenstein L., Williams I., Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5068–5072. doi: 10.1073/pnas.89.11.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Vassar R., Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991 May;5(5):714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Lalor P. A., Cory S., Johnson G. R. In vivo expression of interleukin 5 induces an eosinophilia and expanded Ly-1B lineage populations. Int Immunol. 1990;2(10):965–971. doi: 10.1093/intimm/2.10.965. [DOI] [PubMed] [Google Scholar]
- Wetzel G. D. Interleukin 5 regulation of peritoneal Ly-1 B lymphocyte proliferation, differentiation and autoantibody secretion. Eur J Immunol. 1989 Sep;19(9):1701–1707. doi: 10.1002/eji.1830190926. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Feldmann M., Maini R. N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogensen L., Huang X., Sarvetnick N. Leukocyte extravasation into the pancreatic tissue in transgenic mice expressing interleukin 10 in the islets of Langerhans. J Exp Med. 1993 Jul 1;178(1):175–185. doi: 10.1084/jem.178.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Dunbar C. E., Bodine D. M., Ruscetti S., Nienhuis A. W. Retrovirus-mediated transfer and expression of the interleukin-3 gene in mouse hematopoietic cells result in a myeloproliferative disorder. Mol Cell Biol. 1989 Feb;9(2):798–808. doi: 10.1128/mcb.9.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Are bacterial endotoxins involved in autoimmunity by CD5+ (Ly-1+) B cells? Immunol Today. 1989 Oct;10(10):334–336. doi: 10.1016/0167-5699(89)90189-8. [DOI] [PubMed] [Google Scholar]