Abstract
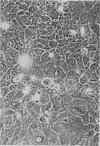
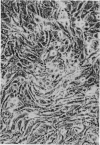
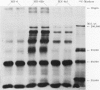
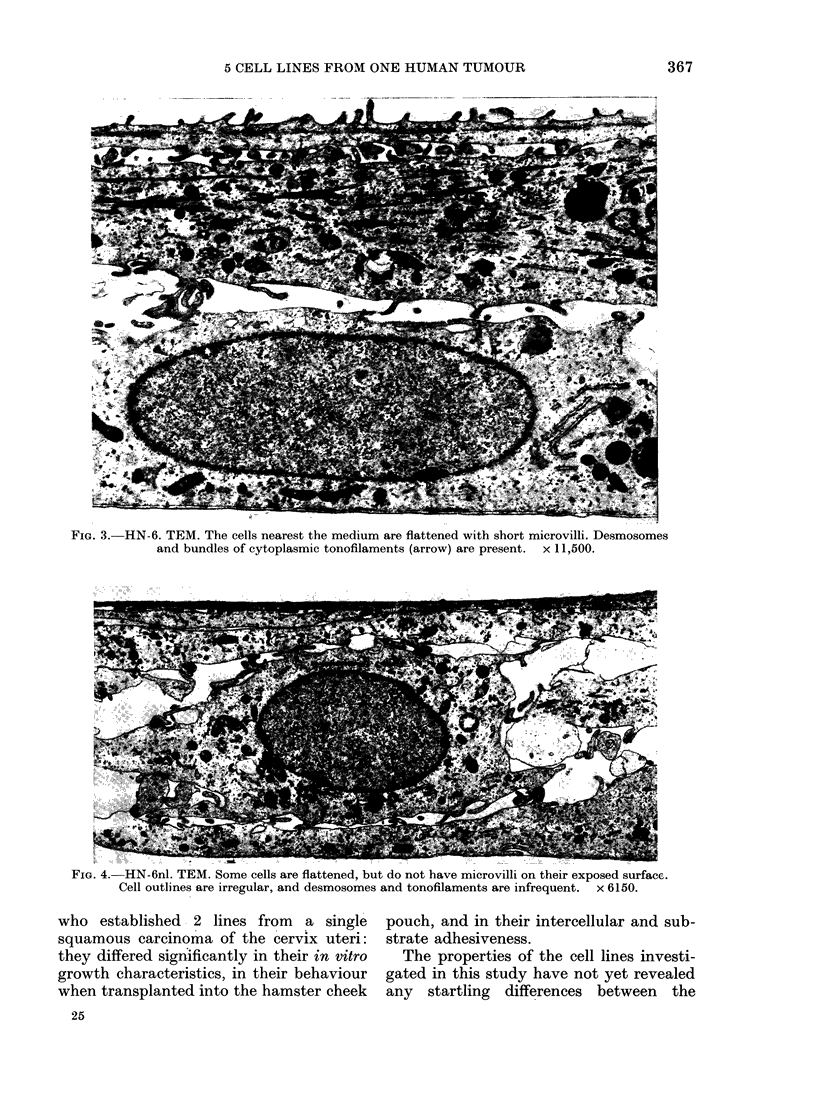
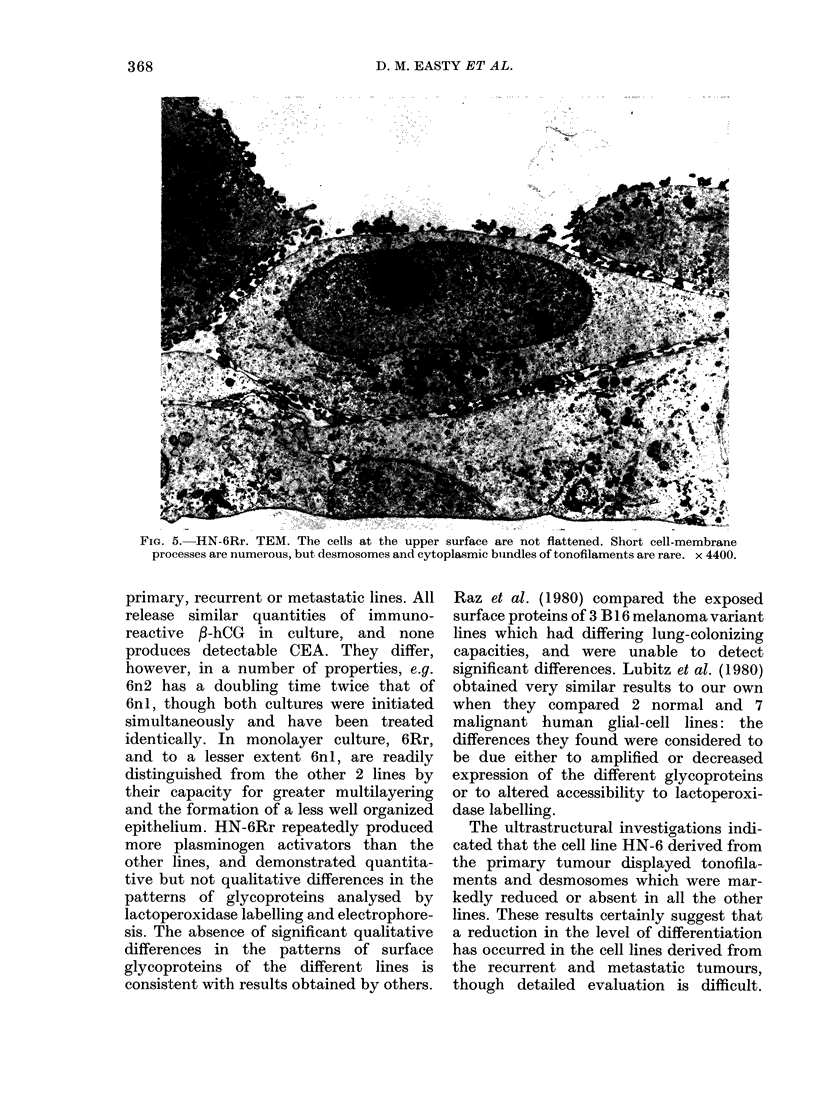
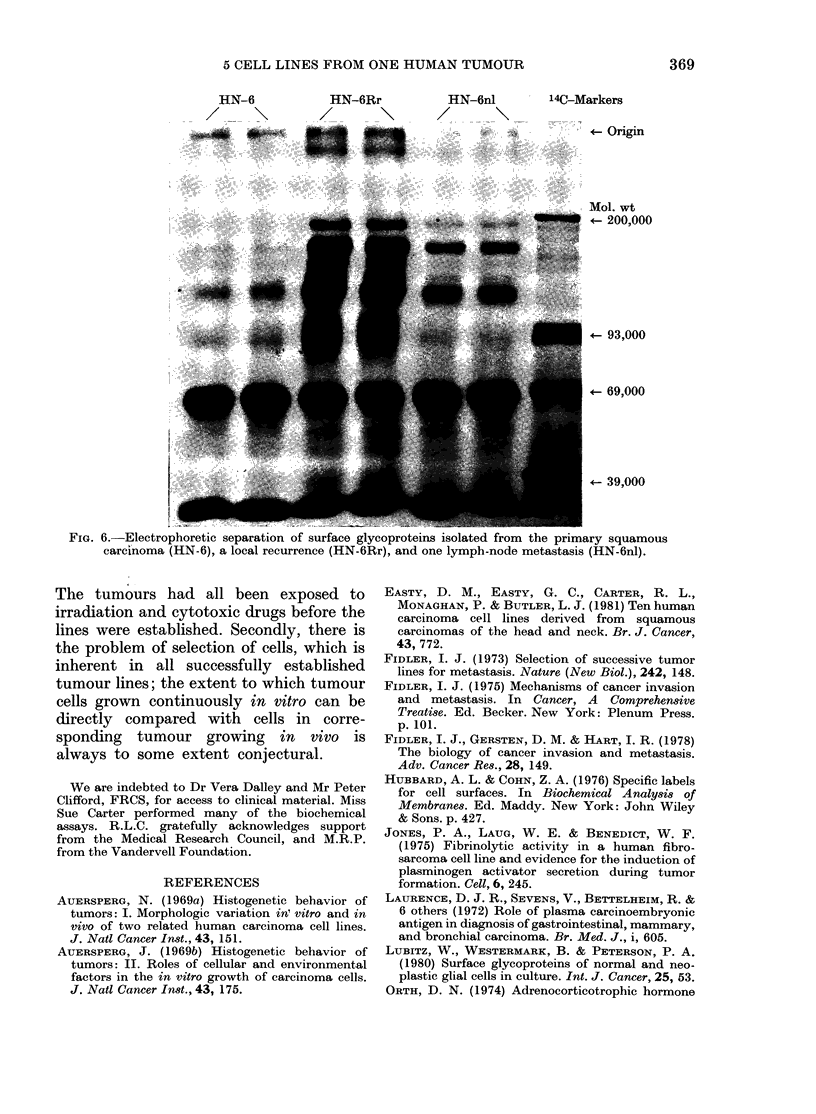
Five tumour cell lines have been derived from a primary squamous carcinoma of the tongue, from 2 subsequent local recurrences, and from 2 lymph-node metastases--all from the same patient. While the cell lines shared many morphological and biochemical characteristics, those derived from recurrences and metastases appeared to be less differentiated, were less well organized in culture, and displayed fewer desmosomes and tonofilaments than cells in the primary tumour line. A recurrent line showing greatest morphological divergence from the primary tumour line also demonstrated the greatest differences at the ultrastructural level, in increased production of plasminogen activator and in the composition of cell-surface glycoproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auersperg N. Histogenetic behavior of tumors. I. Morphologic variation in vitro and in vivo of two related human carcinoma cell lines. J Natl Cancer Inst. 1969 Jul;43(1):151–173. [PubMed] [Google Scholar]
- Auersperg N. Histogenetic behavior of tumors. II. Roles of cellular and environmental factors in the in vitro growth of carcinoma cells. J Natl Cancer Inst. 1969 Jul;43(1):175–190. [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Butler L. J. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br J Cancer. 1981 Jun;43(6):772–785. doi: 10.1038/bjc.1981.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Benedict W. F. Fibrinolytic activity in a human fibrosarcoma cell line and evidence for the induction of plasminogen activator secretion during tumor formation. Cell. 1975 Oct;6(2):245–252. doi: 10.1016/0092-8674(75)90015-x. [DOI] [PubMed] [Google Scholar]
- Laurence D. J., Stevens U., Bettelheim R., Darcy D., Leese C., Turberville C., Alexander P., Johns E. W., Neville A. M. Role of plasma carcinoembryonic antigen in diagnosis of gastrointestinal, mammary, and bronchial carcinoma. Br Med J. 1972 Sep 9;3(5827):605–609. doi: 10.1136/bmj.3.5827.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz W., Westermark B., Peterson P. A. Surface glycoproteins of normal and neoplastic glia cells in culture. Int J Cancer. 1980 Jan 15;25(1):53–58. doi: 10.1002/ijc.2910250107. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Raz A., McLellan W. L., Hart I. R., Bucana C. D., Hoyer L. C., Sela B. A., Dragsten P., Fidler I. J. Cell surface properties of B16 melanoma variants with differing metastatic potential. Cancer Res. 1980 May;40(5):1645–1651. [PubMed] [Google Scholar]
- Weiss L. Dynamic aspects of cancer cell populations in metastasis. Am J Pathol. 1979 Dec;97(3):601–608. [PMC free article] [PubMed] [Google Scholar]