Abstract
Target DNA from the uncultivable Codakia orbicularis endosymbiont was PCR amplified from sea-grass sediment. To confirm that such amplifications originated from intact bacterial cells rather than free DNA, whole-cell hybridization (fluorescence in situ hybridization technique) with the specific probe Symco2 was performed along with experimental infection of aposymbiotic juveniles placed in contact with the same sediment. Taken together, the data demonstrate that the sulfide-oxidizing gill endosymbiont of Codakia orbicularis is present in the environment as a free-living uncultivable form.
All of the thioautotrophic endosymbioses occurring in marine invertebrates that have been examined so far are unambiguously confined to the gamma subdivision of the Proteobacteria, where they form a single coherent cluster (13, 14). These endosymbionts have to infect each new host generation successfully either by a vertical transmission from parents to offspring (6) or by an environmental transmission that involves the infection of the next host generation from an environmental stock of a free-living symbiont form (3).
The endosymbiont transmission mode has been elucidated for only few species due to the difficulty in cultivating symbionts and raising the invertebrate hosts from the egg to the adult stage in the laboratory. The environmental transmission mode was strongly suggested to occur in two oligochaetes (19) and in a few vestimentiferans based on molecular data obtained from mature gonads (8) and on ultrastructural observations of embryonic and larval stages (4, 35, 42). In bivalves, the symbiont transmission mode appears to be family specific, as suggested previously (23): vertical in Solemyidae (7, 33) and Vesicomyidae (8, 9) and environmental in Lucinidae (21, 23, 25, 26).
The shallow-water tropical lucinid Codakia orbicularis is the only marine invertebrate with chemoautotrophic bacterial endosymbionts for which the environmental transmission mode has unequivocally been demonstrated, by using experimental infections of aposymbiotic juveniles (21, 24) with unsterilized sediment collected from sea-grass beds as an inoculum. These experiments have suggested the presence of a free-living but uncultivable form of the gill endosymbiont in the sediment.
Recently, fluorescence in situ hybridization (FISH) with specific labeled oligonucleotide probes that target intracellular rRNA were described for the direct identification of individual bacterial cells within their natural environment (1, 2). This approach offers the advantage of circumventing the requirement for cultivation to identify and distinguish bacterial cells in environmental samples either directly (in situ) or after preliminary extraction (2). FISH probes have been successfully applied to the hydrothermal environment for the identification of yet-unculturable filamentous bacteria (41), ɛ-proteobacteria invertebrate epibionts (39), and thermophilic bacteria from deep-sea hydrothermal chimneys (28) but have never been used to locate chemoautotrophic endosymbionts prior to their colonizing invertebrate hosts.
MATERIALS AND METHODS
Sediment was collected from low-sulfide-containing Thalassia testudinum sea-grass beds and subdivided into three parts. The first part was used for experimental infections, the second was used for DNA extraction, and the third was fixed in 4% paraformaldehyde in 4× phosphate-buffered saline for FISH experiments.
Bacterial cells were extracted from sea-grass bed sediment as described elsewhere (28) before hybridization as described by Heddi et al. (30). Two oligonucleotides probes were used: EUB338 (5′-GCTGCCTCCCGTAGGAGT-3′), targeting most members of the eubacteria (1, 11), and the C. orbicularis symbiont-specific probe Symco2 (5′-TACAGAGGGTCGCCAACCCGTG-3′; Escherichia coli positions 1247 to 1268) (21).
Total nucleic acids were extracted from low and highly reduced sediments according to the protocol for DNA extraction from soil described by Zhou et al. (43) before PCR amplifications with the specific C. orbicularis symbiont primer set (21, 23). To protect against unspecific hybridization that could occur when amplifying total DNA extracted from the environment, each of the symbiont ribosomal DNA (rDNA) targets amplified from sediment was purified and sequenced independently. PCR products from three independent amplifications were pooled, sequenced, and manually aligned with the previously published complete 16S rDNA sequence of the C. orbicularis gill endosymbiont (EMBL accession number X84979) (15). A total of 730 nucleotide positions were utilized in this analysis.
RESULTS AND DISCUSSION
PCR amplifications performed with the specific C. orbicularis symbiont primer set (Symco1-1492r) on sea-grass bed sediments and on mangrove swamp samples produced DNA fragments around the expected size of 872 bp (Fig. 1). No amplification products were obtained from the negative control reactions.
FIG. 1.
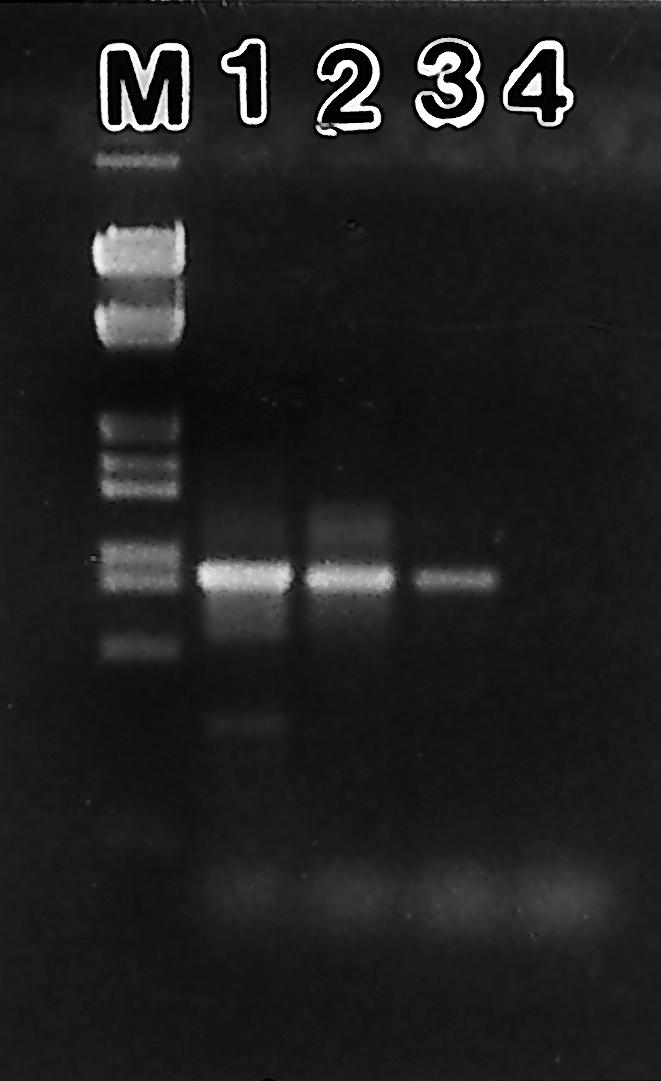
PCR detection of C. orbicularis free-living symbiont form in sediments. Lanes: M, DNA marker; 1, gill; 2, T. testudinum sea-grass sediment; 3, mangrove sediment; 4, negative control. PCR products are located between 831 and 983 bp according to the sizes of standard bands.
The oligonucleotide sequence of the specific primer Symco1 had been designed from 16S rDNA sequences deposited in database libraries. Such analyses could not be sufficient enough to guarantee a strong specificity of the primer sequences for studying the microbial diversity in the environment, as the sequences available represent only a small percentage of the environmental bacteria. Direct sequence analysis indicated that the PCR products amplified from sediment contained a single detectable sequence. A total of 730 nucleotides were sequenced for each sample analyzed, corresponding to positions 638 to 1002 and 1126 to 1492 of the E. coli nomenclature (5). These 16S rDNA sequences were identical at all 730 nucleotide positions determined from the previously examined C. orbicularis symbiont, indicating that the PCR fragments correspond to the gill endosymbiotic DNA.
However, PCR amplifications may also result from free DNA or dead symbionts rather than from free-living intact cells. Such a DNA could be released in the environment from a recently deceased clam close to the sediment collection spot or due to the feeding activity of clam predators that could release symbionts in the sediment. Therefore, FISH experiments were used to confirm the PCR results. Fluorescent probes have successfully been used in various studies of environmental bacteria from soils, lakes, or marine environments (10, 12, 20, 28, 38, 40) and also in studies of various animal symbioses (30, 31, 34).
Here, we used the symbiont-specific probe Symco2, which has good accessibility to its target site along the 16S rRNA sequence (18), i.e., 60%, compared to only 5% for Symco1 (E. coli positions 638 to 656).
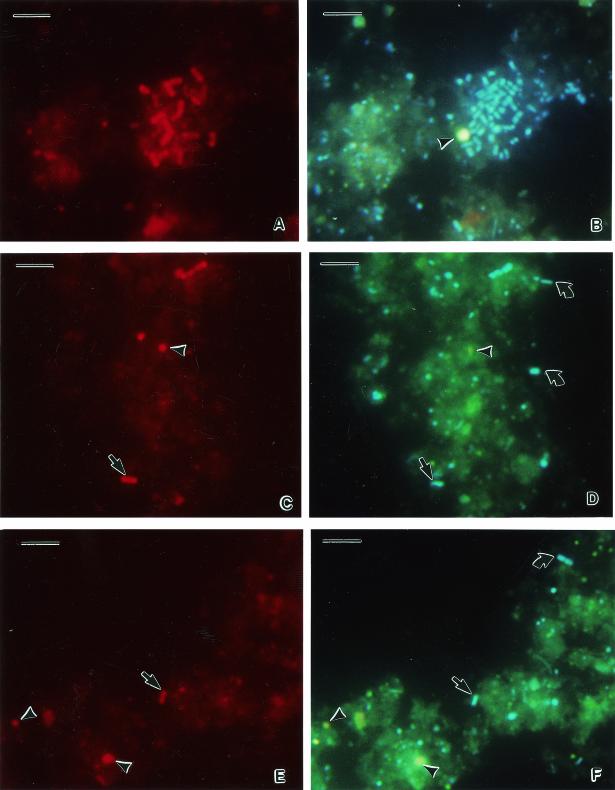
Most of the bacteria extracted hybridized with the universal probe EUB338, which was used as positive control (Fig. 2A), when compared to the cells stained with the DNA fluorescent dye 4′,6′-diamidino-2-phenylindole (DAPI) (Fig. 2B). Only a few bacteria were hybridized with the specific probe Symco 2 (Fig. 2C and E) compared to DAPI staining (Fig. 2D and F). Such cells likely represent the free-living form of the C. orbicularis gill endosymbiont.
FIG. 2.
Whole-cell hybridization of cells extracted from T. testudinum sediment. (A) Hybridization with eubacterial probe EUB338; (B) identical microscopic field for DAPI staining; (C and E) hybridization with the C. orbicularis symbiont-specific probe Symco2; (D and F) identical microscopic fields for DAPI staining. Arrowheads, nonbacterial sediment particles; straight arrows, free-living form of C. orbicularis gill endosymbiont; curved arrows, environmental bacteria. Bars, 5 μm.
The gill endosymbionts of C. orbicularis are generally rod shaped, large (up to 5 μm), and characterized by sulfur granules located in the periplasmic space (17). The free-living form of C. orbicularis gill endosymbiont extracted from T. testudinum sediment appears as small rods (1 to 2 μm) (Fig. 2C and E). Thus, the observation of the symbiont structure appears to reflect modifications that occur from extracellular to intracellular life styles. The most striking difference is in the bacterial size, which can increase two- to fivefold inside the bacteriocytes. This phenomenon seems to occurs in most animal bacteriocyte-inducing symbioses and may result from bacterial growth deregulation under intracellular conditions (37).
Experimental infections of aposymbiotic juveniles of C. orbicularis, obtained as described previously (22), were also performed with crude sediment collected in the same sea-grass beds. All juveniles were infected by symbiosis-competent bacteria from the sediment, while juveniles from the negative control remained aposymbiotic, indicating that no contamination had occurred in the laboratory during the experiments. This demonstrates that the sediment used for in situ hybridization contained viable bacteria that were able to initiate symbiosis with aposymbiotic host juveniles.
The data presented in this study provide the first evidence for the free-living form of this symbiont species in the environment and confirm environmental symbiont acquisition for the other lucinids colonized by the C. orbicularis symbiont (15, 16, 26, 27). Thus, lucinid endosymbionts may exhibit evolutionary features different from those of other invertebrate (solemyid and vesicomyid bivalves) and particularly insect symbioses, where endosymbionts are transmitted strictly vertically (7, 8, 33), which results in bacterial genome A+T bias and severe genome size reduction (29, 32, 36).
REFERENCES
- 1.Amann, R. I., M. Krumholtz, and D. A. Stahl. 1990. Fluorescent oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbial. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, W. D. 1981. Infection of legumes by rhizobia. Annu. Rev. Plant. Physiol. 32:407-422. [Google Scholar]
- 4.Bright, M., H. Keckeis, and C. R. Fisher. 2000. An autoradiographic examination of carbon fixation, transfer and utilization in the Riftia symbiosis. Mar. Biol. 136:621-632. [Google Scholar]
- 5.Brosius, J., T. J. Dull, D. D. Sleeter, and H. F. Noller. 1981. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J. Mol. Biol. 48:107-127. [DOI] [PubMed] [Google Scholar]
- 6.Buchner, P. 1965. Endosymbiosis of animals with plant micro-organisms. Interscience Publishers, New York, N.Y.
- 7.Cary, S. C. 1994. Vertical transmission of a chemoautotrophic symbiont in the protobranch bivalve, Solemya reidi. Mar. Mol. Biol. Biotechnol. 3:121-130. [PubMed] [Google Scholar]
- 8.Cary, S. C., and S. J. Giovannoni. 1993. Transovarial inheritance of endosymbiotic bacteria in clams inhabiting deep-sea hydrothermal vents and cold seeps. Proc. Natl. Acad. Sci. USA 90:5695-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cary, S. C., W. Warren, E. Anderson, and S. J. Giovannoni. 1993. Identification and localization of bacterial endosymbionts in hydrothermal vent taxa with symbiont specific polymerase chain reaction amplification and in situ hybridization techniques. Mar. Mol. Biol. Biotechnol. 2:51-62. [PubMed] [Google Scholar]
- 10.Christensen, H., M. Hansen, and J. Sorensen. 1999. Counting and size classification of active soil bacteria by fluorescence in situ hybridization with an rRNA oligonucleotide probe. Appl. Environ. Microbiol. 65:1753-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daims, H., A. Brühl, R. Amann, K. H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 12.DeLong, E. F., L. T. Taylor, T. L. Marsh, and C. M. Preston. 1999. Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl. Environ. Microbiol. 65:5554-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Distel, D. L., D. J. Lane, G. J. Olsen, H. Felbeck, S. J. Giovannoni, B. Pace, and D. A. Stahl. 1988. Sulfur-oxidizing bacterial endosymbionts: analysis of phylogeny and specificity by 16Sr RNA sequences. J. Bacteriol. 170:2506-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Distel, D. L., H. Felbeck, and C. M. Cavanaugh. 1994. Evidence for phylogenetic congruence among sulfur-oxidizing chemoautotrophic bacterial endosymbionts and their bivalve hosts. J. Mol. E. 38:533-542. [Google Scholar]
- 15.Durand, P., and O. Gros. 1996. Phylogenetic characterization of three Lucinid gill-endosymbionts determined by 16S rRNA gene sequence analysis. FEMS Microbiol. Lett. 140:193-198. [DOI] [PubMed] [Google Scholar]
- 16.Durand, P., O. Gros, L. Frenkiel, and D. Prieur. 1996. Phylogenetic characterization of sulfur-oxidizing bacteria endosymbionts in three tropical Lucinidae by using 16S rDNA sequence. Mol. Mar. Biol. Biotechnol. 5:37-42. [Google Scholar]
- 17.Frenkiel, L., and M. Mouëza. 1995. Gill ultrastructure and symbiotic bacteria in Codakia orbicularis (Bivalvia, Lucinidae). Zoomorphology 115:51-61. [Google Scholar]
- 18.Fuchs, B. M., G. Wallner, W. Beisker, I. Schwippl, W. Ludwig, and R. Amann. 1998. Flow cytometry analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 64:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giere, O., N. M. Conway, G. Gastrock, and C. Schmidt. 1991. Regulation of gutless annelid ecology by endosymbiotic bacteria. Mar. Ecol. Prog. Ser. 68:287-299. [Google Scholar]
- 20.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gros, O., A. Darrasse, P. Durand, L. Frenkiel, and M. Mouëza. 1996. Environmental transmission of a sulfur-oxidizing bacterial gill endosymbiont in the tropical lucinid bivalve Codakia orbicularis. Appl. Environ. Microbiol. 62:2324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gros, O., L. Frenkiel, and M. Mouëza. 1997. Embryonic, larval, and post-larval development in the symbiotic clam Codakia orbicularis (Bivalvia: Lucinidae). Invertebr. Biol. 116:86-101. [Google Scholar]
- 23.Gros, O., P. Durand, L. Frenkiel, and M. Mouëza. 1998. Putative environmental transmission of sulfur-oxidizing gill endosymbionts in four tropical lucinid bivalves, inhabiting various environments. FEMS Microbiol. Lett. 160:257-262. [Google Scholar]
- 24.Gros, O., L. Frenkiel, and M. Mouëza. 1998. Gill filament differentiation and experimental colonization by symbiotic bacteria in aposymbiotic juveniles of Codakia orbicularis (Bivalvia: Lucinidae). Invertebr. Reprod. Dev. 34:219-231. [Google Scholar]
- 25.Gros, O., M. R. Duplessis, and H. Felbeck. 1999. Embryonic development and endosymbiont transmission mode in the symbiotic clam Lucinoma aequizonata (Bivalvia: Lucinidae). Invertebr. Reprod. Dev. 36:93-103. [Google Scholar]
- 26.Gros, O., L. Frenkiel, and H. Felbeck. 2000. Sulfur-oxidizing endosymbiosis in Divaricella quadrisulcata (Bivalvia: Lucinidae): morphological, ultrastructural, and phylogenetic analysis. Symbiosis 29:293-317. [Google Scholar]
- 27.Gros, O., M. Liberge, and H. Felbeck. 2003. Interspecific infection of aposymbiotic juveniles of Codakia orbicularis by various tropical lucinid gill-endosymbionts. Mar. Biol. 142:57-66. [Google Scholar]
- 28.Harmsen, H. J. M., D. Prieur, and C. Jeanthon. 1997. Group-specific 16S rRNA-targeted oligonucleotide probes to identify thermophilic bacteria in marine hydrothermal vents. Appl. Environ. Microbiol. 63:4061-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heddi, A., H. Charles, C. Khatchadourian, G. Bonnot, and P. Nardon. 1998. Molecular characterization of the principal symbiotic bacteria of the weevil Sitophilus oryzae: a peculiar G-C content of an endocytobiotic DNA. J. Mol. E. 47:52-61. [DOI] [PubMed] [Google Scholar]
- 30.Heddi, A., A. M. Grenier, C. Khatchadourian, H. Charles, and P. Nardon. 1999. Four intracellular genomes direct weevil biology: mitochondrial, principal endosymbiont, and Wolbachia. Proc. Natl. Acad. Sci. USA 96:6814-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn, M., M. Wagner, K. D. Muller, E. N. Schmid, T. R. Fritsche, K. H. Schleifer, and R. Michel. 2000. Neochlamydia hartmannellae gen. nov., sp. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology 146:1231-1239. [DOI] [PubMed] [Google Scholar]
- 32.Itoh, T., W. Martin, and M. Nei. 2002. Acceleration of genomic evolution caused by enhanced mutation rate in endocellular symbionts. Proc. Natl. Acad. Sci. USA 99:12944-12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krueger, D. M., R. G. Gustafson, and C. M. Cavanaugh. 1996. Vertical transmission of chemoautotrophic symbionts in the bivalve Solemya velum (Bivalvia: Protobranchia). Biol. Bull. 190:195-202. [DOI] [PubMed] [Google Scholar]
- 34.Manz, W., G. Arp, G. Schumann-Kindel, U. Szewzyk, and J. Reitner. 2000. Widefield deconvolution epifluorescence microscopy combined with fluorescence in situ hybridization reveals the spatial arrangement of bacteria in sponge tissue. J. Microbiol. Methods 40:125-134. [DOI] [PubMed] [Google Scholar]
- 35.Marsh, A. G., L. S. Mullineaux, C. M. Young, and D. T. Manahan. 2001. Larval dispersal potential of the tube worm Riftia pachyptila at deep-sea hydrothermal vents. Nature 411:77-80. [DOI] [PubMed] [Google Scholar]
- 36.Moran, N. A. 1996. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl. Acad. Sci. USA 93:2873-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nardon, P., A. M. Grenier, and A. Heddi. 1998. Endocytobiote control by the host in the weevil Sitophilus oryzae, Coleoptera, Curculionidae. Symbiosis 25:237-250. [Google Scholar]
- 38.Nogales, B., E. R. B. Moore, E. Llobet-Brossa, R. Rosello-Mora, R. Amann, and K. N. Timmis. 2001. Combined use of 16S ribosomal DNA and 16S rRNA to study the bacterial community of polychlorinated biphenyl polluted soil. Appl. Environ. Microbiol. 67:1874-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polz, M. F., and C. M. Cavanaugh. 1995. Dominance of one bacterial phylotype at a Mid-Atlantic ridge hydrothermal vent site. Proc. Natl. Acad. Sci. USA 92:7232-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravenschlag, K., K. Sahm, C. Knoblauch, B. B. Jorgensen, and R. Amann. 2000. Community structure cellular rRNA content and activity of sulfate-reducing bacteria in marine arctic sediments. Appl. Environ. Microbiol. 66:3592-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reysenbach, A. L., G. S. Wickham, and N. R. Pace. 1994. Phylogenetic analysis of the hyperthermophilic pink filament community in Octopus Spring, Yellowstone National Park. Appl. Environ. Microbiol. 60:2113-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, C. M., E. Vazquez, A. Metaxas, and P. A. Tyler. 1996. Embryology of vestimentiferan tube worms from deep-sea methane/sulphide seeps. Nature 381:514-516. [Google Scholar]
- 43.Zhou, J., M. A. Bruns, and J. M. Tiedje. 1996. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62:316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]