Abstract
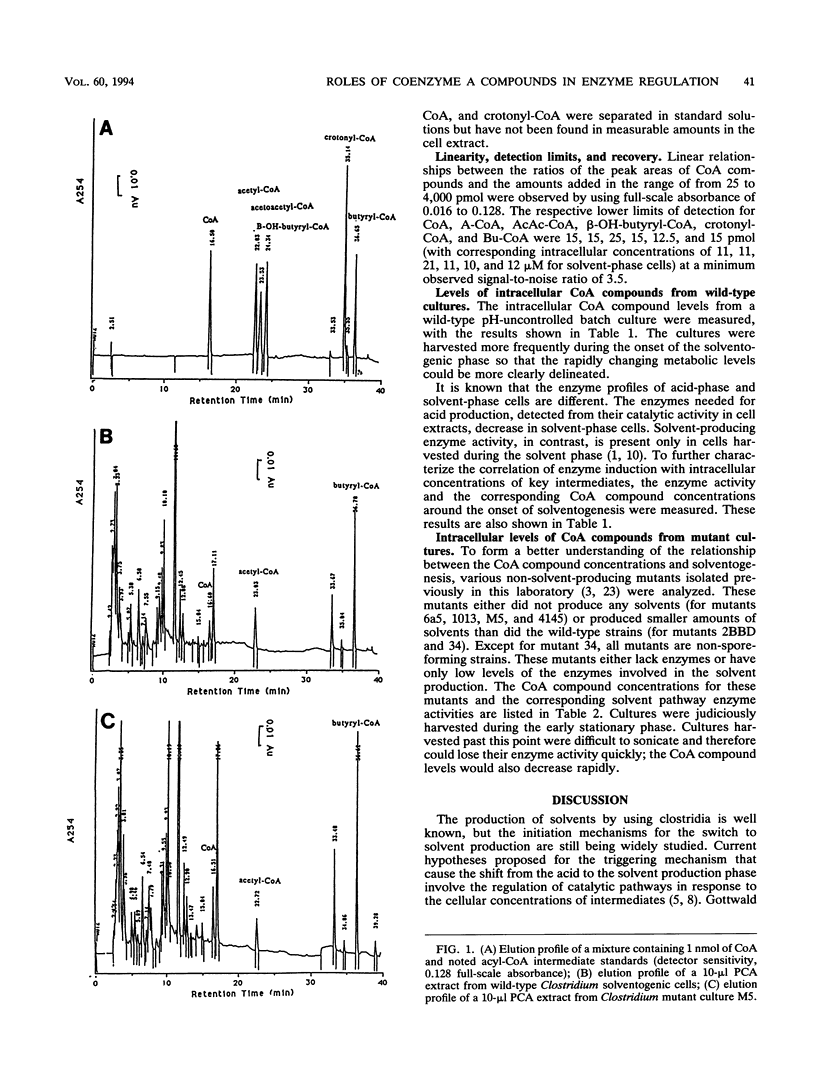
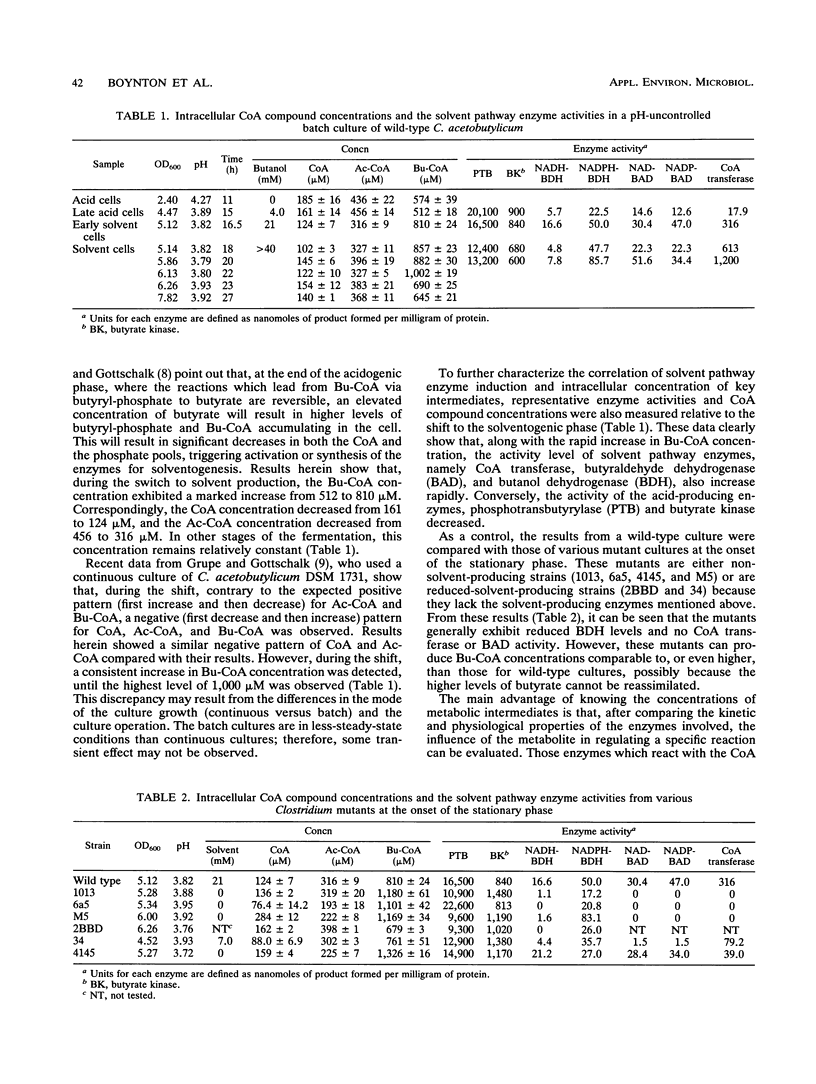
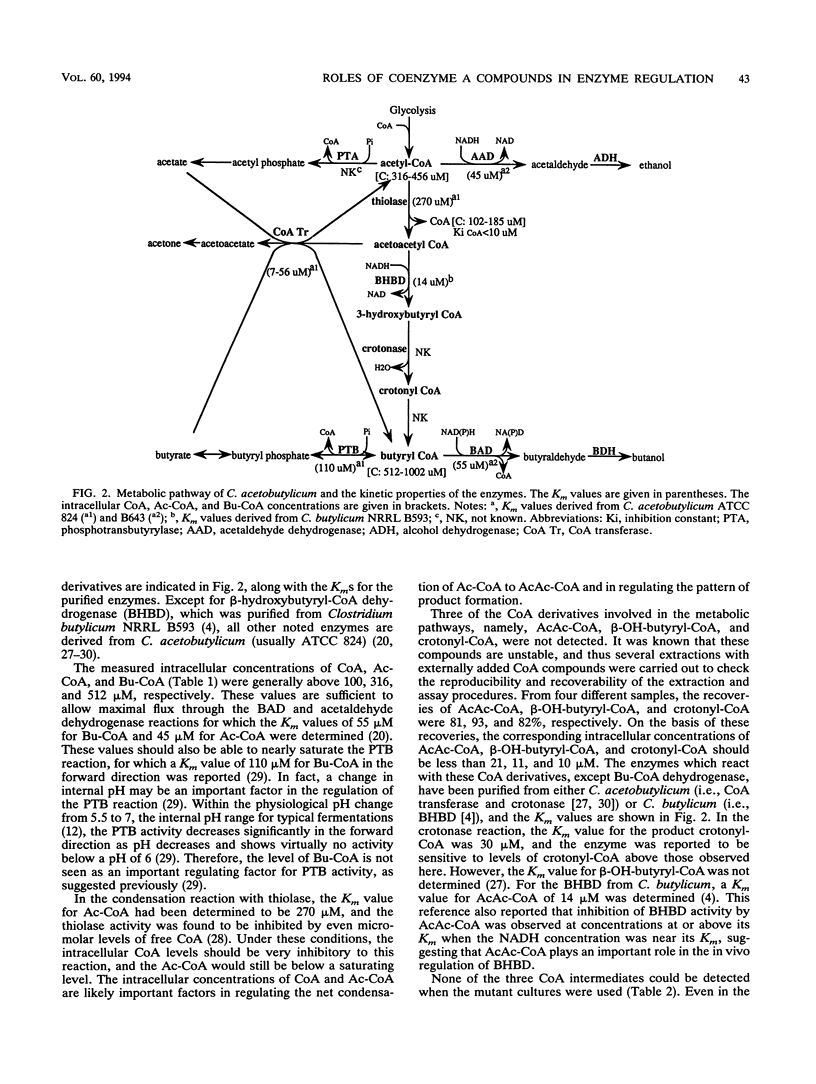
Intracellular levels of coenzyme A (CoA) and its derivatives involved in the metabolic pathways for Clostridium acetobutylicum ATCC 824 were analyzed by using reverse-phase high-performance liquid chromatography (HPLC). During the shift from the acidogenic to the solventogenic or stationary growth phase, the concentration of butyryl-CoA increased rapidly and the concentrations of free CoA and acetyl-CoA decreased. These changes were accompanied by a rapid increase of the solvent pathway enzyme activity and a decrease of the acid pathway enzyme activity. Assays with several non-solvent-producing mutant strains were also carried out. Upon entry of the mutant strains to the stationary phase, the butyryl-CoA concentrations for these mutant strains were comparable to those for the wild type even though the mutants were deficient in solvent-producing enzymes. Levels of acetoacetyl-CoA, β-hydroxy-butyryl-CoA, and crotonyl-CoA compounds in both wild-type and mutant extracts were below HPLC detection thresholds (<21 μM).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark S. W., Bennett G. N., Rudolph F. B. Isolation and Characterization of Mutants of Clostridium acetobutylicum ATCC 824 Deficient in Acetoacetyl-Coenzyme A:Acetate/Butyrate:Coenzyme A-Transferase (EC 2.8.3.9) and in Other Solvent Pathway Enzymes. Appl Environ Microbiol. 1989 Apr;55(4):970–976. doi: 10.1128/aem.55.4.970-976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby G. D., Chen J. S. Purification and properties of 3-hydroxybutyryl-coenzyme A dehydrogenase from Clostridium beijerinckii ("Clostridium butylicum") NRRL B593. Appl Environ Microbiol. 1992 Oct;58(10):3297–3302. doi: 10.1128/aem.58.10.3297-3302.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R., Zeikus J. G. Modulation of acetone-butanol-ethanol fermentation by carbon monoxide and organic acids. Appl Environ Microbiol. 1985 Mar;49(3):522–529. doi: 10.1128/aem.49.3.522-529.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R., Stephenson M. Studies on the acetone-butyl alcohol fermentation: Nutritional and other factors involved in the preparation of active suspensions of Cl. acetobutylicum (Weizmann). Biochem J. 1941 Dec;35(12):1320–1331. doi: 10.1042/bj0351320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. Studies on the acetone-butyl alcohol fermentation: Intermediates in the fermentation of glucose by Cl. acetobutylicum. 3. Potassium as an essential factor in the fermentation of maize meal by Cl. acetobutylicum (BY). Biochem J. 1942 Sep;36(7-9):582–599. doi: 10.1042/bj0360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe H., Gottschalk G. Physiological Events in Clostridium acetobutylicum during the Shift from Acidogenesis to Solventogenesis in Continuous Culture and Presentation of a Model for Shift Induction. Appl Environ Microbiol. 1992 Dec;58(12):3896–3902. doi: 10.1128/aem.58.12.3896-3902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmanis M. G., Gatenbeck S. Intermediary Metabolism in Clostridium acetobutylicum: Levels of Enzymes Involved in the Formation of Acetate and Butyrate. Appl Environ Microbiol. 1984 Jun;47(6):1277–1283. doi: 10.1128/aem.47.6.1277-1283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Gibbins L. N., Forsberg C. W. Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol. 1985 Oct;50(4):1043–1047. doi: 10.1128/aem.50.4.1043-1047.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Rupprecht E., Ohrloff C., Thauer R., Decker K. Regulation of the reduced nicotinamide adenine dinucleotide-ferredoxin reductase system in Clostridium kluyveri. J Biol Chem. 1971 Feb 25;246(4):960–963. [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- King M. T., Reiss P. D. Separation and measurement of short-chain coenzyme-A compounds in rat liver by reversed-phase high-performance liquid chromatography. Anal Biochem. 1985 Apr;146(1):173–179. doi: 10.1016/0003-2697(85)90412-9. [DOI] [PubMed] [Google Scholar]
- Palosaari N. R., Rogers P. Purification and properties of the inducible coenzyme A-linked butyraldehyde dehydrogenase from Clostridium acetobutylicum. J Bacteriol. 1988 Jul;170(7):2971–2976. doi: 10.1128/jb.170.7.2971-2976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENFELD B., SIMON E. The mechanism of the butanol-acetone fermentation. II. Phosphoenolpyruvate as a new intermediate. J Biol Chem. 1950 Sep;186(1):405–410. [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. K., Chen J. S. Purification and properties of an acetoacetyl coenzyme A-reacting phosphotransbutyrylase from Clostridium beijerinckii ("Clostridium butylicum") NRRL B593. Appl Environ Microbiol. 1990 Mar;56(3):607–613. doi: 10.1128/aem.56.3.607-613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson R. M., Castellino F. J., Hass G. M., Hill R. L. Purification and characterization of crotonase from Clostridium acetobutylicum. J Biol Chem. 1972 Aug 25;247(16):5266–5271. [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Coenzyme A transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol. 1989 Feb;55(2):323–329. doi: 10.1128/aem.55.2.323-329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Phosphotransbutyrylase from Clostridium acetobutylicum ATCC 824 and its role in acidogenesis. Appl Environ Microbiol. 1989 Feb;55(2):317–322. doi: 10.1128/aem.55.2.317-322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Thiolase from Clostridium acetobutylicum ATCC 824 and Its Role in the Synthesis of Acids and Solvents. Appl Environ Microbiol. 1988 Nov;54(11):2717–2722. doi: 10.1128/aem.54.11.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


