Abstract
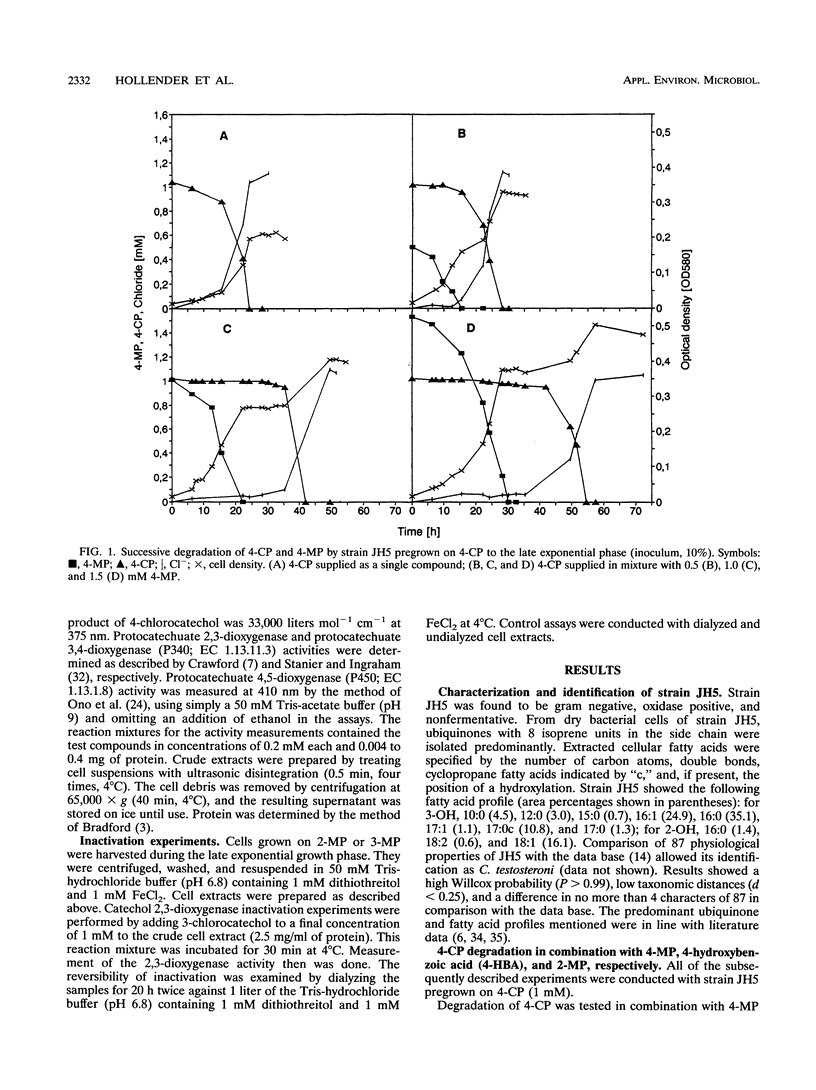
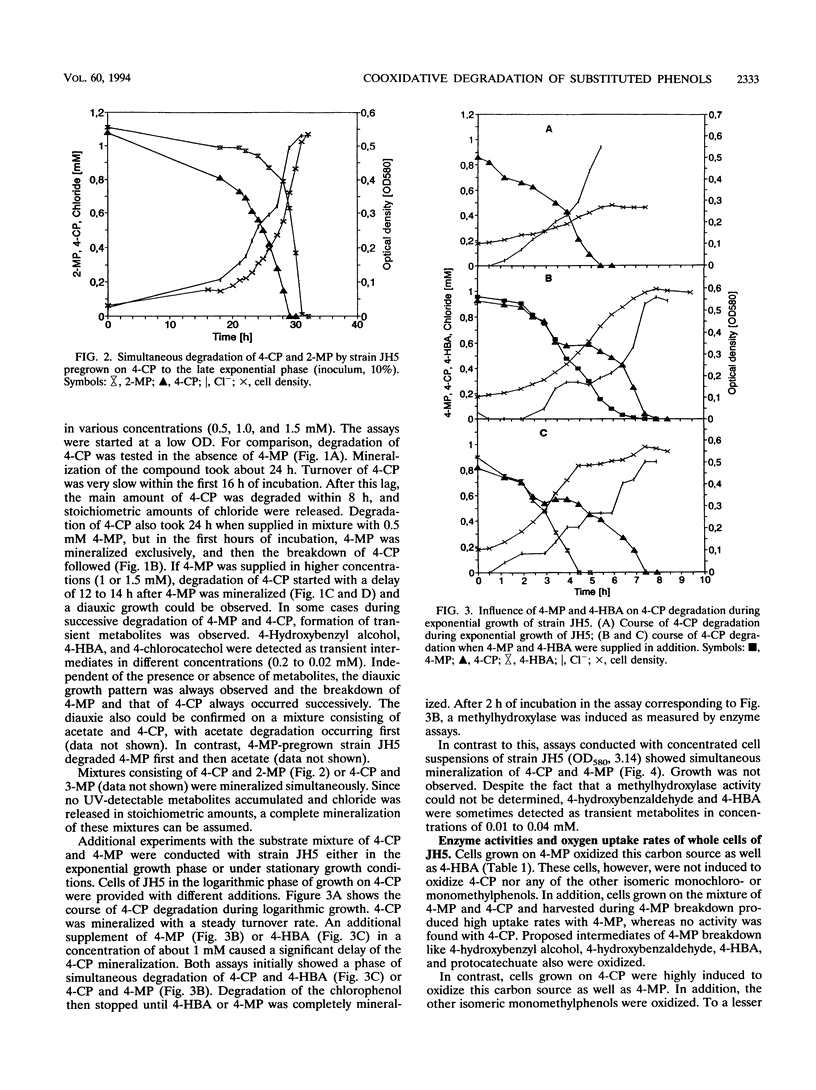
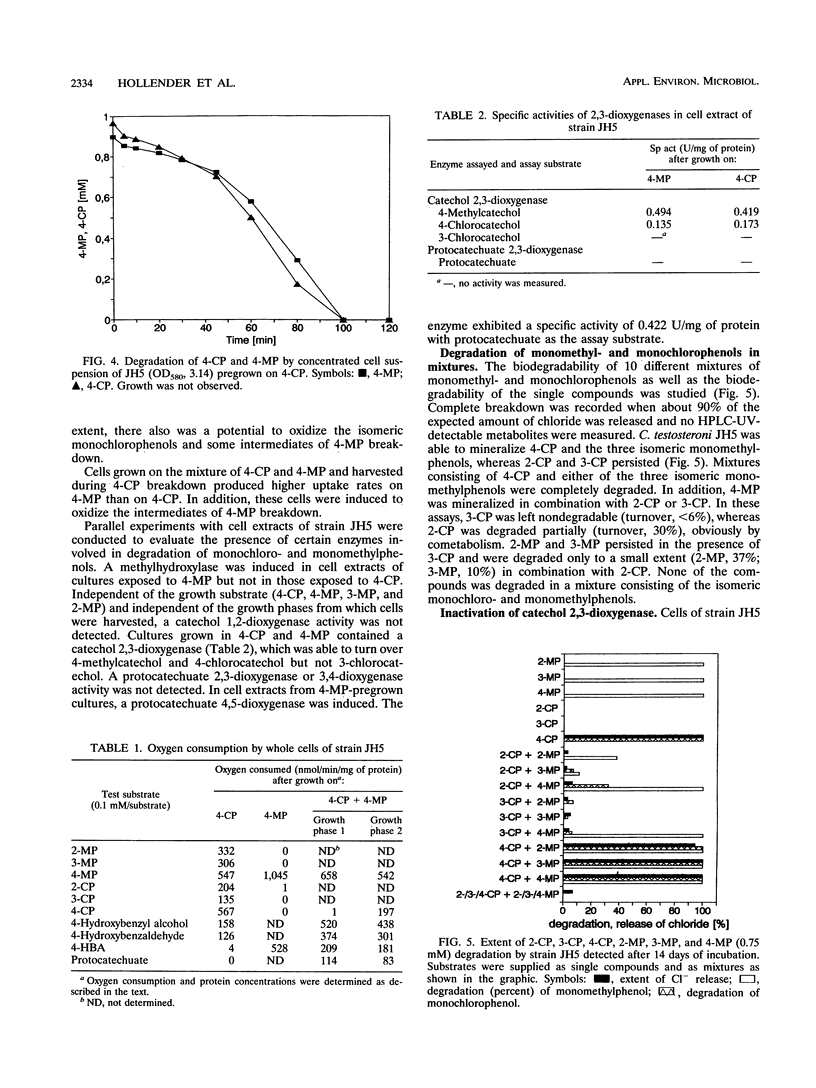
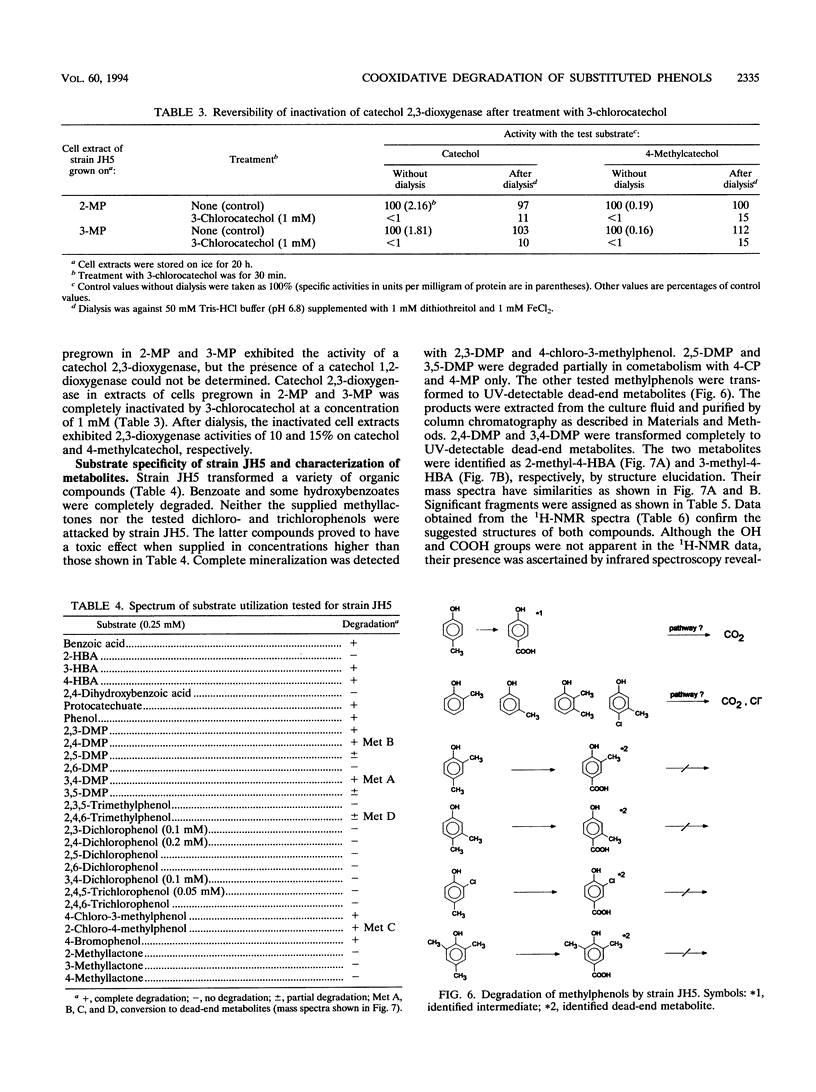
Comamonas testosteroni JH5 was isolated from a mixed bacterial culture enriched on different chloro- and methylphenols. The strain completely mineralized a mixture consisting of 4-chlorophenol (4-CP) and 4-methylphenol (4-MP). During degradation of the mixture, 4-hydroxybenzyl alcohol, 4-hydroxybenzaldehyde, 4-hydroxybenzoic acid, and 4-chlorocatechol were detected as short-lived intermediates. Mineralization of 4-CP and that of 4-MP occurred successively and were accompanied by diauxic growth, whereas 4-CP and 2-methylphenol were mineralized simultaneously. It was ascertained that neither a reversible enzyme inhibition nor potential toxic intermediates caused the observed diauxie. Some facts support the hypothesis that the successive degradation of 4-CP and 4-MP is regulated on the level of transcription. C. testosteroni JH5 contained a meta-cleaving enzyme when pregrown on 4-CP and the isomeric monomethylphenols. Inactivation of this enzyme in the presence of 3-chlorocatechol was observed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels I., Knackmuss H. J., Reineke W. Suicide Inactivation of Catechol 2,3-Dioxygenase from Pseudomonas putida mt-2 by 3-Halocatechols. Appl Environ Microbiol. 1984 Mar;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L. Novel pathway for degradation of protocatechuic acid in Bacillus species. J Bacteriol. 1975 Feb;121(2):531–536. doi: 10.1128/jb.121.2.531-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers J., Rubio M. A., Knackmuss H. J., Freier-Schröder D. Bacterial metabolism of 2,6-xylenol. Appl Environ Microbiol. 1989 Nov;55(11):2904–2908. doi: 10.1128/aem.55.11.2904-2908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Pettigrew C. A., Spain J. C. Biodegradation of mixtures of substituted benzenes by Pseudomonas sp. strain JS150. Appl Environ Microbiol. 1992 Jul;58(7):2237–2244. doi: 10.1128/aem.58.7.2237-2244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Taylor D. G. Pathways for the degradation of m-cresol and p-cresol by Pseudomonas putida. J Bacteriol. 1975 Apr;122(1):1–6. doi: 10.1128/jb.122.1.1-6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Chapman P. J., Dagley S. Enzymatic release of halogens or methanol from some substituted protocatechuic acids. J Bacteriol. 1985 May;162(2):693–697. doi: 10.1128/jb.162.2.693-697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Inhibition of catechol 2,3-dioxygenase from Pseudomonas putida by 3-chlorocatechol. Appl Environ Microbiol. 1981 May;41(5):1159–1165. doi: 10.1128/aem.41.5.1159-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackmuss H. J., Hellwig M. Utilization and cooxidation of chlorinated phenols by Pseudomonas sp. B 13. Arch Microbiol. 1978 Apr 27;117(1):1–7. doi: 10.1007/BF00689343. [DOI] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Singer T. P. p-Cresol methylhydroxylase. Assay and general properties. Biochem J. 1985 Jun 1;228(2):325–335. doi: 10.1042/bj2280325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Nozaki M., Hayaishi O. Purification and some properties of protocatechuate 4,5-dioxygenase. Biochim Biophys Acta. 1970 Nov 11;220(2):224–238. doi: 10.1016/0005-2744(70)90008-2. [DOI] [PubMed] [Google Scholar]
- Pettigrew C. A., Haigler B. E., Spain J. C. Simultaneous biodegradation of chlorobenzene and toluene by a Pseudomonas strain. Appl Environ Microbiol. 1991 Jan;57(1):157–162. doi: 10.1128/aem.57.1.157-162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo F., Pieper D. H., Engesser K. H., Knackmuss H. J., Timmis K. N. Assemblage of ortho cleavage route for simultaneous degradation of chloro- and methylaromatics. Science. 1987 Dec 4;238(4832):1395–1398. doi: 10.1126/science.3479842. [DOI] [PubMed] [Google Scholar]
- Rudolphi A., Tschech A., Fuchs G. Anaerobic degradation of cresols by denitrifying bacteria. Arch Microbiol. 1991;155(3):238–248. doi: 10.1007/BF00252207. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., INGRAHAM J. L. Protocatechuic acid oxidase. J Biol Chem. 1954 Oct;210(2):799–808. [PubMed] [Google Scholar]
- Veys A., Callewaert W., Waelkens E., Van den Abbeele K. Application of gas-liquid chromatography to the routine identification of nonfermenting gram-negative bacteria in clinical specimens. J Clin Microbiol. 1989 Jul;27(7):1538–1542. doi: 10.1128/jcm.27.7.1538-1542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser M., Eberspächer J., Vogler B., Lingens F. Metabolism of 4-chlorophenol by Azotobacter sp. GP1: structure of the meta cleavage product of 4-chlorocatechol. FEMS Microbiol Lett. 1994 Feb 1;116(1):73–78. doi: 10.1111/j.1574-6968.1994.tb06678.x. [DOI] [PubMed] [Google Scholar]


