Abstract
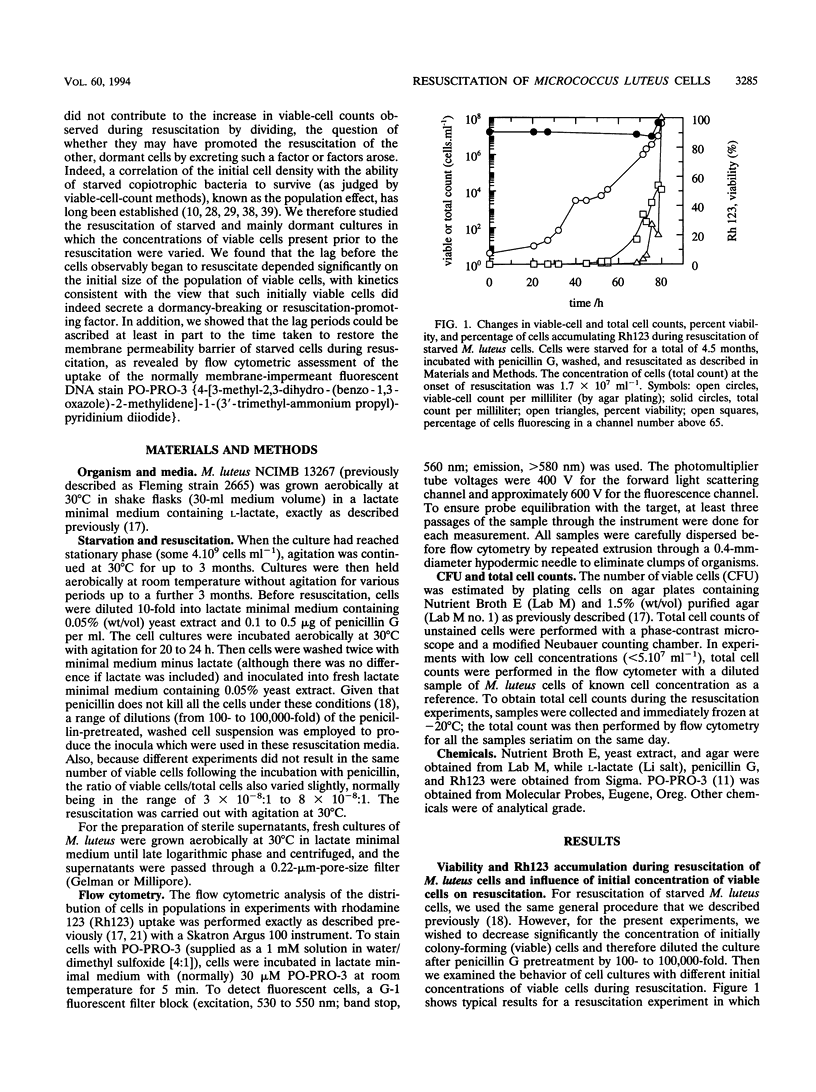
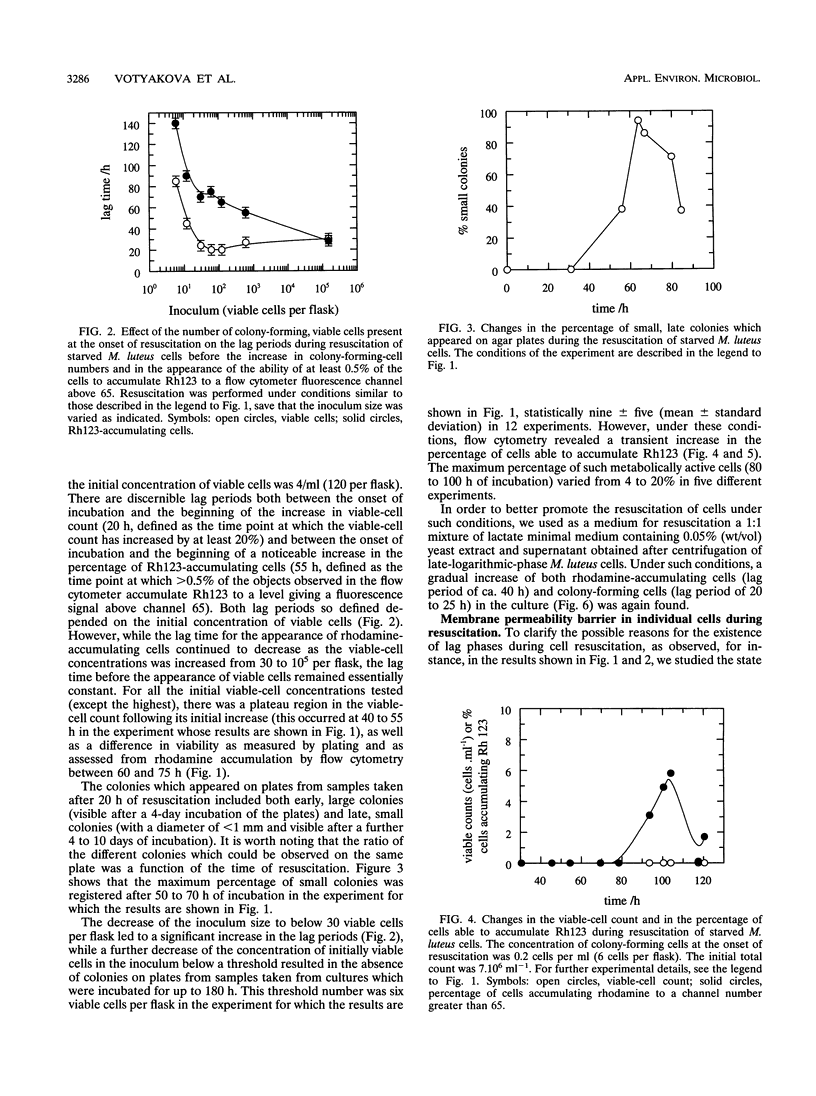
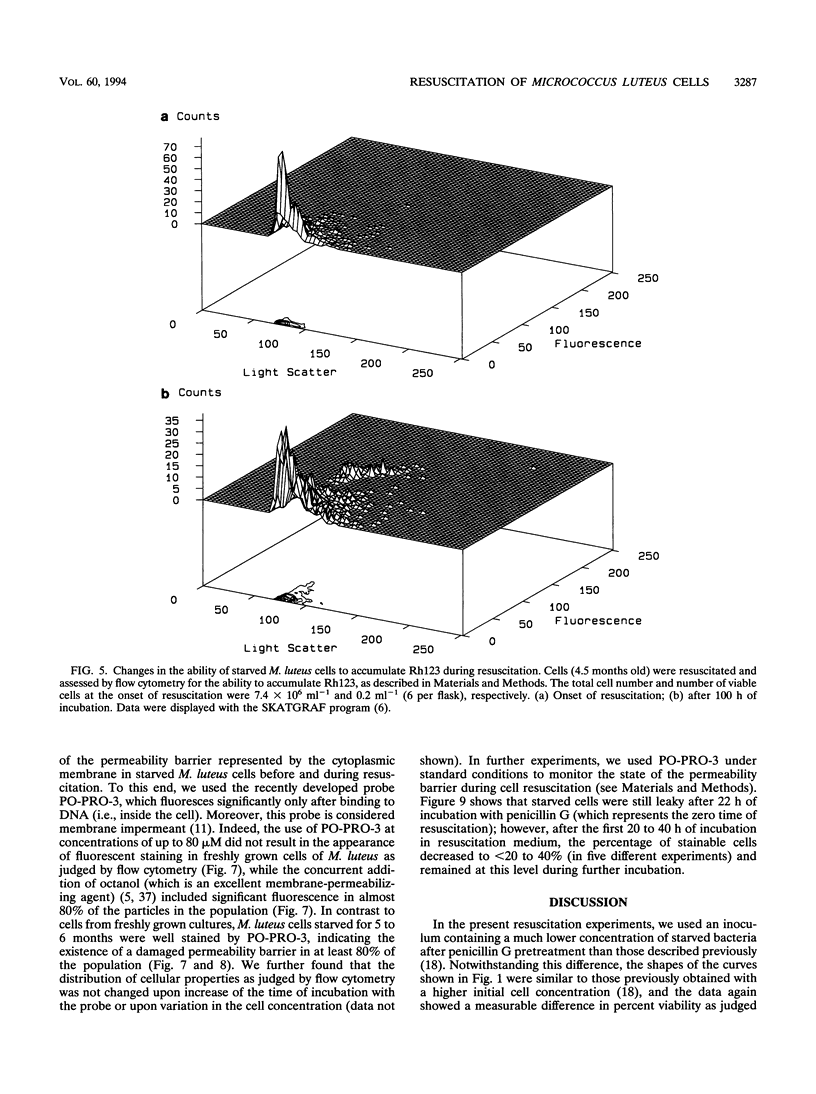
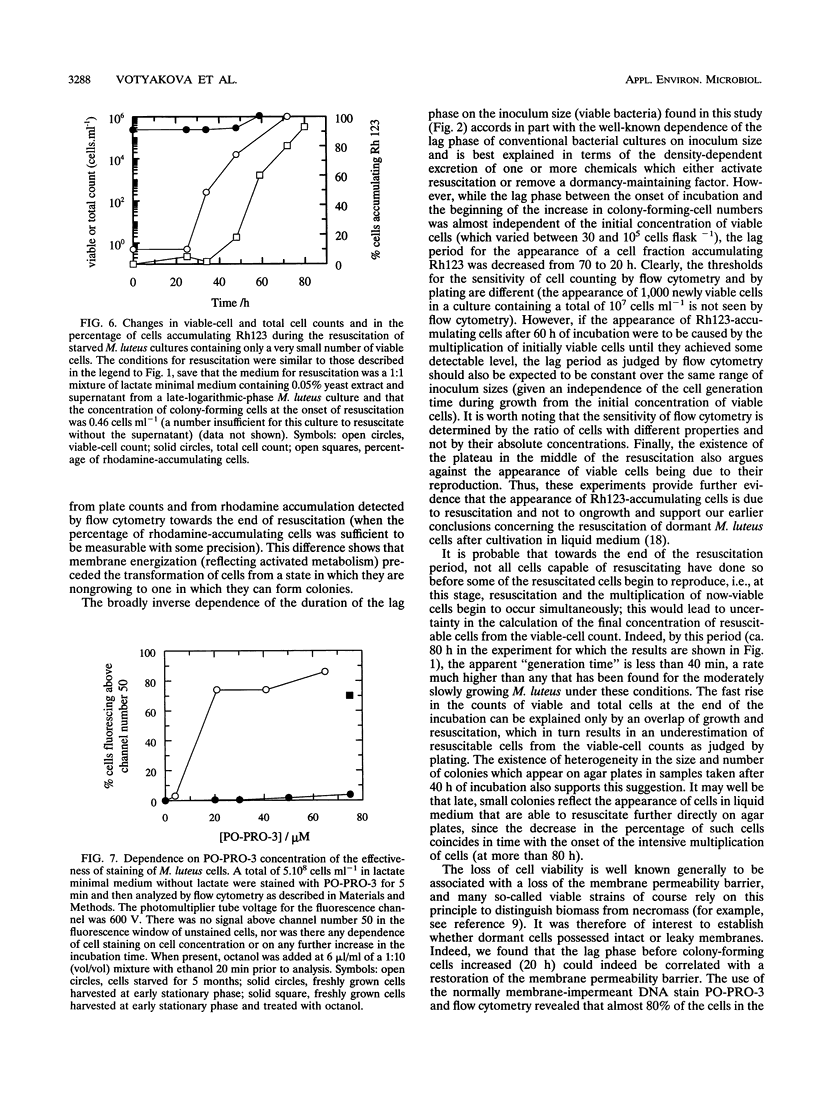
A high proportion of Micrococcus luteus cells in cultures which had been starved for 3 to 6 months lost the ability to grow and form colonies on agar plates but could be resuscitated from their dormancy by incubation in an appropriate liquid medium (A. S. Kaprelyants and D. B. Kell, Appl. Environ. Microbiol. 59:3187-3196, 1993). In the present work, such cultures were studied by both flow cytometry and conventional microbiological methods and were found to contain various numbers of viable cells. Pretreatment of such cultures with penicillin G, and subsequent dilution, was used to vary this number. When the initial number of colony-forming cells per 30-ml flask was approximately nine (±five) or more, resuscitation of 10 to 40% of the cells, and thus culture growth, was observed. The lag period before the appearance of a population of cells showing significant accumulation of the fluorescent dye rhodamine 123 (i.e., of cells with measurable membrane energization) decreased from 70 to 27 h when the number of viable cells was increased from 30 to 105 per flask, while the lag period before an observable increase in the number of colony-forming cells occurred was almost constant (at some 20 h). Provided there were more than nine (±five) initially viable cells per flask, the number of initially viable cells did not affect the final percentage of resuscitable cells in the culture. The lag period could be ascribed in part to the time taken to restore the membrane permeability barrier of starved cells during resuscitation, as revealed by flow cytometric assessment of the uptake of the normally membrane-impermeant fluorescent DNA stain PO-PRO-3 {4-[3-methyl-2, 3-dihydro-(benzo-1, 3-oxazole)-2-methylidene]-1-(3′-trimethylammonium propyl)-pyridinium diiodide}. Although cell populations which contained fewer than nine ±five viable cells per flask failed to grow, 4 to 20% of the cells (of 1.2 X 106) were able to accumulate rhodamine 123 after 80 to 100 h of incubation, showing the ability of a significant number of the cells in the population at least to display “metabolic resuscitation.” Resuscitation and cell growth under such conditions were favored by the use of a 1:1 mixture of fresh lactate medium and supernatant from late-logarithmic-phase M. luteus cultures as the resuscitation medium. We conclude that the presence of a small fraction of viable cells at the onset of resuscitation facilitates the recovery of the majority of the remaining (dormant) cells. The cell density dependence of the kinetics, or population effect, suggests that this recovery is due to the excretion of some factor(s) which promoted the transition of cells from a state in which they are incapable of growth and division to one in which they are capable of colony formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton N. J., Bycroft B. W., Chhabra S. R., Stead P., Gledhill L., Hill P. J., Rees C. E., Winson M. K., Salmond G. P., Stewart G. S. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992 Jul 1;116(1):87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Bacterial sex pheromone-induced plasmid transfer. Cell. 1993 Apr 9;73(1):9–12. doi: 10.1016/0092-8674(93)90153-h. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Van Brunt J. Circulation of H+ and K+ across the plasma membrane is not obligatory for bacterial growth. Science. 1977 Jul 22;197(4301):372–373. doi: 10.1126/science.69317. [DOI] [PubMed] [Google Scholar]
- Harris C. M., Kell D. B. The estimation of microbial biomass. Biosensors. 1985;1(1):17–84. doi: 10.1016/0265-928x(85)85005-7. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Beppu T. Autoregulatory factors of secondary metabolism and morphogenesis in actinomycetes. Crit Rev Biotechnol. 1990;10(3):191–204. doi: 10.3109/07388559009038207. [DOI] [PubMed] [Google Scholar]
- Jones S., Yu B., Bainton N. J., Birdsall M., Bycroft B. W., Chhabra S. R., Cox A. J., Golby P., Reeves P. J., Stephens S. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993 Jun;12(6):2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Losick R. How and why bacteria talk to each other. Cell. 1993 Jun 4;73(5):873–885. doi: 10.1016/0092-8674(93)90268-u. [DOI] [PubMed] [Google Scholar]
- Kaprelyants A. S., Gottschal J. C., Kell D. B. Dormancy in non-sporulating bacteria. FEMS Microbiol Rev. 1993 Apr;10(3-4):271–285. doi: 10.1111/j.1574-6968.1993.tb05871.x. [DOI] [PubMed] [Google Scholar]
- Kaprelyants A. S., Kell D. B. Dormancy in Stationary-Phase Cultures of Micrococcus luteus: Flow Cytometric Analysis of Starvation and Resuscitation. Appl Environ Microbiol. 1993 Oct;59(10):3187–3196. doi: 10.1128/aem.59.10.3187-3196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B., Ryder H. M., Kaprelyants A. S., Westerhoff H. V. Quantifying heterogeneity: flow cytometry of bacterial cultures. Antonie Van Leeuwenhoek. 1991 Oct-Nov;60(3-4):145–158. doi: 10.1007/BF00430362. [DOI] [PubMed] [Google Scholar]
- Kuspa A., Plamann L., Kaiser D. A-signalling and the cell density requirement for Myxococcus xanthus development. J Bacteriol. 1992 Nov;174(22):7360–7369. doi: 10.1128/jb.174.22.7360-7369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonell M. T., Hood M. A. Isolation and characterization of ultramicrobacteria from a gulf coast estuary. Appl Environ Microbiol. 1982 Mar;43(3):566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Cranwell P. A., Pickup R. W. Survival of Aeromonas salmonicida in lake water. Appl Environ Microbiol. 1991 Jun;57(6):1777–1782. doi: 10.1128/aem.57.6.1777-1782.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Piper K. R., Beck von Bodman S., Farrand S. K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993 Apr 1;362(6419):448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rose A. S., Ellis A. E., Munro A. L. Evidence against dormancy in the bacterial fish pathogen Aeromonas salmonicida subsp. salmonicida. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):105–107. doi: 10.1016/0378-1097(90)90133-b. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Effect of chilling on Aerobacter aerogenes in aqueous suspension. J Gen Microbiol. 1962 Dec;29:719–730. doi: 10.1099/00221287-29-4-719. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., SHON M. EFFECTS OF THERMAL STRESS ON VIABILITY AND RIBONUCLEIC ACID OF AEROBACTER AEROGENES IN AQUEOUS SUSPENSION. J Gen Microbiol. 1964 Jan;34:99–114. doi: 10.1099/00221287-34-1-99. [DOI] [PubMed] [Google Scholar]
- Stephens K. Pheromones among the procaryotes. Crit Rev Microbiol. 1986;13(4):309–334. doi: 10.3109/10408418609108741. [DOI] [PubMed] [Google Scholar]
- Sternberg P. W. Intercellular signaling and signal transduction in C. elegans. Annu Rev Genet. 1993;27:497–521. doi: 10.1146/annurev.ge.27.120193.002433. [DOI] [PubMed] [Google Scholar]
- Weichart D., Oliver J. D., Kjelleberg S. Low temperature induced non-culturability and killing of Vibrio vulnificus. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):205–210. doi: 10.1111/j.1574-6968.1992.tb14041.x. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F., Weinhold K. J., Levich J. The tumor dormant state. Adv Cancer Res. 1981;34:107–140. doi: 10.1016/s0065-230x(08)60240-7. [DOI] [PubMed] [Google Scholar]
- Williams P., Bainton N. J., Swift S., Chhabra S. R., Winson M. K., Stewart G. S., Salmond G. P., Bycroft B. W. Small molecule-mediated density-dependent control of gene expression in prokaryotes: bioluminescence and the biosynthesis of carbapenem antibiotics. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):161–167. doi: 10.1111/j.1574-6968.1992.tb14035.x. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis and the regulation of cell numbers in normal and neoplastic tissues: an overview. Cancer Metastasis Rev. 1992 Sep;11(2):95–103. doi: 10.1007/BF00048057. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Picker L. J., Scheuermann R. H., Tucker T. F., Vitetta E. S., Uhr J. W. Cancer dormancy: isolation and characterization of dormant lymphoma cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1829–1833. doi: 10.1073/pnas.90.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Murphy P. J., Kerr A., Tate M. E. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993 Apr 1;362(6419):446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]


