Abstract
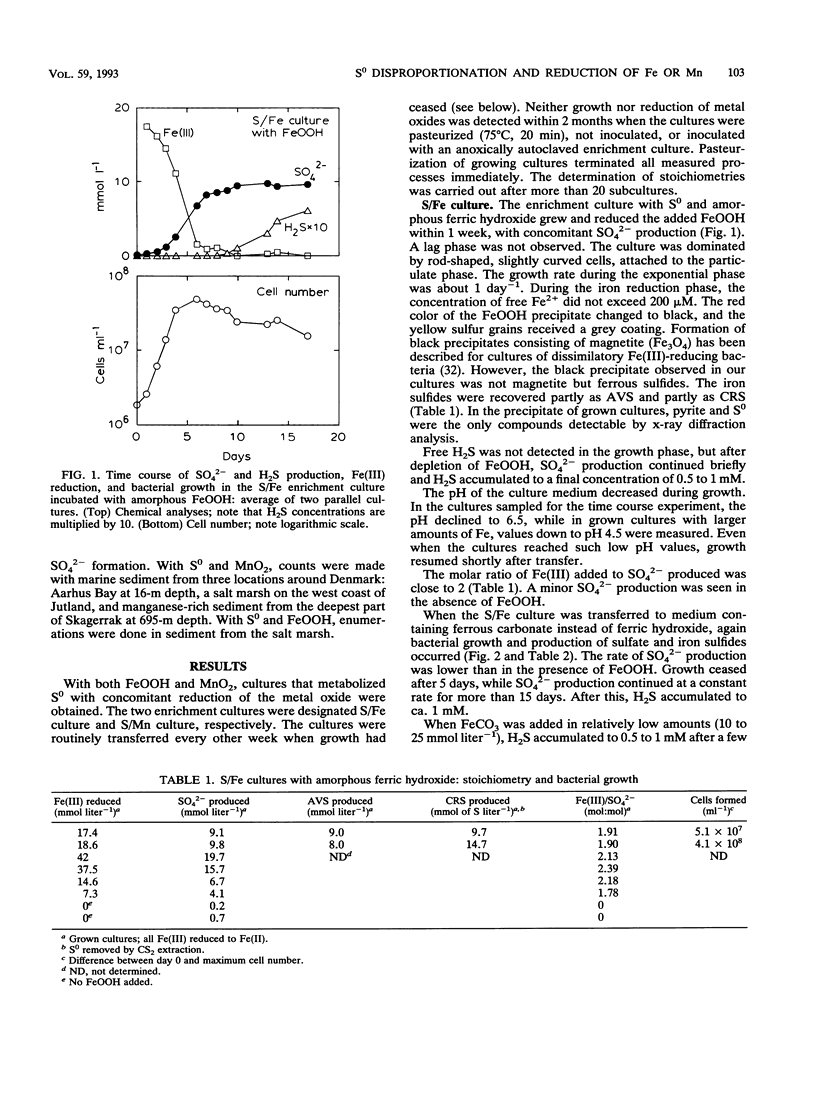
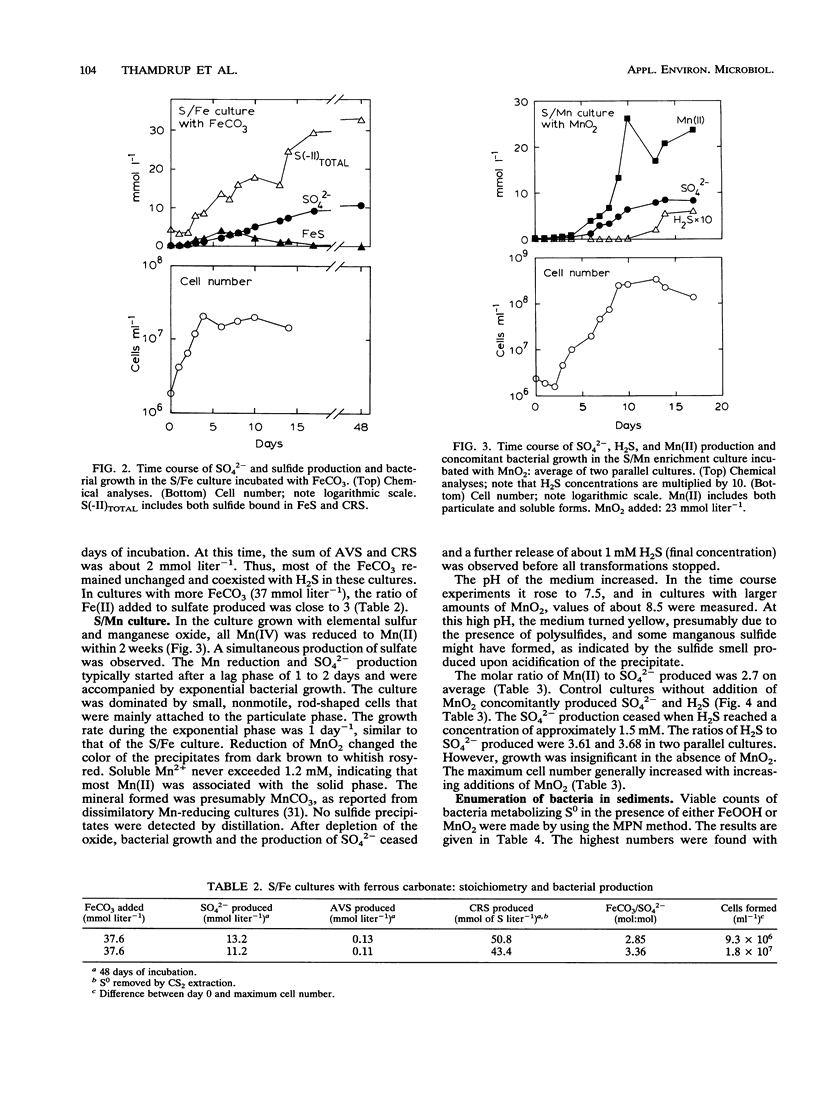
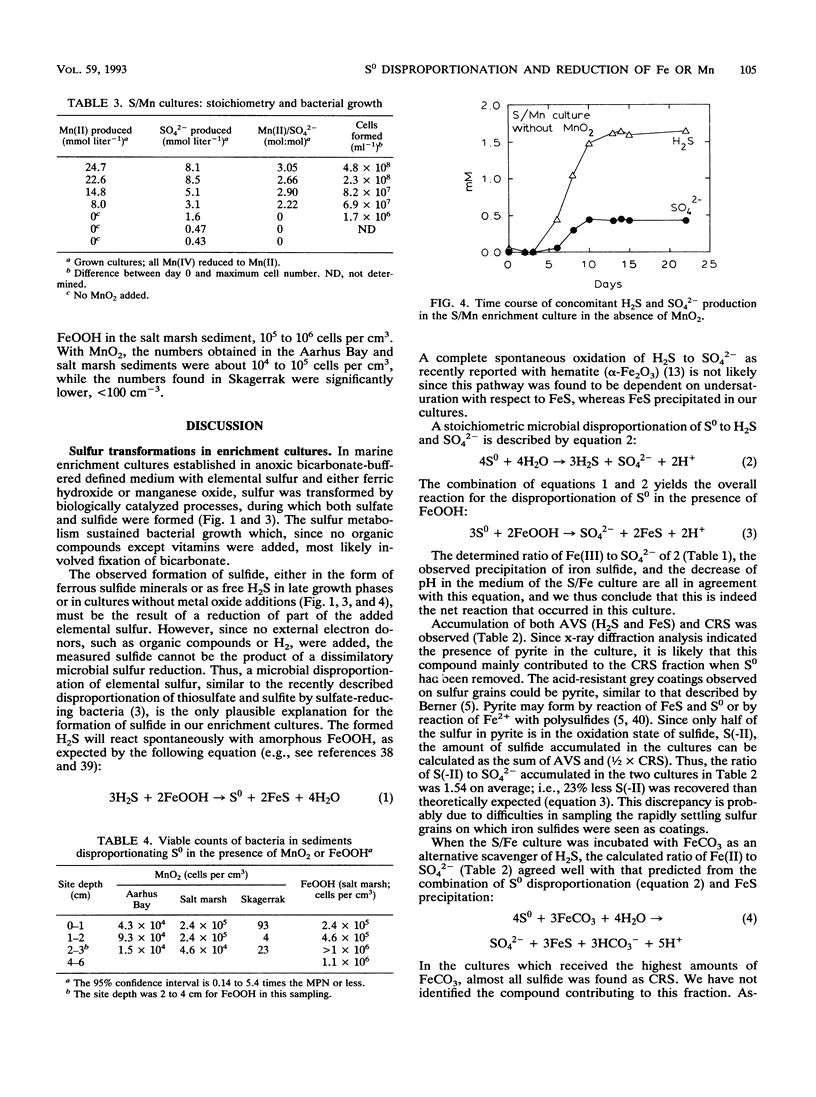
A new chemolithotrophic bacterial metabolism was discovered in anaerobic marine enrichment cultures. Cultures in defined medium with elemental sulfur (S0) and amorphous ferric hydroxide (FeOOH) as sole substrates showed intense formation of sulfate. Furthermore, precipitation of ferrous sulfide and pyrite was observed. The transformations were accompanied by growth of slightly curved, rod-shaped bacteria. The quantification of the products revealed that S0 was microbially disproportionated to sulfate and sulfide, as follows: 4S0 + 4H2O → SO42- + 3H2S + 2H+. Subsequent chemical reactions between the formed sulfide and the added FeOOH led to the observed precipitation of iron sulfides. Sulfate and iron sulfides were also produced when FeOOH was replaced by FeCO3. Further enrichment with manganese oxide, MnO2, instead of FeOOH yielded stable cultures which formed sulfate during concomitant reduction of MnO2 to Mn2+. Growth of small rod-shaped bacteria was observed. When incubated without MnO2, the culture did not grow but produced small amounts of SO42- and H2S at a ratio of 1:3, indicating again a disproportionation of S0. The observed microbial disproportionation of S0 only proceeds significantly in the presence of sulfide-scavenging agents such as iron and manganese compounds. The population density of bacteria capable of S0 disproportionation in the presence of FeOOH or MnO2 was high, > 104 cm-3 in coastal sediments. The metabolism offers an explanation for recent observations of anaerobic sulfide oxidation to sulfate in anoxic sediments.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belkin S., Wirsen C. O., Jannasch H. W. Biological and abiological sulfur reduction at high temperatures. Appl Environ Microbiol. 1985 May;49(5):1057–1061. doi: 10.1128/aem.49.5.1057-1061.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield D. E. Reactive iron in marine sediments. Geochim Cosmochim Acta. 1989;53:619–632. doi: 10.1016/0016-7037(89)90005-7. [DOI] [PubMed] [Google Scholar]
- Jorgensen B. B., Des Marais D. J. The diffusive boundary layer of sediments: oxygen microgradients over a microbial mat. Limnol Oceanogr. 1990;35(6):1343–1355. doi: 10.4319/lo.1990.35.6.1343. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B. A thiosulfate shunt in the sulfur cycle of marine sediments. Science. 1990 Jul 13;249(4965):152–154. doi: 10.1126/science.249.4965.152. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B., Bak F. Pathways and microbiology of thiosulfate transformations and sulfate reduction in a marine sediment (kattegat, denmark). Appl Environ Microbiol. 1991 Mar;57(3):847–856. doi: 10.1128/aem.57.3.847-856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Revsbech N. P., Jørgensen B. B. Microoxic-Anoxic Niche of Beggiatoa spp.: Microelectrode Survey of Marine and Freshwater Strains. Appl Environ Microbiol. 1986 Jul;52(1):161–168. doi: 10.1128/aem.52.1.161-168.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger H., Paschinger J., Gaffron H. Photochemical disproportionation of sulfur into sulfide and sulfate by Chlorobium limicola forma thiosulfatophilum. Arch Mikrobiol. 1974 Mar 28;96(4):341–351. [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


