Abstract
β-chemokines play an important role in the development of immunologic reactions. Macrophages are major β-chemokine-producing cells during T-cell directed, delayed-type hypersensitivity reactions in tissues, and have been reported to be important producers of β-chemokines in the lymph nodes of HIV-1-infected individuals. However, the physiological signals responsible for inducing macrophages to produce β-chemokines have not been established. Two soluble T cell products, interferon-γ and granulocyte-macrophage colony stimulating factor, were added to cultured macrophages, but failed to stimulate the production of macrophage inflammatory protein-1α and -1β; regulated upon activation, normal T cell expressed and secreted (RANTES); or monocyte chemoattractant protein-1. Instead, direct cell–cell contact between macrophages and cells engineered to express CD40L (also known as CD154) resulted in the production of large amounts of macrophage inflammatory protein-1α and -1β, and RANTES (all ligands for CCR5), and monocyte chemoattractant protein-1 (a ligand for CCR2). Supernatants from CD40L-stimulated macrophages protected CD4+ T cells from infection by a nonsyncytium-inducing strain of HIV-1 (which uses CCR5 as a coreceptor). These results have implications for granulomatous diseases, and conditions such as atherosclerosis and multiple sclerosis, where CD40L-bearing cells have been found in the macrophage-rich lesions where β-chemokines are being produced. Overall, these findings define a pathway linking the specific recognition of antigen by T cells to the production of β-chemokines by macrophages. This pathway may play a role in anti-HIV-1 immunity and the development of immunologic reactions or lesions.
β-chemokines are required to induce immunologically important cells to migrate from blood into tissue (1–3). Included in this family of proteins are the macrophage inflammatory proteins (MIP), 1α (MIP-1α) and 1β (MIP-1β), which were first identified as the products of stimulated macrophages (4), and RANTES (regulated upon activation, normal T cell expressed and secreted) and monocyte chemoattractant protein-1 (MCP-1), which were first described as the products of activated T cells (5, 6). A number of microbial agents have been shown to induce the production of these β-chemokines by macrophages, including bacterial lipopolysaccharide (LPS) (4, 7) and viruses such as HIV-1 (8–10). In addition, macrophages in delayed-typed hypersensitivity reactions produce β-chemokines (11–14), but it has not been previously determined how the specific recognition of antigen by T cells could be linked to the production of β-chemokines by macrophages. In this study, we found that two well-known soluble macrophage stimulants, interferon-γ (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were unable to induce macrophages to produce β-chemokines. Instead, direct cell–cell contact with cells expressing CD40L was found to induce macrophages to produce large amounts of these four β-chemokines.
MATERIALS AND METHODS
Reagents.
Phenol-extracted LPS from Escherichia coli 0111:B4 (Sigma) was dissolved in ethanol and water (1:1, vol/vol) at 10 mg/ml and stored at −20°C. The following were purchased from Genzyme: IFN-γ (1 × 107/mg), GM-CSF (1.25 × 107 units/mg), and murine mAbs against CD40L (clone M90) and CD40 (clone M3). Recombinant interleukin (IL)-2 and G418 were from Life Technologies (Gaithersburg, MD). OKT3 anti-CD3 mAb was from Ortho Diagnostics. Unlabeled and phycoerythrin (PE)-labeled mAb against human CD40L (clone 24–31) was from Ancell (Bayport, MN). PE-labeled mAb against murine CD40L (clone MR1) was from PharMingen. The medium for 293 cells consisted of enriched MEM with Earle’s salts (no. 112–039-101, Quality Biologicals, Gaithersburg, MD) containing 2 mM l-glutamine and 10% heat-inactivated fetal bovine serum (FBS; Summit Biotechnology, Ft. Collins, CO) (E10). The medium for peripheral blood mononuclear cells (PBMC) and monocyte-derived macrophages (MDM) was RPMI 1640 medium containing 2 mM l-glutamine (BioWhittaker) and 10% FBS (R10).
MDM Cultures.
Venous blood was collected with informed consent from healthy donors, and PBMC were prepared by centrifugation over Ficoll/Hypaque. Monocytes were then isolated by the fibronectin adherence method and plated in 48-well culture plates at 4 × 105 cells per well in 1.0 ml of RPMI 1640 medium containing 10% fresh, unheated, autologous serum. After 5–7 days, the medium and any residual nonadherent cells were removed, and the MDM monolayers (2 × 105 cells per well) were refed with R10 and used 3–7 days later when they were >99% positive for nonspecific esterase (15). All culture components were prescreened to select nonactivating, low endotoxin lots of media and serum as described (15).
Generation of Normal and Mutant CD40L-Expressing 293 Cells.
To clone the cDNA for human CD40L, PBMC were stimulated with plate-immobilized anti-CD3 (OKT3) overnight, fed with 10 units/ml IL-2, and selected for surface CD40L expression by using an anti-CD40L mAb (clone 24–31) and magnetic beads coated with anti-mouse IgG antibodies (Dynal, Oslo). Two additional weekly cycles of restimulation with anti-CD3 and IL-2 and magnetic bead selection were performed. mRNA was then prepared, reverse-transcribed into cDNA by using random hexamer primers, and amplified by PCR using Pfu polymerase (Stratagene). The PCR primers for human CD40L (originally designed for a previous generation of expression vector) were sense, 5′-GGGGACTAGTAGATACCATTTCAACTTTAACACAG-3′ (containing an underlined SpeI site) and antisense, 5′-GGGCTCGAGCGGCCGCAGTTCTACATGCCTTGGAGTGTATAAT-3′ (containing underlined XhoI and NotI sites). Hot-start PCR was performed beginning with 94°C for 2.5 min followed by 45 cycles of the following program: 94°C for 10 sec, 43°C for 30 sec, and 76°C for 5 min. The product was then digested with SpeI and NotI and cloned into the NheI and NotI sites of the expression vector, pcDNA3.1(+) (Invitrogen) to create the plasmid, pcDNA3.1-CD40L. The final construct was verified by sequencing both DNA strands. Following transfection of pcDNA3.1-CD40L into 293 cells and selection in E-10 containing G418 (500 μg/ml) cells were further selected by repeated flow sorting using PE-conjugated mAb 24–31 and growth for 1 week, for a total of three cycles. The “control 293 cells” used in this study were prepared by transfection with the empty expression vector, pcDNA3.1(+), and selection in G418.
To clone the cDNA for murine CD40L, mRNA from a BALB/c mouse spleen was reverse-transcribed into cDNA by using random hexamer primers, and amplified by hemi-nested PCR using Pfu polymerase. The PCR primers for the first 30 cycles were sense, 5′-GGGGAAGCTTGCCTCTGTCCCATTCGTTGGTCAG-3′ (containing an underlined HindIII site) and antisense, 5′-CAGGGCAGGTCCTAACTAACTGACTTGC-3′. A 1:1,000 dilution was made for the second 30 cycle PCR, which used the same sense primer and an internally placed antisense primer, 5′-GGGGTCTAGACTGCTGCAGCCTAGGACAGCGCAC-3′ (containing an underlined XbaI site). Both PCR reactions were performed by hot-start beginning with 94°C for 2.5 min followed by the following cycling program: 94°C for 10 sec, 53°C for 30 sec, and 76°C for 5 min. The product was then digested with HindIII and XbaI and cloned into pcDNA3.1(+) to create the plasmid, pcDNA3.1-mCD40L. The final construct was verified by sequencing both DNA strands. Following transfection of pcDNA3.1-mCD40L into 293 cells and selection in E-10 containing G418 (500 μg/ml), cells were further selected by repeated flow sorting using PE-conjugated MR1 and growth for 1 week, for a total of three cycles.
As an additional control for human CD40L-293 cells, cells expressing an inactive mutant of CD40L were prepared. This mutant encodes the abnormal T147N protein, which is found in a subset of patients with X-linked hyper-IgM syndrome (X-HIM) (16). The codon for amino acid 147 in CD40L was changed from Thr (ACC) to Asn (AAC) by overlapping PCR mutagenesis (17) using 5′-GCTGAAAAAGGATACTACAACATGAGCAACAACTTGG-3′ (mutated codon shown in boldface) and its complementary inverse sequence. The final plasmid, pcDNA3.1-T147N-CD40L, was verified by sequencing both DNA strands and expressed in 293 cells using G418 selection and flow sorting as described to create T147N-CD40L-293 cells. When evaluated by flow cytometry using PE-conjugated mAb 24–31, the resulting T147N-CD40L-293 cells expressed CD40L protein at a level comparable to the CD40L-293 cells shown in Fig. 1A.
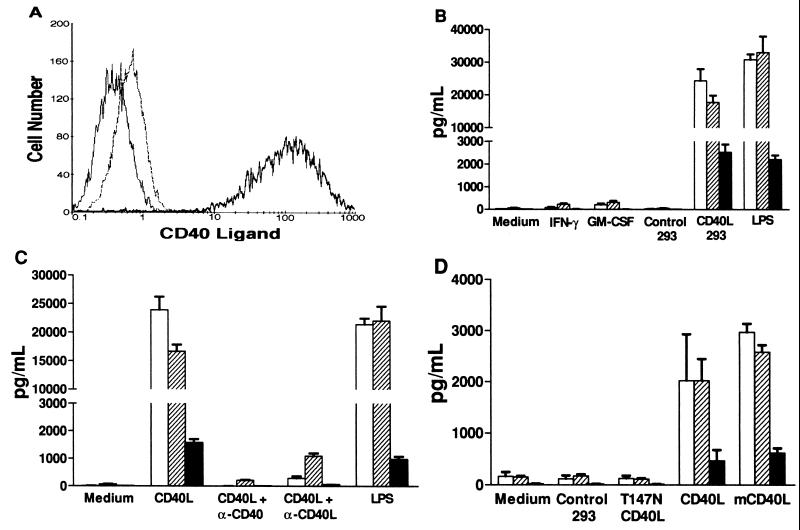
Figure 1.
CD40L induction of β-chemokine production by macrophages. (A) Flow cytometry measurement of CD40L surface expression. Isotype control staining of CD40L-293 cells with PE-conjugated anti-CD8 control mAb (thin solid line). Expression of human CD40L on control 293 cells (thin broken line) and CD40L-293 cells (thick solid line) assessed by using PE-conjugated anti-CD40L mAb 24–31. (B) β-chemokine production by macrophages. MDM were cultured in medium alone, with added IFN-γ or GM-CSF, or with added control 293 cells or CD40L-293 cells. LPS was used as a positive control. The mean chemokine concentrations (picogram per milliliter) of the supernatants from quadruplicate wells 24 hr later are shown (±SD). □, MIP-1α; ▨, MIP-1β; ▪, RANTES. (C) Abrogation of CD40L stimulation by anti-CD40 or anti-CD40L mAbs. In an experiment similar to B, neutralizing mAbs were added at the initiation of the CD40L-293 cell-MDM cocultures. Anti-CD40 mAb blocked >99% and anti-CD40L mAb blocked 94% of the β-chemokine release. (D) Stimulation of macrophages by CD40L-bearing plasma membranes. Acellular preparations of membranes from control 293 cells, 293 cells expressing a nonfunctional mutant of human CD40L (T147N), human CD40L-293 cells (CD40L), and murine CD40L-293 cells (mCD40L) were added to MDM in an experiment similar to B.
Preparation of Plasma Membranes from CD40L-Expressing 293 Cells.
Membranes were prepared from roller bottle cultures of stably transfected 293 cells by ultracentrifugation of a crude membrane preparation over a 35–73% sucrose step gradient as described (18, 19). The resulting membranes were washed in PBS by repeated centrifugation at 90,000 × g and stored at −80°C until used. To quantify the membranes, they were first solubilized in 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and then assayed for protein content using the bicinchoninic acid method.
Stimulation of MDM.
To study the influence of soluble factors, cytokines were added to MDM and the supernatant media were collected 24 hr later. To study the cell contact pathway of macrophage activation, control 293 cells or CD40L-293 cells were added directly to MDM cultures at a 1:1 cell-to-cell ratio, and the media were collected 24 hr later. In some experiments, membranes from the 293 cells were added instead of intact cells. In all cases, the MDM supernatants were stored frozen at −80°C until assayed for β-chemokines by ELISA (R & D Systems). All cultures were set up in quadruplicate and the results are expressed as the mean (±SD).
HIV-1 Isolates.
HIV-1SF-162 (20) was propagated in PBMC cultures that had been stimulated with 3 μg/ml phytohemagglutinin (PHA) and fed with R10 containing 50 μM β-mercaptoethanol and 10 units per ml IL-2. The LAV-1 strain of HIV-1LAI was propagated in CEM cells and titered using MT-2 cell cultures. The macrophage–tropic strain, HIV-1BaL, was propagated and titered in MDM as described (15). p24 Gag protein was measured by ELISA (Coulter).
Anti-HIV-1 Effects of MDM Supernatants.
The HIV-1 suppressive effects of MDM supernatants were detected essentially as described by Paxton et al. (21). Activated CD4-enriched T cells were prepared by stimulating PBMC with PHA (3 μg/ml) and IL-2 (10 units per ml) in RPMI 1640 medium containing 10% unheated autologous serum and 50 μM 2-mercaptoethanol. Contaminating CD8+ T cells were removed by using anti-CD8-coated magnetic beads (Dynal), and the remaining T cells were typically about 90% CD4+ by flow cytometry. The CD4+ T cells were infected with the nonsyncytium-inducing (NSI) isolate, HIV-1SF162, at an multiplicity of infection of 0.01 for 1 hr at room temperature, extensively washed, and distributed into the wells of a 48-well plate at 104/ml. Supernatants from macrophages cultured with either control 293 cells, CD40L 293 cells, or LPS (100 ng/ml) were added at a final dilution of 1:20. In some experiments, β-chemokines in the supernatants were neutralized by preincubating them for 30 min at room temperature with a mixture of 20 μg/ml each of goat polyclonal anti-MIP-1α IgG, anti-MIP-1β mAb, and anti-RANTES mAb (R & D Systems). The cultures were fed every 3–4 days with medium containing IL-2 and the respective MDM supernatants and antibodies. On day 14, the CD4+ T cell supernatants were collected and tested for p24 antigen.
RESULTS
T Cell Soluble Factors, IFN-γ and GM-CSF, Fail to Induce β-Chemokine Production by Macrophages.
To study the regulation of β-chemokine production in macrophages, conditions were established in which unstimulated MDM cultured only in R10 medium failed to produce significant levels of MIP-1α, MIP-1β, or RANTES (Fig. 1B). As expected (4, 7), LPS (100 ng/ml) strongly induced chemokine production. However, IFN-γ (300 ng/ml) and GM-CSF (100 ng/ml), two classic macrophage-stimulating lymphokines (22), did not induce significant amounts of these β-chemokines (<0.25 ng/ml).
Direct Cell–Cell Contact with CD40L-Expressing Cells Induces Macrophages to Produce Large Amounts of β-Chemokines.
We next examined the effects of CD40L, a membrane molecule expressed on the surface of certain activated T cells, which acts as a major regulatory signal in humoral and cellular immunity (23–27). Recent reports have indicated that CD40L is also the primary molecule responsible for the cell–cell contact pathway of macrophage activation by T cells (28–31). By exposing MDM to 293 cells engineered to express CD40L (CD40L-293 cells, Fig. 1A), large amounts of MIP-1α (15–25 ng/ml), MIP-1β (15–25 ng/ml), and RANTES (1.5–5 ng/ml) were generated (Fig. 1B) (ranges given are from 14 independent experiments by using MDM from 6 different donors). β-chemokine production was detectable within 3 hr of CD40L stimulation (data not shown). In contrast, control 293 cells were inactive. As an additional control, 293 cells expressing the T147N mutant form of human CD40L were prepared. This mutation results in a protein that is recognized by several anti-CD40L mAbs yet is nonfunctional because it is found in a subset of humans with CD40L deficiency disease, the X-linked hyper-IgM (X-HIM) syndrome (16). These T147N-CD40L-293 cells were completely inactive in inducing MDM to produce the three β-chemokines (data not shown).
To prove that a CD40L/CD40 interaction was necessary for chemokine production, neutralizing mAbs directed against either CD40L or CD40 were added to the cocultures of CD40L-293 cells and MDM (32). Antibody against CD40 (1 μg/ml) prevented >99% and antibody against CD40L (10 μg/ml) prevented 94% of the induced production of the β-chemokines (Fig. 1C). Furthermore, direct cell–cell contact was necessary because CD40L-293 cell supernatant or CD40L-293 cells separated from MDM by a porous membrane (Transwell, Costar) were unable to induce β-chemokine production (data not shown).
Isolated CD40L-Bearing Plasma Membranes Induce Macrophages to Produce β-Chemokines.
Because the above experiments all used live 293 cells in addition to MDM, it was important to consider the possibility that the β-chemokines might be produced by the 293 cells rather than the MDM. Consequently, acellular plasma membranes were prepared from the 293 cells and used to stimulate MDM (250 μg/ml). Only membranes prepared from 293 cells expressing human or murine CD40L were capable of stimulating MDM to produce MIP-1α, MIP-1β, and RANTES. Membranes prepared from control 293 cells or from T147N-CD40L-293 cells were unable to stimulate MDM (Fig. 1D).
Suppression of NSI HIV-1 Replication in CD4+ T Cells by Supernatants from CD40L-Stimulated Macrophages.
The three β-chemokines initially selected for study were chosen because they suppress the replication of NSI strains of HIV-1 (33) by binding to CC chemokine receptor 5 (CCR5), the β-chemokine receptor that has been shown to be critical for the acquisition of HIV-1 infection (34–36). To determine whether the numbers of β-chemokines produced by CD40L-stimulated macrophages were sufficient to inhibit the replication of a virus that uses CCR5 as a coreceptor, purified CD4+ T cells were stimulated with PHA and IL-2 and infected with the NSI isolate, HIV-1SF162. The CD4+ T cells were fed twice a week for 2 weeks and their supernatants were evaluated for HIV-1 p24 antigen as a measure of virus replication. As expected, supernatants from MDM cultured only in medium or MDM cocultured with control 293 cells (both of which contained almost none of the three β-chemokines (Fig. 1B)) did not affect HIV-1 replication in the CD4+ T cells (Fig. 2). However, supernatants from CD40L-stimulated MDM (which contained high levels of the β-chemokines) were highly effective at preventing the replication of this NSI HIV-1 strain. To determine whether the three β-chemokines were responsible for this protection, supernatants from CD40L-stimulated MDM were preincubated with neutralizing antibodies against MIP-1α, MIP-1β, and RANTES, which eliminated their anti-HIV-1 activity (Fig. 2).
Figure 2.
Inhibition of NSI HIV-1 replication in CD4+ T cells by CD40L-stimulated macrophage supernatants. CD4+ T cells (depleted of CD8+ T cells) were stimulated by PHA and IL-2, infected with the NSI isolate, (HIV-1SF162), and cultured for 14 days in either R10 medium or a 1:20 dilution of MDM supernatants. Virus production was measured by ELISA for HIV-1 p24 antigen. To demonstrate that the β-chemokines in the CD40L-stimulated MDM supernatant accounted for the HIV-1 suppressive activity, the supernatant was pretreated with a mixture of neutralizing antibodies against MIP-1α, MIP-1β, and RANTES, which abrogated its effectiveness.
Unlike NSI HIV-1 strains, syncytium-inducing (SI) strains use CXCR4 as a coreceptor rather than CCR5, and their infectivity is not affected by MIP-1α, MIP-1β, or RANTES (33). Instead, SDF-1, an α-chemokine that is the ligand for CXCR4, is capable of blocking the infection of CD4+ T cells by many SI strains (34–36). To evaluate the effects of CD40L-stimulated MDM supernatants against an SI strain of HIV-1, these supernatants were added to CD4+ T cells exposed to HIV-1LAI. Unlike the results with the NSI strain, the CD40L-stimulated MDM supernatants failed to protect CD4+ T cells from infection by this SI virus. Consistent with these results, a reverse transcriptase-PCR analysis showed only trace expression of SDF-1 mRNA in MDM, and this was not upregulated by CD40L stimulation (data not shown). Also, consistent with other reports that β-chemokines do not protect macrophages from HIV-1 infection (37, 38), the β-chemokine-containing CD40L-stimulated MDM supernatants were unable to protect MDM from infection by a macrophage-tropic NSI strain, HIV-1BaL, under conditions similar to those reported previously (15).
CD40L Stimulation of MCP-1 Production by Macrophages.
Because it was not feasible to examine all of the numerous other chemokines of immunologic significance, MCP-1 was chosen as an additional β-chemokine for study. MCP-1 is particularly chemotactic for monocytes and has been found in atherosclerotic plaques (39) and granulomatous lesions (40). As with the other three β-chemokines examined, CD40L-293 cells, but not control cells, stimulated MDM to produce large amounts of MCP-1 (Fig. 3).
Figure 3.
CD40L stimulation of MCP-1 production by MDM. Culture conditions were identical to that in Fig. 1B.
DISCUSSION
These results have particular relevance to HIV-1 infection and imply that T cells that express CD40L upon activation could play a special role in controlling HIV-1 replication (Fig. 4). Immune responses that activate CD4+ T cells are known to make them more vulnerable to HIV-1 infection (41). However, a subset of CD4+ T cells can express CD40L on their cell surfaces within a few hours following T cell activation (42). Because the production of β-chemokines by MDM is also rapid and begins within 3 hr after CD40L stimulation, it is possible that such CD40L+CD4+ T cells could induce enough chemokine production by macrophages to protect both themselves and any adjacent CD4+ T cells from infection by NSI strains of HIV-1. [In this context, it should be noted that dendritic cells, another but rarer type of antigen-presenting cell, have also been reported to produce large amounts of MIP-1α after CD40L stimulation (43).] Overall, our data indicate that T cells (both CD4+ and CD8+), which recognize HIV-1 antigens and express CD40L, could form an additional β-chemokine-mediated pathway of acquired immunologic resistance to HIV-1 infection, along with anti-HIV-1 antibodies, cytotoxic T cells, and interferons (15). However, under the conditions tested, CD40L stimulated macrophages were not themselves protected from HIV infection nor did their supernatants protect CD4+ T cells from infection by an SI strain of HIV-1. Thus, the expression of CD40L alone might not completely protect the host from all modes of HIV-1 replication.
Figure 4.
CD40L-macrophage pathway for the generation of β-chemokines. Stimulation of the T cell receptor (TCR) by peptidic antigen presented by major histocompatibility complex (MHC) molecules (Signal 1) is sufficient to stimulate a subset of T cells to rapidly express CD40L (42). Macrophages bearing CD40 respond to cell–cell contact with such T cells by producing β-chemokines within 3 hr. The resulting β-chemokines may then act to attract additional lymphocytes and monocyte/macrophages to the site of the developing immune reaction or block the infection of nearby CD4+ T cells by NSI strains of HIV-1.
Previously, only CD8+ T cells (33, 38) and CD4+ T cells (44, 45) have been considered as important immunologic sources of the HIV-1-suppressive β-chemokines. However, CD40L-stimulated MDM produced 5–20 times more MIP-1α and MIP-1β and only slightly less RANTES than any untransformed activated T cell yet described (33, 44, 45), when production is considered on a per cell per day basis. More importantly, Tedla et al. (46) used in situ hybridization and immunohistochemistry to show that the lymph nodes of HIV-1-infected individuals contain abundant macrophages (most of which were not infected by HIV-1), which are producing MIP-1α and MIP-1β. They also demonstrated RANTES expression by macrophages (although at a lower level than MIP-1α and MIP-1β, similar to our in vitro results in Fig. 1B), as well as in perivascular cells. Although large numbers of T cells (mainly CD8+ T cells) were also present in these lymph nodes, they were not significant producers of any of these three β-chemokines in these tissues (46). Therefore, macrophages should be considered as a major source of the HIV-1-suppressive β-chemokines in vivo. In this context, our data show that the level of macrophage β-chemokine production can be high enough to protect CD4+ T cells against many of the NSI strains of HIV-1 that predominate during the early phase of HIV-1 infection (47), and we have identified CD40L as a stimulus for macrophage β-chemokine production.
Although we studied MCP-1 production because of its importance for immunologic lesions involving macrophages, two recent studies suggest that there may be a role for MCP-1 in anti-HIV-1 immunity. An epidemiological study of the MCP-1 receptor, CCR2, revealed a delayed progression to AIDS in HIV-1-infected individuals who were homozygous for a mutation in the first transmembrane region (CCR2–64I) (48). Another study found that MCP-1 at 1 nM (8,700 pg/ml) was found to suppress the replication of both NSI and SI strains of HIV-1 in primary CD4+ T cell cultures (49). Although our antibody neutralization studies demonstrated that MIP-1α, MIP-1β, and RANTES could account for almost all of the HIV-1 suppressive activity in the supernatants of CD40L-stimulated MDM (Fig. 2), our experiments tested relatively high dilutions (1:20), which may have minimized any contribution from MCP-1. Similarly, the dilutions tested may have minimized any protective effects from the newly described HIV-suppressive β-chemokine, macrophage-derived chemokine (50).
CD8+ T cell clones that express CD40L have been isolated with high frequency from the peripheral blood of a subset of HIV-1-infected individuals (51), and it is possible that such cells contribute to the control of HIV-1 replication in early infection. Additional studies are needed to determine whether current anti-HIV-1 vaccines generate CD40L-expressing T cells in uninfected individuals or whether special immunization procedures are needed for their expansion. However, because the control of HIV-1 replication by the immune system is typically incomplete even in early infection, it is relevant to note that HIV-1 infection and purified gp120 envelope protein have been reported to inhibit the expression of CD40L on CD4+ T cells in vitro (52, 53), and that HIV infection is associated with a preferential loss of CD40L-expressing CD4+ T cells in vivo (54). This suggests that HIV-1 may have evolved a means to counter the CD40L-macrophage pathway for β-chemokine production as a strategy to avoid the suppressive effects of these β-chemokines on viral replication.
These results are also important for immune diseases where CD40L/CD40 interactions may be involved. In multiple sclerosis, a disease in which CD40L is required for disease activity in the extrinsic allergic encephalitis animal model (55, 56), T cells expressing CD40L have been identified in direct contact with CD40-expressing macrophages in human brain lesions (55) and MIP-1α has been identified in extrinsic allergic encephalitis lesions (57). CD40L has also been found in atherosclerotic plaques (58), where MCP-1 production by macrophages has also been demonstrated (39). Further indirect evidence suggesting a linkage between CD40L expression and β-chemokine production comes from animal studies where anti-CD40L antibodies have been used to block cardiac, skin, and renal transplant rejection (59) and chemically induced immune colitis (60). In each case, not only was the deleterious immunologic reaction prevented, but the infiltration of immune cells was markedly reduced as well. CD40L has already been shown to be critical for the elaboration of a cellular immune response (23–27). In addition, our data now suggest that CD40L may act at the earliest phases of immune reactivity to induce the β-chemokine-mediated recruitment of additional cells into developing immunologic reactions or lesions.
Acknowledgments
We thank Sharon Wilcox, Darica Smith, Theresa Jackson, and Carol Latham for administrative support, and Violetta Alvarado, Sara Albanil, and Jeanne Aufderheide for p24 ELISA results. HIV-1SF162, from Drs. Cecilia Cheng-Mayer and Jay A. Levy, was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). This work was supported by NIH Grants RO1 AI35258 and RO1 HL57911 to R.S.K.; Grant Supplement P30 CA23100–15S1 to the University of California, San Diego (UCSD) Cancer Center; and the State of California University-wide AIDS Research Program. Other support was provided by the UCSD Center for AIDS Research (NIH Grant P30 AI36214) and the Research Center on AIDS and HIV Infection of the San Diego Veterans Affairs Medical Center.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CCR, CC chemokine receptor; MIP-1, macrophage inflammatory protein-1; MCP-1, monocyte chemoattractant protein-1; MDM, monocyte-derived macrophages; NSI, nonsyncytium-inducing; PBMC, peripheral blood mononuclear cells; RANTES, regulated upon activation, normal T cell expressed and secreted; PE, phycoerythrin; IL, interleukin; LPS, lipopolysaccharide; IFN-γ; interferon γ; GM-CSF, granulocyte-macrophage colony-stimulating factor; PHA, phytohemagglutinin; SI, syncytium inducing.
References
- 1.Taub D D, Oppenheim J J. Therapeutic Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- 2.Schall T J, Bacon K B. Curr Opin Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy P M. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 4.Sherry B, Horii Y, Manogue K R, Widmer U, Cerami A. Cytokines. 1992;4:117–130. [PubMed] [Google Scholar]
- 5.Schall T J, Jongstra J, Dyer B J, Jorgensen J, Clayberger C, Davis M M, Krensky A M. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 6.Altman L C, Snyderman R, Oppenheim J J, Mergenhagen S E. J Immunol. 1973;110:801–810. [PubMed] [Google Scholar]
- 7.Verani A, Scarlatti G, Comar M, Tresoldi E, Polo S, Giacca M, Lusso P, Siccardi A G, Vercelli D. J Exp Med. 1997;185:805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis M, Ghadirian E. Clin Exp Immunol. 1994;96:187–192. doi: 10.1111/j.1365-2249.1994.tb06540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canque B, Rosenzwajg M, Gey A, Tartour E, Fridman W H, Gluckman J C. Blood. 1996;87:2011–2019. [PubMed] [Google Scholar]
- 11.Lukacs N W, Kunkel S L, Strieter R M, Warmington K, Chensue S W. J Exp Med. 1993;177:1551–1559. doi: 10.1084/jem.177.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devergne O, Marfaing-Koka A, Schall T J, Leger-Ravet M B, Sadick M, Peuchmaur M, Crevon M C, Kim K J, Galanaud P, Emilie D. J Exp Med. 1994;179:1689–1694. doi: 10.1084/jem.179.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denis M. Am J Respir Crit Care Med. 1995;151:164–169. doi: 10.1164/ajrccm.151.1.7812548. [DOI] [PubMed] [Google Scholar]
- 14.Petrek M, Pantelidis P, Southcott A M, Lympany P, Safranek P, Black C M, Kolek V, Weigl E, du Bois R M. Eur Respir J. 1997;10:1207–1216. doi: 10.1183/09031936.97.10061207. [DOI] [PubMed] [Google Scholar]
- 15.Kornbluth R S, Oh P S, Munis J R, Cleveland P H, Richman D D. J Exp Med. 1989;169:1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajorath J, Seyama K, Nonoyama S, Ochs H D, Aruffo A. Protein Sci. 1996;5:531–534. doi: 10.1002/pro.5560050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho S D, Hunt H D, Horton R M, Pullen J K, Pease L R. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 18.Noelle R J, Daum J, Bartlett W C, McCann J, Shepherd D J. J Immunol. 1991;146:1118–1124. [PubMed] [Google Scholar]
- 19.Suttles J, Evans M, Miller R W, Poe J C, Stout R D, Wahl L M. J Leukocyte Biol. 1996;60:651–657. doi: 10.1002/jlb.60.5.651. [DOI] [PubMed] [Google Scholar]
- 20.Cheng-Mayer C, Levy J A. Ann Neurol. 1988;23:S58–S61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 21.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton J A. Immunol Today. 1993;14:18–24. doi: 10.1016/0167-5699(93)90319-G. [DOI] [PubMed] [Google Scholar]
- 23.Kroczek R A, Graf D, Brugnoni D, Giliani S, Korthuer U, Ugazio A, Senger G, Mages H W, Villa A, Notarangelo L D. Immunol Rev. 1994;138:39–59. doi: 10.1111/j.1600-065x.1994.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh N, Morio T, Fuleihan R, Worm M, Horner A, Tsitsikov E, Castigli E, Geha R S. Clin Immunol Immunopathol. 1995;76:S208–S213. doi: 10.1016/s0090-1229(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 25.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 26.Van Kooten C, Banchereau J. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 27.Grewal I S, Borrow P, Pamer E G, Oldstone M B A, Flavell R A. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 28.Alderson M, Armitage R J, Tough T W, Strockbine L, Fanslow W C, Spriggs M K. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiener P A, Moran-Davis P, Rankin B M, Wahl A F, Aruffo A, Hollenbaugh D. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 30.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 31.Stout R D, Suttles J. Immunol Today. 1996;17:487–492. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 32.Wagner D H, Jr, Stout R D, Suttles J. Eur J Immunol. 1994;24:3148–3154. doi: 10.1002/eji.1830241235. [DOI] [PubMed] [Google Scholar]
- 33.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 34.D’Souza M P, Harden V A. Nat Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 35.Moore J P, Trkola A, Dragic T. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 36.Broder C C, Collman R G. J Leukocyte Biol. 1997;62:20–29. doi: 10.1002/jlb.62.1.20. [DOI] [PubMed] [Google Scholar]
- 37.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 38.Moriuchi M, Moriuchi H, Combadiere C, Murphy P M, Fauci A S. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yla-Herttuala S, Lipton B A, Rosenfeld M E, Sarkioja T, Yoshimura T, Leonard E J, Witztum J L, Steinberg D. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chensue S W, Warmington K S, Ruth J H, Sanghi P S, Lincoln P, Kunkel S L. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- 41.Pinchuk L M, Polacino P S, Agy M B, Klaus S J, Clark E A. Immunity. 1994;1:317–325. doi: 10.1016/1074-7613(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 42.Casamayor-Palleja M, Khan M, MacLennan I C. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conlon K, Lloyd A, Chattopadhyay U, Lukacs N, Kunkel S, Schall T, Taub D, Morimoto C, Osborne J, Oppenheim J, Young H, Kelvin D, Ortaldo J. Eur J Immunol. 1995;25:751–756. doi: 10.1002/eji.1830250319. [DOI] [PubMed] [Google Scholar]
- 45.Kinter A L, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci A S. Proc Natl Acad Sci USA. 1996;93:14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tedla N, Palladinetti P, Kelly M, Kumar R K, DiGirolamo N, Chattophadhay U, Cooke B, Truskett P, Dwyer J, Wakefield D, Lloyd A. Am J Pathol. 1996;148:1367–1373. [PMC free article] [PubMed] [Google Scholar]
- 47.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith M W, Dean M, Carrington M, Winkler C, Huttler G A, Lomb D A, Goedert J J, O’Brien T R, Jacobson L P, et al. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 49.Frade J M R, Llorante M, Mellado M, Alcami J, Gutierrez-Ramos J C, Zaballos A, del Real G, Martinez-A C. J Clin Invest. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 51.Paganelli R, Scala E, Ansotegui I J, Ausiello C M, Halapi E, Fanales-Belasio E, D’Offizi G, Mezzaroma I, Pandolfi F, Fiorilli M, Cassone A, Aiuti F. J Exp Med. 1995;181:423–428. doi: 10.1084/jem.181.1.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macchia D, Almerigogna F, Parronchi P, Ravina A, Maggi E, Romagnani S. Nature (London) 1993;363:464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 53.Chirmule N, McCloskey T W, Hu R, Kalyanaraman V S, Pahwa S. J Immunol. 1995;155:917–924. [PubMed] [Google Scholar]
- 54.Wolthers K C, Otto S A, Lens S M, Van Lier R A, Miedema F, Meyaard L. AIDS Res Human Retroviruses. 1997;13:1023–1029. doi: 10.1089/aid.1997.13.1023. [DOI] [PubMed] [Google Scholar]
- 55.Gerritse K, Laman J D, Noelle R J, Aruffo A, Ledbetter J A, Boersma W J, Claassen E. Proc Natl Acad Sci USA. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grewal I S, Foellmer H G, Grewal K D, Xu J, Hardardottir F, Baron J L, Janeway C A, Jr, Flavell R A. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 57.Glabinski A R, Tani M, Strieter R M, Tuohy V K, Ransohoff R M. Am J Pathol. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- 58.Mach F, Schonbeck U, Sukhova G K, Bourcier T, Bonnefoy J Y, Pober J S, Libby P. Proc Natl Acad Sci USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsen C P, Elwood E T, Alexander D Z, Ritchie S C, Hendrix R, Tucker-Burden C, Cho H R, Aruffo A, Hollenbaugh D, Linsley P S, Winn K J, Pearson T C. Nature (London) 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 60.Stuber E, Strober W, Neurath M. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]