Abstract
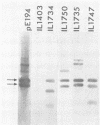
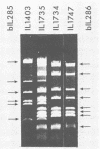
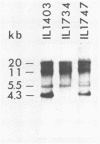
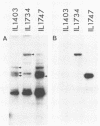
The plasmid pE194 is unable to replicate in Lactococcus lactis subsp. lactis (formerly Streptococcus lactis). When linked to resident bacteriophage sequences, pE194 was able to integrate into the L. lactis subsp. lactis chromosome either by Campbell-like recombination or by double crossing over with deletion. Integration occurred into the DNA of the prophage and prevented its multiplication. When a selective pressure was applied to an integrant in which pE194 was flanked by two direct repeats of prophage fragment, amplification of pE194 and the prophage fragment was observed. The pE194 copy number was assessed at six to nine, and amplification was stable upon growth under nonselective conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin A., Chopin M. C., Moillo-Batt A., Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984 May;11(3):260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Connors M. J., Howard S., Hoch J., Setlow P. Determination of the chromosomal locations of four Bacillus subtilis genes which code for a family of small, acid-soluble spore proteins. J Bacteriol. 1986 May;166(2):412–416. doi: 10.1128/jb.166.2.412-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Mechanism of integrating foreign DNA during transformation of Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3664–3668. doi: 10.1073/pnas.75.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterson N. I., Koshland D. E., Jr Replacement and amplification of bacterial genes with sequences altered in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4894–4898. doi: 10.1073/pnas.80.16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi K., Tanimoto A., Nomura S., Yamane K., Yoda K., Harada S., Mori M., Furusato T., Takatsuki A., Yamasaki M. Amplification of the amyE-tmrB region on the chromosome in tunicamycin-resistant cells of Bacillus subtilis. Mol Gen Genet. 1986 Jul;204(1):36–43. doi: 10.1007/BF00330184. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannière L., Niaudet B., Pierre E., Ehrlich S. D. Stable gene amplification in the chromosome of Bacillus subtilis. Gene. 1985;40(1):47–55. doi: 10.1016/0378-1119(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B., Ehrlich S. D. Recombination efficiency is a quadratic function of the length of homology during plasmid transformation of Bacillus subtilis protoplasts and Escherichia coli competent cells. EMBO J. 1984 Dec 1;3(12):2879–2884. doi: 10.1002/j.1460-2075.1984.tb02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Trombe M. C., Hayden M. K., Waszak G. A., Chen J. D. Isolation of transformation-deficient Streptococcus pneumoniae mutants defective in control of competence, using insertion-duplication mutagenesis with the erythromycin resistance determinant of pAM beta 1. J Bacteriol. 1984 Sep;159(3):870–876. doi: 10.1128/jb.159.3.870-876.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean V., Claverys J. P., Vasseghi H., Sicard A. M. Rapid cloning of specific DNA fragments of Streptococcus pneumoniae by vector integration into the chromosome followed by endonucleolytic excision. Gene. 1981 Nov;15(2-3):289–293. doi: 10.1016/0378-1119(81)90139-6. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Goze A., Ehrlich S. D. Insertional mutagenesis in Bacillus subtilis: mechanism and use in gene cloning. Gene. 1982 Oct;19(3):277–284. doi: 10.1016/0378-1119(82)90017-8. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Jannière L., Ehrlich S. D. Integration of linear, heterologous DNA molecules into the Bacillus subtilis chromosome: mechanism and use in induction of predictable rearrangements. J Bacteriol. 1985 Jul;163(1):111–120. doi: 10.1128/jb.163.1.111-120.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G., Guild W. R. Modes of integration of heterologous plasmid DNA into the chromosome of Streptococcus pneumoniae. J Bacteriol. 1985 Mar;161(3):909–912. doi: 10.1128/jb.161.3.909-912.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Mock M., Schwartz M. A technique for integrating any DNA fragment into the chromosome of Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):231–241. doi: 10.1016/0378-1119(84)90183-5. [DOI] [PubMed] [Google Scholar]
- Reyrolle J., Chopin M. C., Letellier F., Novel G. Lysogenic strains of lactic Acid streptococci and lytic spectra of their temperate bacteriophages. Appl Environ Microbiol. 1982 Feb;43(2):349–356. doi: 10.1128/aem.43.2.349-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988 Apr;70(4):559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- Simon D., Rouault A., Chopin M. C. High-efficiency transformation of Streptococcus lactis protoplasts by plasmid DNA. Appl Environ Microbiol. 1986 Aug;52(2):394–395. doi: 10.1128/aem.52.2.394-395.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseghi H., Claverys J. P. Amplification of a chimeric plasmid carrying an erythromycin-resistance determinant introduced into the genome of Streptococcus pneumoniae. Gene. 1983 Mar;21(3):285–292. doi: 10.1016/0378-1119(83)90012-4. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M. N., Priebe S. D., Pozzi G., Hageman J. M., Guild W. R. Cloning and physical characterization of chromosomal conjugative elements in streptococci. J Bacteriol. 1986 Jun;166(3):972–977. doi: 10.1128/jb.166.3.972-977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. E., Hoch J. A., Bott K. Genetic mapping of a linked cluster of ribosomal ribonucleic acid genes in Bacillus subtilis. J Bacteriol. 1981 Nov;148(2):624–628. doi: 10.1128/jb.148.2.624-628.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. Gene amplification in Bacillus subtilis. J Gen Microbiol. 1984 Jul;130(7):1613–1621. doi: 10.1099/00221287-130-7-1613. [DOI] [PubMed] [Google Scholar]
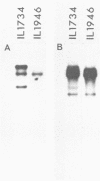
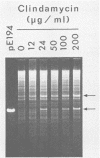
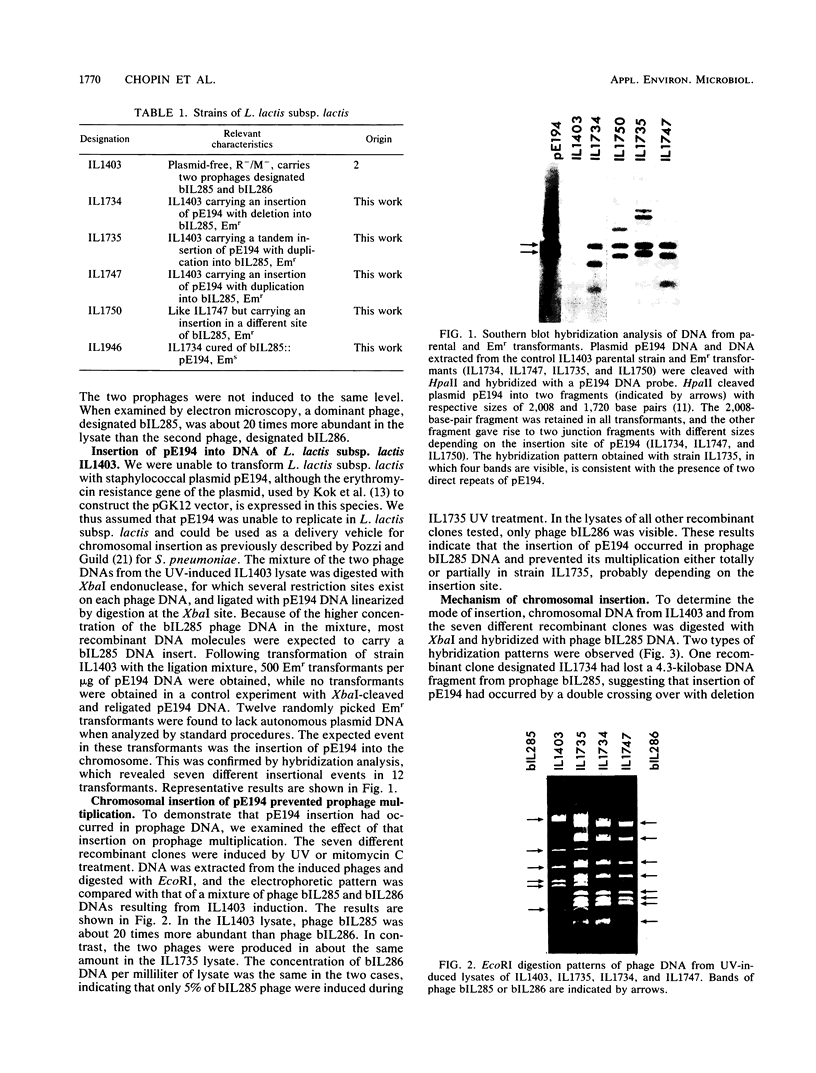
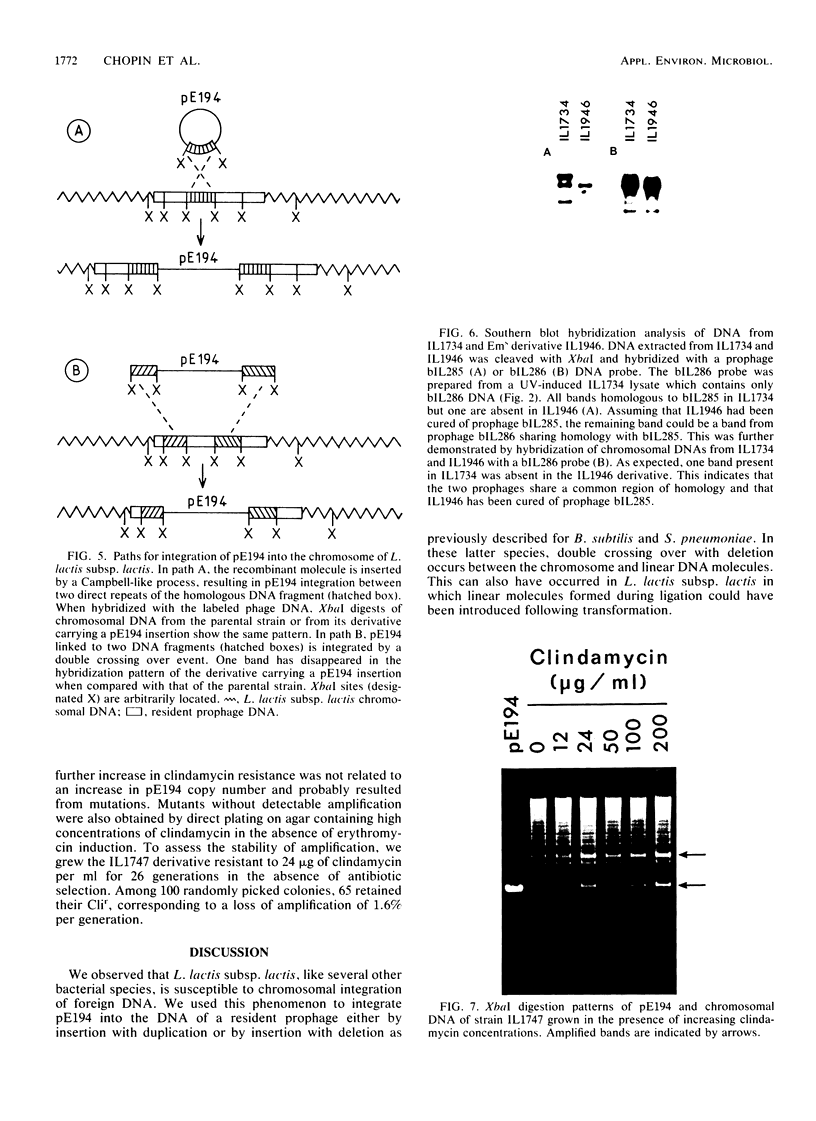
- Young M. The mechanism of insertion of a segment of heterologous DNA into the chromosome of Bacillus subtilis. J Gen Microbiol. 1983 May;129(5):1497–1512. doi: 10.1099/00221287-129-5-1497. [DOI] [PubMed] [Google Scholar]