Abstract
The general characteristics of the preneoplastic lesions of the human mammary gland, as they are known through histologic description, are outlined, and data obtained from the experimental analysis of mammary gland preneoplasia in five areas of endeavor are discussed. Results obtained with transplantation procedures and aimed at defining the growth potential of hyperplastic outgrowths are reported. Information derived from the study of events able to induce benign hyperplastic outgrowths or their malignant transformation in the murine mammary gland is summarized. Attempts to predict neoplastic transformation in morphologically hyperplastic epithelium of human and rodent glands are discussed. The present status of efforts toward the prophylaxis of preneoplastic lesions of the mammary gland is described. Considerations of the relationship between preneoplasia and tumor dormancy conclude the presentation.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abell C. W., Hodgins D. S., Stith W. J. An in vitro evaluation of the chemotherapeutic potency of phenylalanine ammonia-lyase. Cancer Res. 1973 Oct;33(10):2529–2532. [PubMed] [Google Scholar]
- Ankerst J., Jonsson N., Kjellén L., Norrby E., Sjögren H. O. Induction of mammary fibroadenomas in rats by adenovirus type 9. Int J Cancer. 1974 Mar 15;13(3):286–290. doi: 10.1002/ijc.2910130303. [DOI] [PubMed] [Google Scholar]
- Ashley D. J. On the incidence of carcinoma of the prostate. J Pathol Bacteriol. 1965 Jul;90(1):217–224. doi: 10.1002/path.1700900123. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. B., PERRIN E. V. IN SITU NEUROBLASTOMAS: A CONTRIBUTION TO THE NATURAL HISTORY OF NEURAL CREST TUMORS. Am J Pathol. 1963 Dec;43:1089–1104. [PMC free article] [PubMed] [Google Scholar]
- Basombrío M. A., Prehn R. T. Studies on the basis for diversity and time of appearance of antigens in chemically induced tumors. Natl Cancer Inst Monogr. 1972 Dec;35:117–124. [PubMed] [Google Scholar]
- Ben-David M., Heston W. E., Rodbard D. Mammary tumor virus potentiation of endogenous prolactin effect on mammary gland differentiation. J Natl Cancer Inst. 1969 Feb;42(2):207–218. [PubMed] [Google Scholar]
- Blair P. B., Kripke M. L., Lappé M. A., Bonhag R. S., Young L. Immunologic deficiency associated with mammary tumor virus (MTV) infection in mice: hemagglutinin response and allograft survival. J Immunol. 1971 Feb;106(2):364–370. [PubMed] [Google Scholar]
- Brem H., Folkman J. Inhibition of tumor angiogenesis mediated by cartilage. J Exp Med. 1975 Feb 1;141(2):427–439. doi: 10.1084/jem.141.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
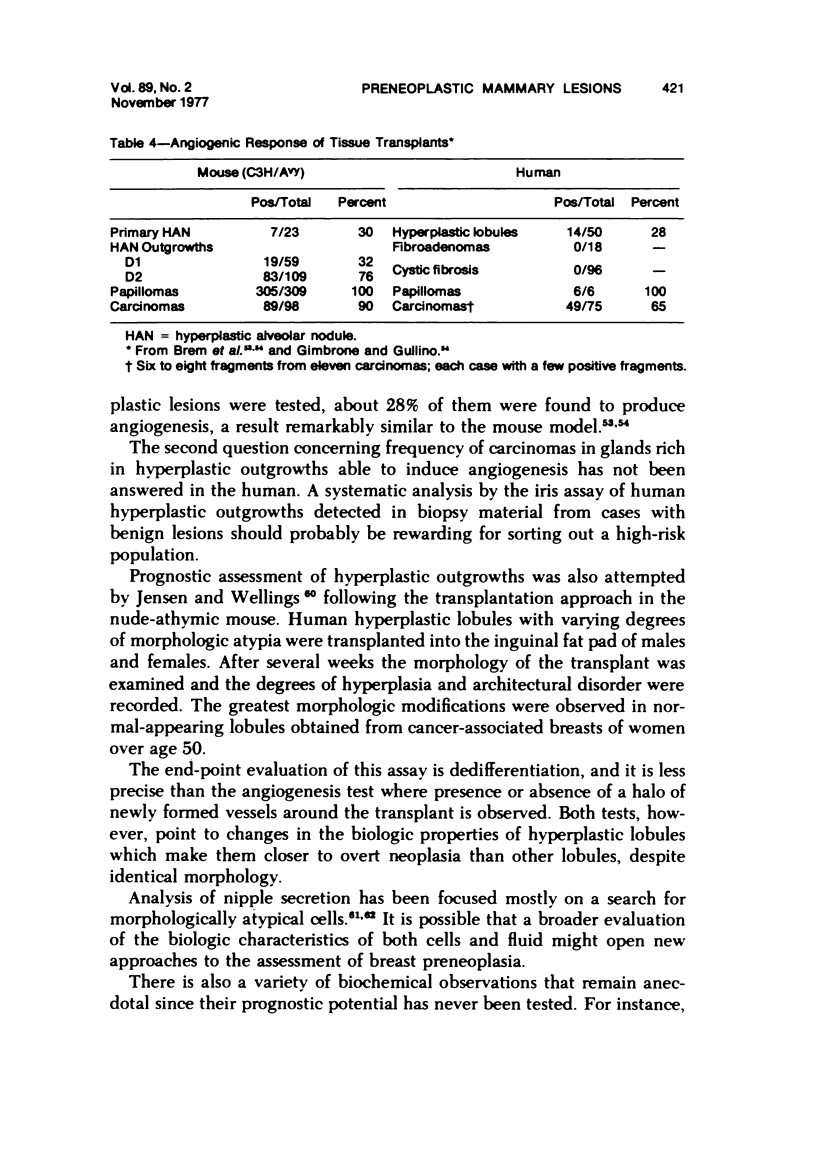
- Brem S. S., Gullino P. M., Medina D. Angiogenesis: a marker for neoplastic transformation of mammary papillary hyperplasia. Science. 1977 Mar 4;195(4281):880–882. doi: 10.1126/science.402692. [DOI] [PubMed] [Google Scholar]
- Brem S., Brem H., Folkman J., Finkelstein D., Patz A. Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Res. 1976 Aug;36(8):2807–2812. [PubMed] [Google Scholar]
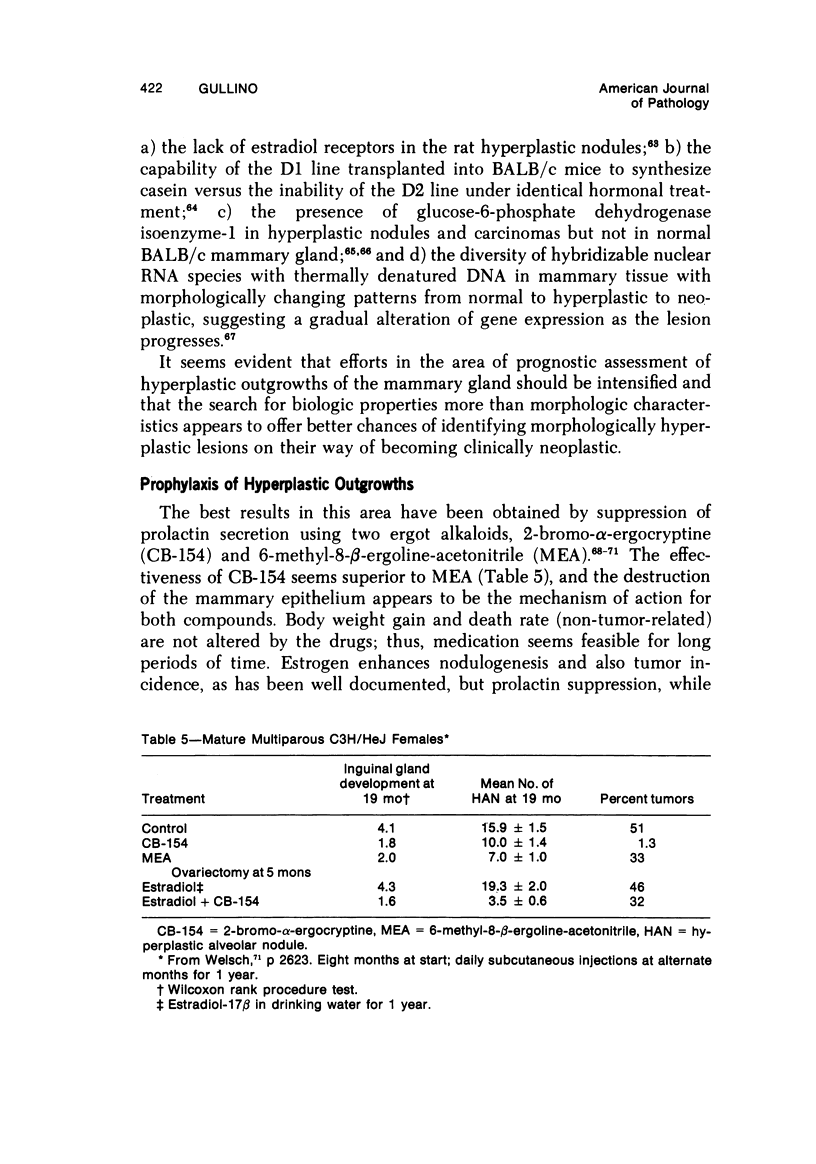
- Brooks C. L., Welsch C. W. Inhibition of mammary dysplasia in estrogen-treated C3H-HeJ female mice by treatment with 2-bromo-alpha-ergocryptine. Proc Soc Exp Biol Med. 1974 Feb;145(2):484–487. doi: 10.3181/00379727-145-37836. [DOI] [PubMed] [Google Scholar]
- Brooks C. L., Welsch C. W. Reduction of serum prolactin in rats by 2 ergot alkaloids and 2 ergoline derivatives: a comparison. Proc Soc Exp Biol Med. 1974 Jul;146(3):863–867. doi: 10.3181/00379727-146-38207. [DOI] [PubMed] [Google Scholar]
- Cameron A. M., Faulkin L. J., Jr Hyperplastic and inflammatory nodules in the canine mammary gland. J Natl Cancer Inst. 1971 Dec;47(6):1277–1287. [PubMed] [Google Scholar]
- Cameron A. M., Faulkin L. T., Jr Subgross evaluation of the nonhuman primate mammary gland: method and initial observations. J Med Primatol. 1974;3(5):298–310. doi: 10.1159/000460031. [DOI] [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- Daniel C. W., Aidells B. D., Medina D., Faulkin L. J., Jr Unlimited division potential of precancerous mouse mammary cells after spontaneous or carcinogen-induced transformation. Fed Proc. 1975 Jan;34(1):64–67. [PubMed] [Google Scholar]
- Dao T. L., Chistakos S. S., Varela R. Biochemical characterization of carcinogen-induced mammary hyperplastic aveolar nodule and tumor in the rat. Cancer Res. 1975 May;35(5):1128–1134. [PubMed] [Google Scholar]
- Diezel P. B., Heilmann K. Zytologische und zytochemische Untersuchungen am Mamillensekret. Verh Dtsch Ges Pathol. 1973;57:347–350. [PubMed] [Google Scholar]
- Eisenstein R., Kuettner K. E., Neapolitan C., Soble L. W., Sorgente N. The resistance of certain tissues to invasion. III. Cartilage extracts inhibit the growth of fibroblasts and endothelial cells in culture. Am J Pathol. 1975 Nov;81(2):337–348. [PMC free article] [PubMed] [Google Scholar]
- Eisenstein R., Sorgente N., Soble L. W., Miller A., Kuettner K. E. The resistance of certain tissues to invasion: penetrability of explanted tissues by vascularized mesenchyme. Am J Pathol. 1973 Dec;73(3):765–774. [PMC free article] [PubMed] [Google Scholar]
- FAULKIN L. J., Jr, DEOME K. B. Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J Natl Cancer Inst. 1960 Apr;24:953–969. [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
- Fenig J., Arlen M., Livingston S. F., Levowitz B. S. The potential for carcinoma existing synchronously on a microscopic level within the second breast. Surg Gynecol Obstet. 1975 Sep;141(3):394–396. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19(0):331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- Foote F. W., Stewart F. W. Lobular carcinoma in situ: A rare form of mammary cancer. Am J Pathol. 1941 Jul;17(4):491–496.3. doi: 10.3322/canjclin.32.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz R. R., Hodgins D. S., Abell C. W. Phenylalanine ammonia-lyase. Induction and purification from yeast and clearance in mammals. J Biol Chem. 1976 Aug 10;251(15):4646–4650. [PubMed] [Google Scholar]
- GODWIN J. T. Chronology of lobular carcinoma of the breast; report of a case. Cancer. 1952 Mar;5(2):259–266. doi: 10.1002/1097-0142(195203)5:2<259::aid-cncr2820050210>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Gallager H. S., Martin J. E. Early phases in the development of breast cancer. Cancer. 1969 Dec;24(6):1170–1178. doi: 10.1002/1097-0142(196912)24:6<1170::aid-cncr2820240615>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Leapman S. B., Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974 Feb;52(2):413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Gullino P. M. Neovascularization induced by intraocular xenografts of normal, preneoplastic, and neoplastic mouse mammary tissues. J Natl Cancer Inst. 1976 Feb;56(2):305–318. doi: 10.1093/jnci/56.2.305. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumors. J Natl Cancer Inst. 1973 Jan;50(1):219–228. doi: 10.1093/jnci/50.1.219. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972 Aug 1;136(2):261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf R., Ickowicz R., Bartley J. C., Abraham S. Multiple molecular forms of glucose-6-phosphate dehydrogenase in normal, preneoplastic, and neoplastic mammary tissues of mice. Cancer Res. 1975 Aug;35(8):2109–2116. [PubMed] [Google Scholar]
- Hilf R., Rector W., Abraham S. A glucose-6-phosphate dehydrogenase isoenzyme characteristic of preneoplastic and neoplastic mouse mammary tissue. J Natl Cancer Inst. 1973 May;50(5):1395–1398. doi: 10.1093/jnci/50.5.1395. [DOI] [PubMed] [Google Scholar]
- Hohmann P., Bern H. A., Cole R. D. Responsiveness of preneoplastic and neoplastic mouse mammary tissues to hormones: casein and histone syntheses. J Natl Cancer Inst. 1972 Aug;49(2):355–360. [PubMed] [Google Scholar]
- Hui Y. H., DeOme K. B., Briggs G. M. Inhibition of spontaneous development of hyperplastic alveolar nodules and mammary tumors in C3H mice fed phenylalanine-deficient diets. J Natl Cancer Inst. 1971 Sep;47(3):687–695. [PubMed] [Google Scholar]
- Hui Y. H., DeOme K. B., Briggs G. M. Inhibition of transformation of mammary preneoplastic nodules to tumor in C3H mice fed a phenylalanine-deficient diet. J Natl Cancer Inst. 1971 Jul;47(1):245–251. [PubMed] [Google Scholar]
- Jensen H. M., Wellings S. R. Preneoplastic lesions of the human mammary gland transplanted into the nude athymic mouse. Cancer Res. 1976 Jul;36(7 Pt 2):2605–2610. [PubMed] [Google Scholar]
- Kern W. H., Dermer G. B. The cytopathology of hyperplastic and neoplastic mammary duct epithelium. Cytologic and ultrastructural studies. Acta Cytol. 1972 Mar-Apr;16(2):120–129. [PubMed] [Google Scholar]
- Langer R., Brem H., Falterman K., Klein M., Folkman J. Isolations of a cartilage factor that inhibits tumor neovascularization. Science. 1976 Jul 2;193(4247):70–72. doi: 10.1126/science.935859. [DOI] [PubMed] [Google Scholar]
- Lavrin D. H., Blair P. B., Weiss D. W. Immunology of spontaneous mammary carcinomas in mice IV. Association of the mammary tumor virus with the immunogenicity of C3H nodules and tumors. Cancer Res. 1966 May;26(5):929–934. [PubMed] [Google Scholar]
- Lavrin D. H., Blair P. B., Weiss D. W. Immunology of spontaneous mammary carcinomas in mice. 3. Immunogenicity of C3H preneoplastic hyperplastic alveolar nodules in C3Hf hosts. Cancer Res. 1966 Feb;26(2):293–304. [PubMed] [Google Scholar]
- Lavrin D. H. Immunology of spontaneous mammary carcinomas in mice: immunogenicity of mammary tumor virus-contaning tissues in mammary tumor virus-free C3H-2 hosts. Cancer Res. 1970 Apr;30(4):1156–1162. [PubMed] [Google Scholar]
- MORTENSEN J. D., WOOLNER L. B., BENNETT W. A. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol Metab. 1955 Oct;15(10):1270–1280. doi: 10.1210/jcem-15-10-1270. [DOI] [PubMed] [Google Scholar]
- MUHLBOCK O., BOOT L. M. Induction of mammary cancer in mice without the mammary tumor agent by isografts of hypophyses. Cancer Res. 1959 May;19(4):402–412. [PubMed] [Google Scholar]
- Medina D., DeOme K. B., Young L. Tumor-producing capabilities of hyperplastic alveolar nodules in virgin and hormone-stimulated BALB/c f. C3H and C3Hf mice. J Natl Cancer Inst. 1970 Jan;44(1):167–174. [PubMed] [Google Scholar]
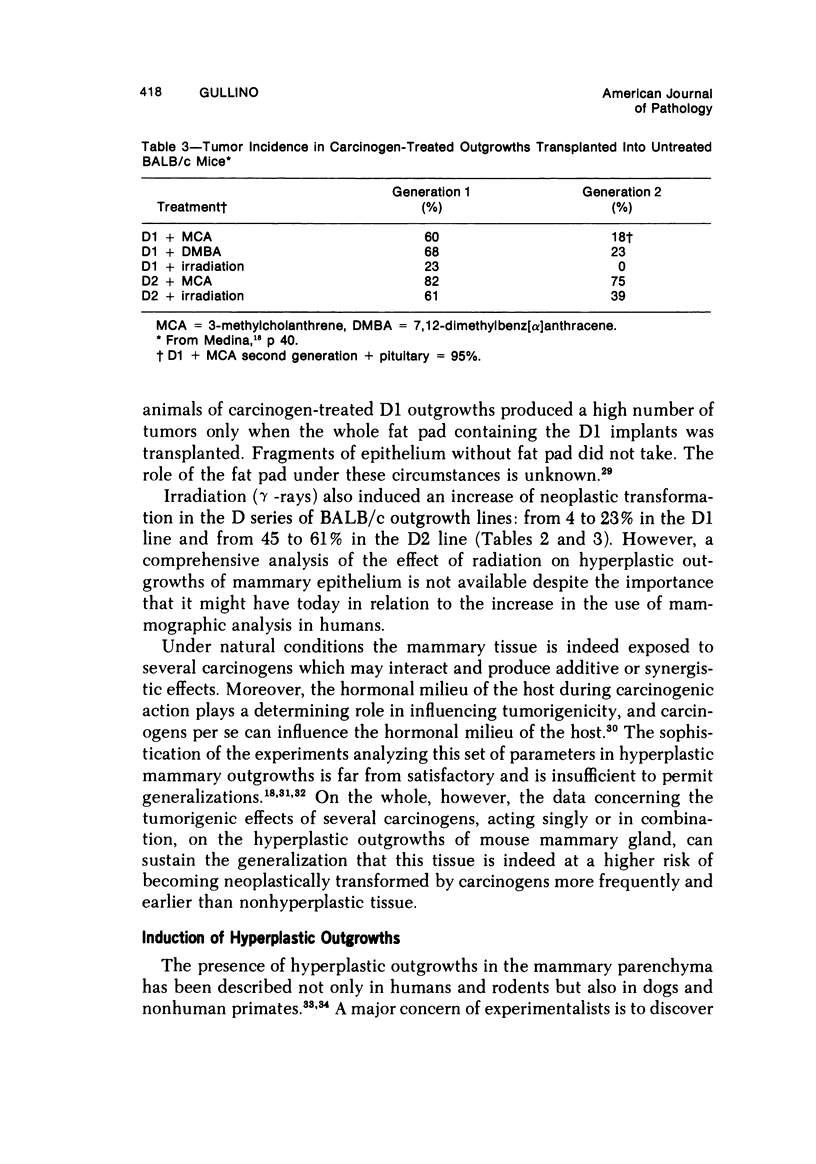
- Medina D. Effect of hormone stimulation, dose, and time of administration of carcinogen on carcinogen-induced mammary tumors from preneoplastic nodule outgrowths. J Natl Cancer Inst. 1971 Apr;46(4):909–916. [PubMed] [Google Scholar]
- Medina D. Serial transplantation of methylcholanthrene-treated mammary nodule outgrowth line D1. J Natl Cancer Inst. 1972 May;48(5):1363–1370. [PubMed] [Google Scholar]
- Middleton P. J. The histogenesis of mammary tumours induced in the rat by chemical carcinogens. Br J Cancer. 1965 Dec;19(4):830–839. doi: 10.1038/bjc.1965.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., Iype P. T., Griesbach L. M., Heidelberger C. Antigenicity of cells derived from mouse prostate cells after malignant transformation in vitro by carcinogenic hydrocarbons. Cancer Res. 1970 Jun;30(6):1593–1597. [PubMed] [Google Scholar]
- Munsie W. J., Foster E. A. Unsuspected very small foci of carcinoma of the prostate in transurethral resection specimens. Cancer. 1968 Apr;21(4):692–698. doi: 10.1002/1097-0142(196804)21:4<692::aid-cncr2820210421>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- NANDI S. Effect of hormones on maintenance of hyperplastic alveolar nodules in mammary glands of various strains of mice. J Natl Cancer Inst. 1961 Jul;27:187–201. [PubMed] [Google Scholar]
- NANDI S. NEW METHOD FOR DETECTION OF MOUSE MAMMARY TUMOR VIRUS. I. INFLUENCE OF FOSTER NURSING ON INCIDENCE OF HYPERPLASTIC MAMMARY NODULES IN BALB/CCRGL MICE. J Natl Cancer Inst. 1963 Jul;31:57–73. [PubMed] [Google Scholar]
- NANDI S. NEW METHOD FOR DETECTION OF MOUSE MAMMARY TUMOR VIRUS. II. EFFECT OF ADMINISTRATION OF LACTATING MAMMARY TISSUE EXTRACTS ON INCIDENCE OF HYPERPLASTIC MAMMARY NODULES IN BALB/CCRGL MICE. J Natl Cancer Inst. 1963 Jul;31:75–89. [PubMed] [Google Scholar]
- Petrakis N. L., Mason L., Lee R., Sugimoto B., Pawson S., Catchpool F. Association of race, age, menopausal status, and cerumen type with breast fluid secretion in nonlactating women, as determined by nepple aspiration. J Natl Cancer Inst. 1975 Apr;54(4):829–834. [PubMed] [Google Scholar]
- ROBBINS G. F., BERG J. W. BILATERAL PRIMARY BREAST CANCER; A PROSPECTIVE CLINICOPATHOLOGICAL STUDY. Cancer. 1964 Dec;17:1501–1527. doi: 10.1002/1097-0142(196412)17:12<1501::aid-cncr2820171202>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
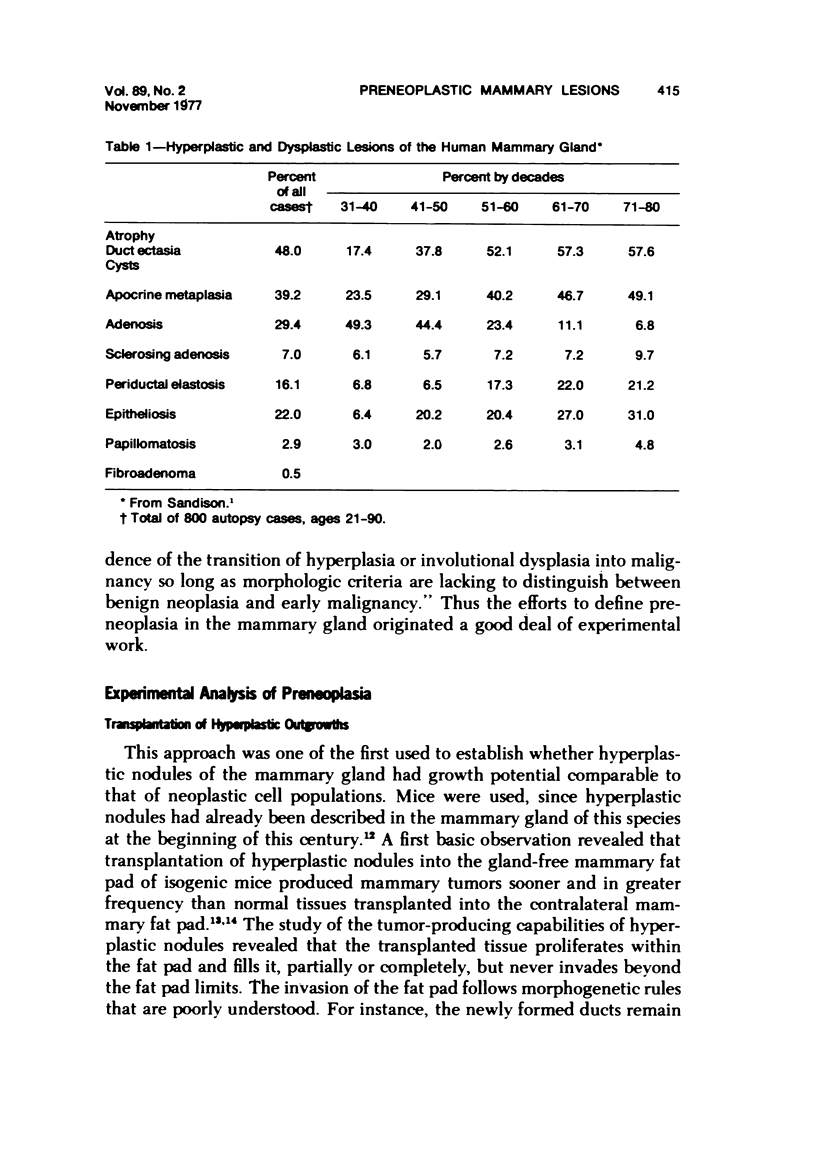
- SANDISON A. T. An autopsy study of the adult human breast: with special reference to proliferative epithelial changes of importance in the pathology of the breast. Natl Cancer Inst Monogr. 1962;4:1–145. [PubMed] [Google Scholar]
- Shellabarger C. J. Effect of 3-methylcholanthrene and x- irradiation, given singly or combined, on rat mammary carcinogenesis. J Natl Cancer Inst. 1967 Jan;38(1):73–77. [PubMed] [Google Scholar]
- Shellabarger C. J., Straub R. F. Effect of 3-methylcholanthrene and fission neutron irradiation, given singly or combined, on rat mammary carcinogenesis. J Natl Cancer Inst. 1972 Jan;48(1):185–187. [PubMed] [Google Scholar]
- Sinha D., Dao T. L. A direct mechanism of mammary carcinogenesis induced by 7,12-dimethyl-benz(alpha)anthracene. J Natl Cancer Inst. 1974 Sep;53(3):841–846. doi: 10.1093/jnci/53.3.841. [DOI] [PubMed] [Google Scholar]
- Sinha D., Dao T. L. Hyperplastic alveolar nodules of the rat mammary gland: tumor-producing capability in vivo and in vitro. Cancer Lett. 1977 Jan;2(3):153–160. doi: 10.1016/s0304-3835(77)80005-0. [DOI] [PubMed] [Google Scholar]
- Sinha D., Dao T. L. Site of origin of mammary tumors induced by 7,12-dimethylbenz(a)anthracene in the rat. J Natl Cancer Inst. 1975 Apr;54(4):1007–1009. [PubMed] [Google Scholar]
- Slemmer G. Host response to premalignant mammary tissues. Natl Cancer Inst Monogr. 1972 Dec;35:57–71. [PubMed] [Google Scholar]
- Slemmer G. Host response to premalignant mammary tissues. Natl Cancer Inst Monogr. 1972 Dec;35:57–71. [PubMed] [Google Scholar]
- Slemmer G. Proceedings: Interactions of separate types of cells during normal and neoplastic mammary gland growth. J Invest Dermatol. 1974 Jul;63(1):24–47. doi: 10.1111/1523-1747.ep12678071. [DOI] [PubMed] [Google Scholar]
- Sorgente N., Kuettner K. E., Soble L. W., Eisenstein R. The resistance of certain tissues to invasion. II. Evidence for extractable factors in cartilage which inhibit invasion by vascularized mesenchyme. Lab Invest. 1975 Feb;32(2):217–222. [PubMed] [Google Scholar]
- Toker C. Small cell dysplasia and in-situ carcinoma of the mammary ducts and lobules. J Pathol. 1974 Sep;114(1):47–52. doi: 10.1002/path.1711140109. [DOI] [PubMed] [Google Scholar]
- Turkington R. W. Regulation of gene expression in normal and neoplastic mammary cells: a review. J Natl Cancer Inst. 1972 Apr;48(4):1231–1234. [PubMed] [Google Scholar]
- Wellings S. R., Jensen H. M., Marcum R. G. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975 Aug;55(2):231–273. [PubMed] [Google Scholar]
- Wellings S. R., Jensen H. M. On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst. 1973 May;50(5):1111–1118. doi: 10.1093/jnci/50.5.1111. [DOI] [PubMed] [Google Scholar]
- Welsch C. W. Prophylaxis of early preneoplastic lesions of the mammary gland. Cancer Res. 1976 Jul;36(7 Pt 2):2621–2625. [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T., Caroline N. L., Sibal L. R., Fink M. A., Beverley P. C., Allison A. C. Suppression of established Friend virus leukemia by statolon. IV. Role of humoral antibody in the development of a dormant infection. J Natl Cancer Inst. 1972 Mar;48(3):665–673. [PubMed] [Google Scholar]
- Yanai R., Nagasawa H. Enhancement by pituitary isografts of mammary hyperplastic nodules in adreno-ovariectomized mice. J Natl Cancer Inst. 1971 Jun;46(6):1251–1255. [PubMed] [Google Scholar]
- Young L. J., Medina D., DeOme K. B., Daniel C. W. The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol. 1971 Feb 1;6(1):49–56. doi: 10.1016/0531-5565(71)90048-9. [DOI] [PubMed] [Google Scholar]


