Abstract
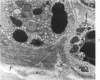
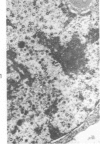
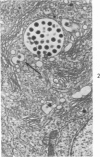
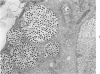
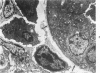
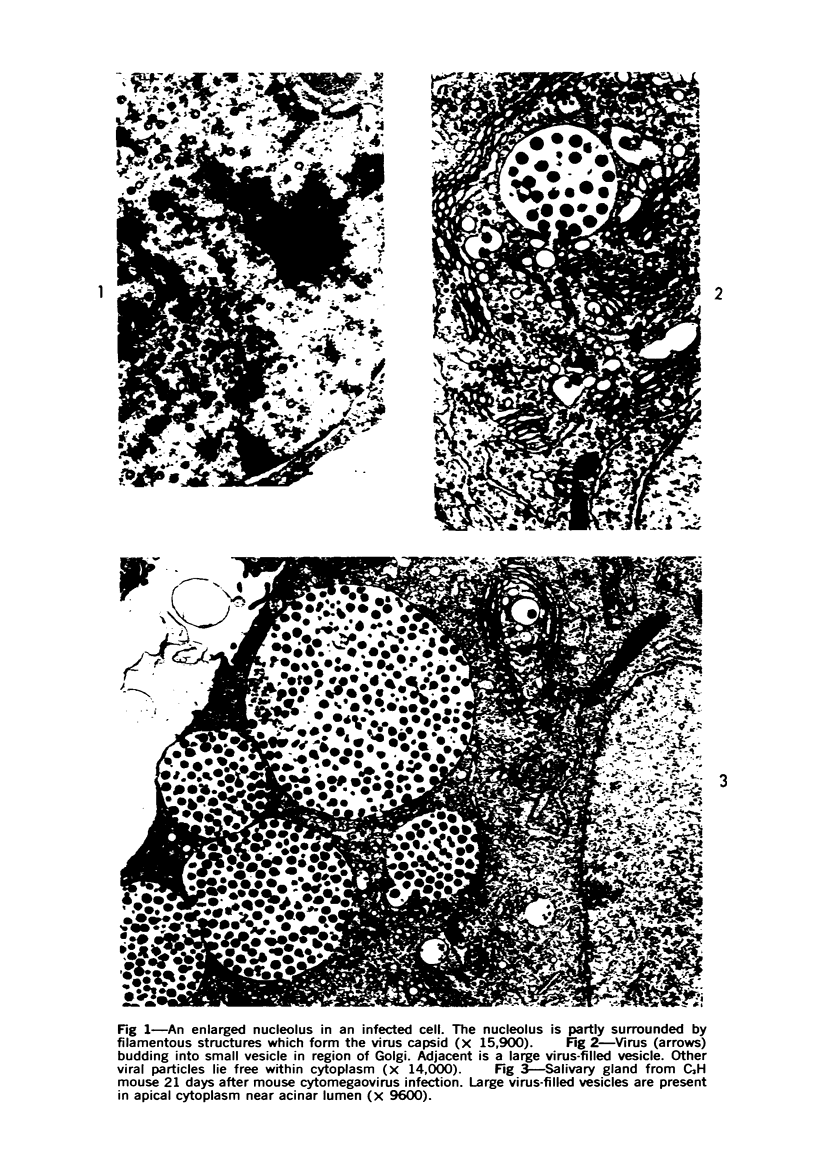
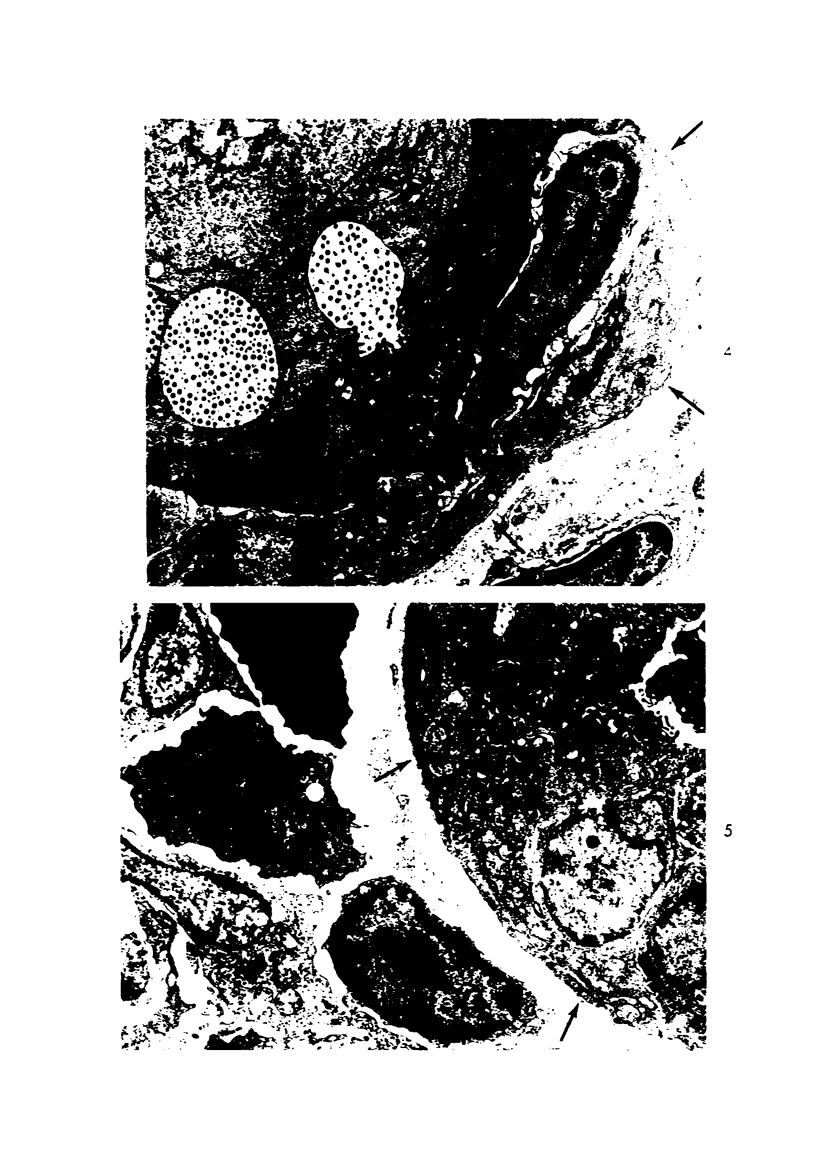
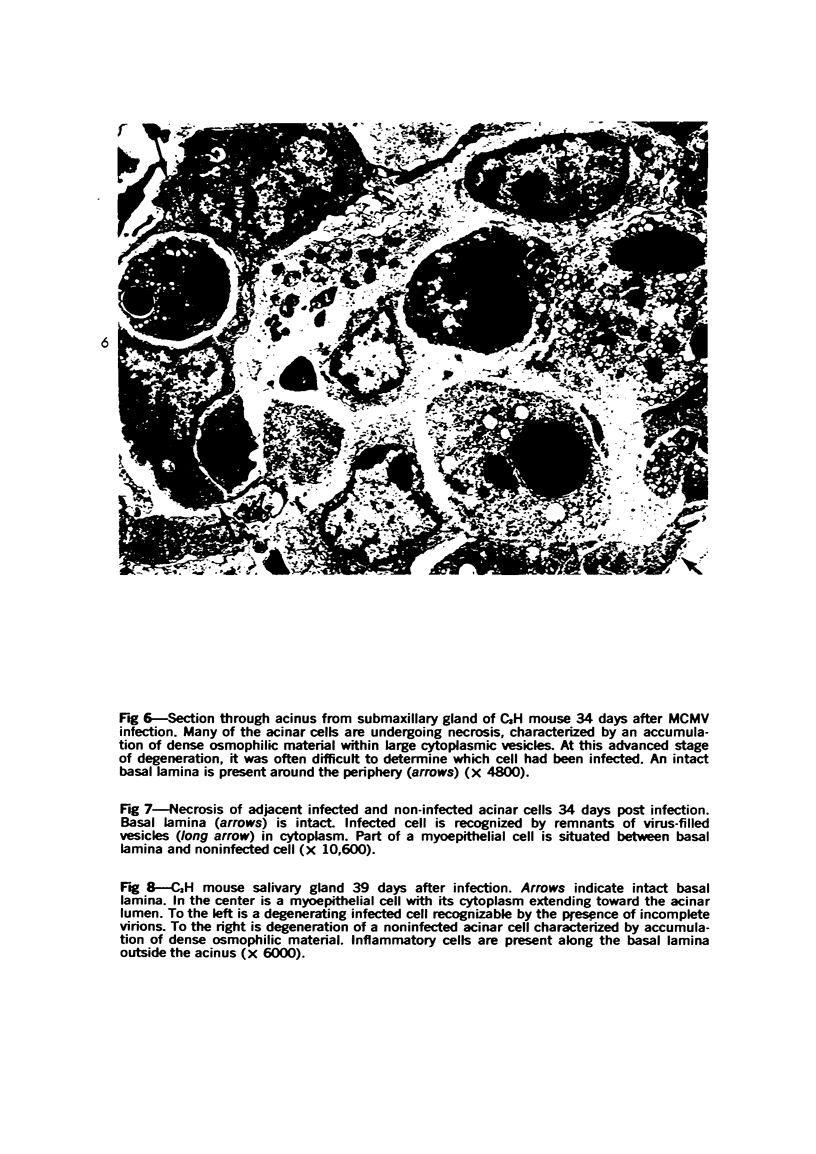
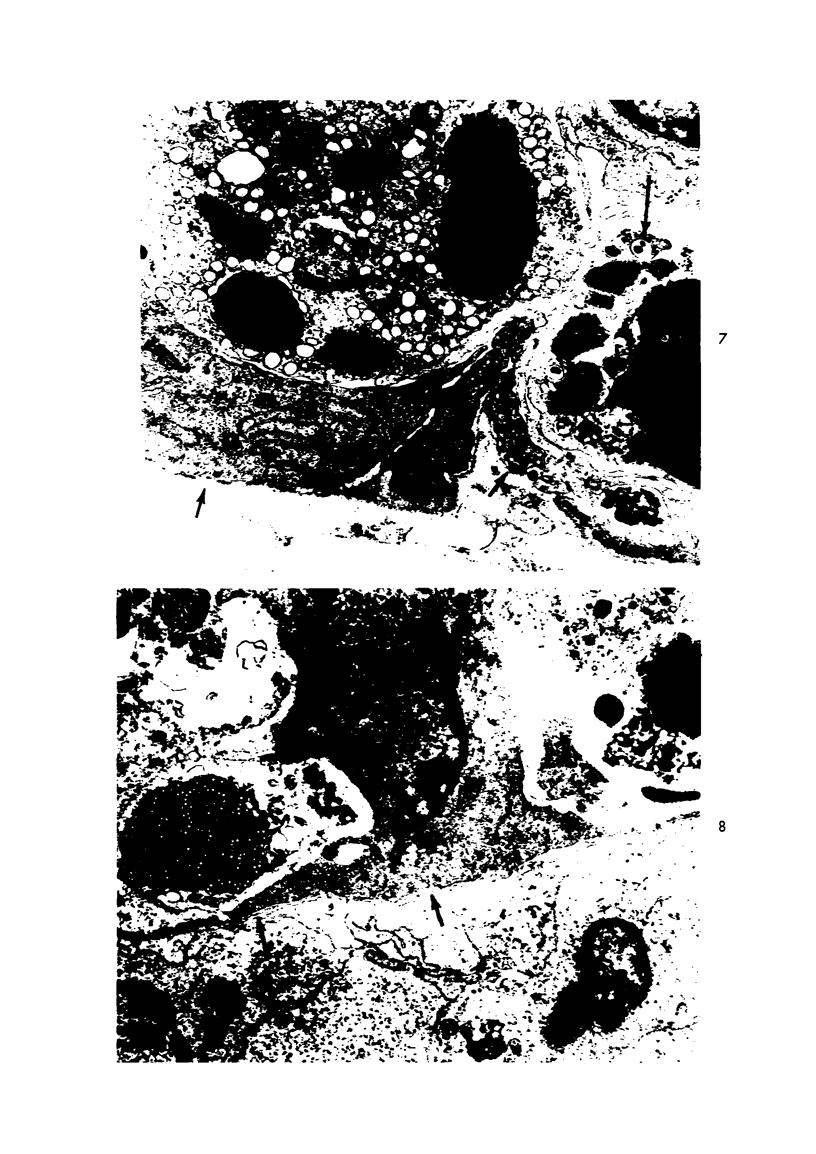
The ultrastructural lesions in the submaxillary glands of C3H mice chronically infected with the murine cytomegalovirus are reported. Virus was synthesized in the nucleus of acinar glandular cells. After passage into the cytoplasm, virus was located in large vesicles which were derived from the Golgi apparatus. These vesicles, which were periodic acid-Schiff positive, migrated to the apex of the cell and released virus into the acinar lumen or canaliculi. Eventually, lymphocytes infiltrated the interstitium and surrounded the basal lamina of acini which contained infected cells. In acini encompassed by lymphocytes, both infected cells and morphologically normal acinar cells simultaneously degenerated, producing a small focus of necrosis. Physical contact between lymphocytes and necrotic cells did not occur for an intact basal lamina was always found interposed between them. Degeneration of infected cells coincided with a decrease in virus titer in the salivary glands. Degeneration of infected and normal acinar cells also occurred in DBA 2 mice which lack the fifth component of complement. In mice conditioned with cortisone to suppress inflammation, neither infected nor normal acinar cells degenerated. We concluded from the electron microscope observations that lymphocytes terminate chronic MCMV infection, that MCMV infection of acinar epithelium is not cytolytic and that normal cells also undergo necrosis during termination of chronic MCMV infection. It is postulated that lymphocytes in responding to infection release a cytotoxic substance which diffuses into the acini and causes indiscriminate necrosis of acinar cells.
Full text
PDF













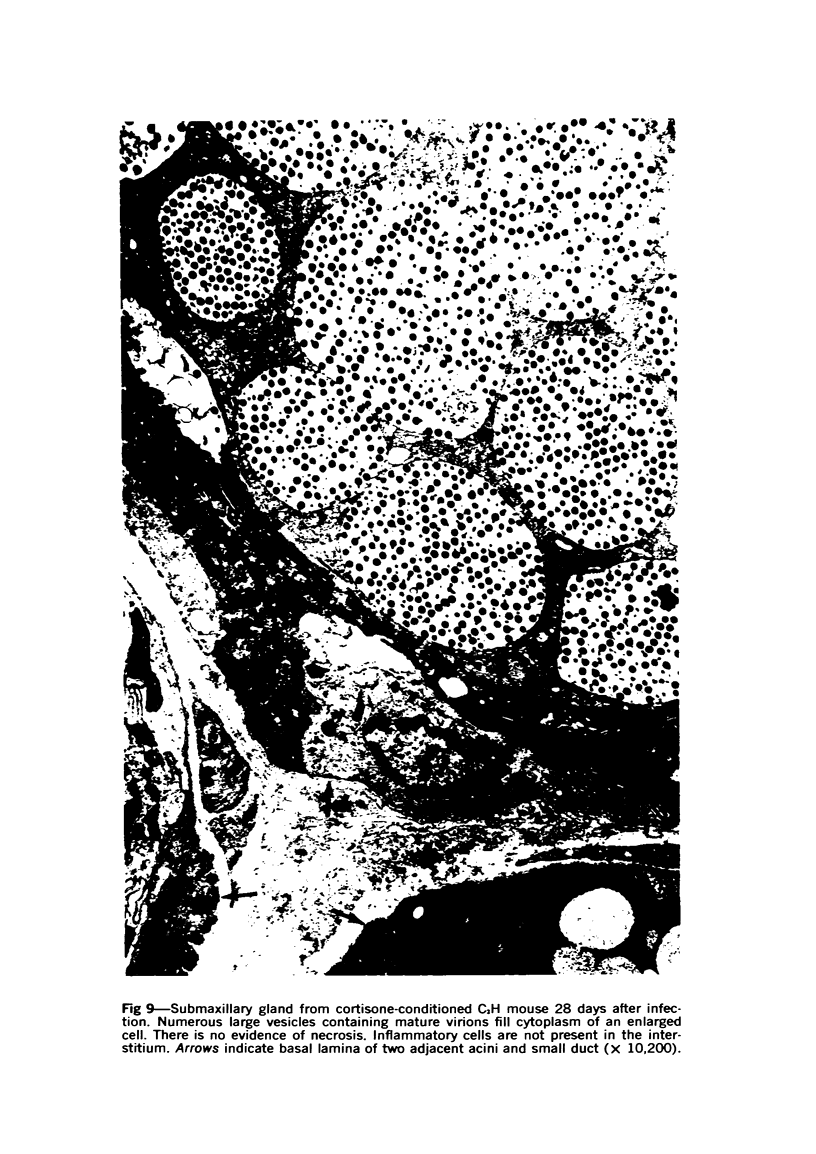
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRDSONG M., COREY J. H., Jr, MITCHELL F. N., SMITH D. E. Generalized cytomegalic inclusion disease in newborn infants. J Am Med Assoc. 1956 Dec 1;162(14):1305–1308. doi: 10.1001/jama.1956.02970310033008. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. R. Cytomegalovirus disease presenting as hepatitis. Br Med J. 1968 Sep 28;3(5621):786–786. doi: 10.1136/bmj.3.5621.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Pulmonary cytomegalovirus infection in the adult. Am J Pathol. 1971 Jun;63(3):487–504. [PMC free article] [PubMed] [Google Scholar]
- DIOSI P., ROSIU N. CYTOMEGALIC INFECTION IN THE SUBMAXILLARY GLANDS OF AN ADULT. Pathol Microbiol (Basel) 1965;28:420–424. doi: 10.1159/000161793. [DOI] [PubMed] [Google Scholar]
- Daniels J. C., Ritzman S. E., Levin W. C. Lymphocytes: morphological, developmental, and functional characteristics in health, disease, and experimental study--an analytical review. Tex Rep Biol Med. 1968 Spring;26(1):5–93. [PubMed] [Google Scholar]
- Granger G. A., Kolb W. P. Lymphocyte in vitro cytotoxicity: mechanisms of immune and non-immune small lymphocyte mediated target L cell destruction. J Immunol. 1968 Jul;101(1):111–120. [PubMed] [Google Scholar]
- Granger G. A., Moore G. E., White J. G., Matzinger P., Sundsmo J. S., Shupe S., Kolb W. P., Kramer J., Glade P. R. Production of lymphotoxin and migration inhibitory factor by established human lymphocytic cell lines. J Immunol. 1970 Jun;104(6):1476–1485. [PubMed] [Google Scholar]
- HANSHAW J. B., BETTS R. F., SIMON G., BOYNTON R. C. ACQUIRED CYTOMEGALOVIRUS INFECTION: ASSOCIATION WITH HEPATOMEGALY AND ABNORMAL LIVER-FUNCTION TESTS. N Engl J Med. 1965 Mar 25;272:602–609. doi: 10.1056/NEJM196503252721202. [DOI] [PubMed] [Google Scholar]
- HENSON D., PINKERTON H. CHARACTERISTICS OF A PLAQUE METHOD FOR THE MURINE SALIVARY GLAND VIRUS. Proc Soc Exp Biol Med. 1963 Oct;114:130–133. doi: 10.3181/00379727-114-28605. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J., WEIGAND H. The effects of pretreatment with x-rays on the pathogenesis of lymphocytic choriomeningitis in mice. I. Host survival, virus multiplication and leukocytosis. J Immunol. 1961 Dec;87:675–681. [PubMed] [Google Scholar]
- Henson D. Cytomegalovirus hepatitis in an adult. An autopsy report. Arch Pathol. 1969 Aug;88(2):199–203. [PubMed] [Google Scholar]
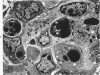
- Henson D., Neapolitan C. Pathogenesis of chronic mouse cytomegalovirus infection in submaxillary glands of C3H mice. Am J Pathol. 1970 Feb;58(2):255–267. [PMC free article] [PubMed] [Google Scholar]
- Henson D., Smith R. D., Gehrke J. Non-fatal mouse cytomegalovirus hepatitis. Combined morphologic, virologic and immunologic observations. Am J Pathol. 1966 Nov;49(5):871–888. [PMC free article] [PubMed] [Google Scholar]
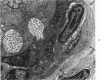
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Kajima M., Pollard M. Ultrastructural pathology of glomerular lesions in gnotobiotic mice with congenital lymphocytic choriomeningitis (LCM) virus infection. Am J Pathol. 1970 Nov;61(2):117–140. [PMC free article] [PubMed] [Google Scholar]
- Klemola E., Käriäinen L. Cytomegalovirus as a possible cause of a disease resembling infectious mononucleosis. Br Med J. 1965 Nov 6;2(5470):1099–1102. doi: 10.1136/bmj.2.5470.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemola E., von Essen R., Wager O., Haltia K., Koivuniemi A., Salmi I. Cytomegalovirus mononucleosis in previously healthy individuals. Five new cases and follow-up of 13 previously published cases. Ann Intern Med. 1969 Jul;71(1):11–19. doi: 10.7326/0003-4819-71-1-11. [DOI] [PubMed] [Google Scholar]
- Kongshavn P. A., Bliss J. Q. Effect of mouse submandibular gland extracts on survival of H-2 incompatible skin allografts. Immunology. 1970 Aug;19(2):363–367. [PMC free article] [PubMed] [Google Scholar]
- MARGILETH A. M. The diagnosis and treatment of generalized cytomegalic inclusion disease of the newborn. Pediatrics. 1955 Mar;15(3):270–283. [PubMed] [Google Scholar]
- MEDEARIS D. N., Jr MOUSE CYTOMEGALOVIRUS INFECTION. II. OBSERVATIONS DURING PROLONGED INFECTIONS. Am J Hyg. 1964 Jul;80:103–112. [PubMed] [Google Scholar]
- Naughton M. A., Koch J., Hoffman H., Bender V., Hagopian H. Isolation and activity of a thymocyte-trasforming factor from the mouse submaxillary gland. Exp Cell Res. 1969 Sep;57(1):95–103. doi: 10.1016/0014-4827(69)90371-1. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M., Hsu K. C. Electron microscopy of herpes simplex virus. IV. Studies with ferritin-conjugated antibodies. J Virol. 1968 Oct;2(10):1172–1184. doi: 10.1128/jvi.2.10.1172-1184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Tissue injury in lymphocytic choriomeningitis viral infection: virus-induced immunologically specific release of a cytotoxic factor from immune lymphoid cells. Virology. 1970 Dec;42(4):805–813. doi: 10.1016/0042-6822(70)90330-2. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Jr, Klintworth G. K., Graham D. G., Griffith J. F. Uncommon morphologic features in subacute sclerosing panencephalitis (SSPE). Report of two cases with virus recovery from one autopsy brain specimen. Am J Pathol. 1970 Nov;61(2):275–292. [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Herpesvirus antigens on cell membranes detected by centrifugation of membrane-antibody complexes. Science. 1971 Jan 22;171(3968):298–300. doi: 10.1126/science.171.3968.298. [DOI] [PubMed] [Google Scholar]
- Ruebner B. H., Hirano T., Slusser R., Osborn J., Medearis D. N., Jr Cytomegalovirus infection. Viral ultrastructure with particular reference to the relationship of lysosomes to cytoplasmic inclusions. Am J Pathol. 1966 Jun;48(6):971–989. [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Yamasaki Y., Yamabe H., Suzuki Y., Haebara H., Irino T., Grollman A. Atrophy of the lymphoid tissues of mice induced by extracts of the submaxillary gland. Proc Soc Exp Biol Med. 1967 Oct;126(1):212–216. doi: 10.3181/00379727-126-32404. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. J., Craighead J. E. Infection of adult mouse macrophages in vitro with cytomegalovirus. Proc Soc Exp Biol Med. 1968 Dec;129(3):690–694. doi: 10.3181/00379727-129-33399. [DOI] [PubMed] [Google Scholar]
- VAN GELDEREN H. H. Successfully treated case of cytomegalic disease in a newborn infant. Acta Paediatr. 1959 Mar;48(2):169–174. [PubMed] [Google Scholar]
- Williams T. W., Granger G. A. Lymphocyte in vitro cytotoxicity: mechanism of lymphotoxin-induced target cell destruction. J Immunol. 1969 Apr;102(4):911–918. [PubMed] [Google Scholar]