Abstract
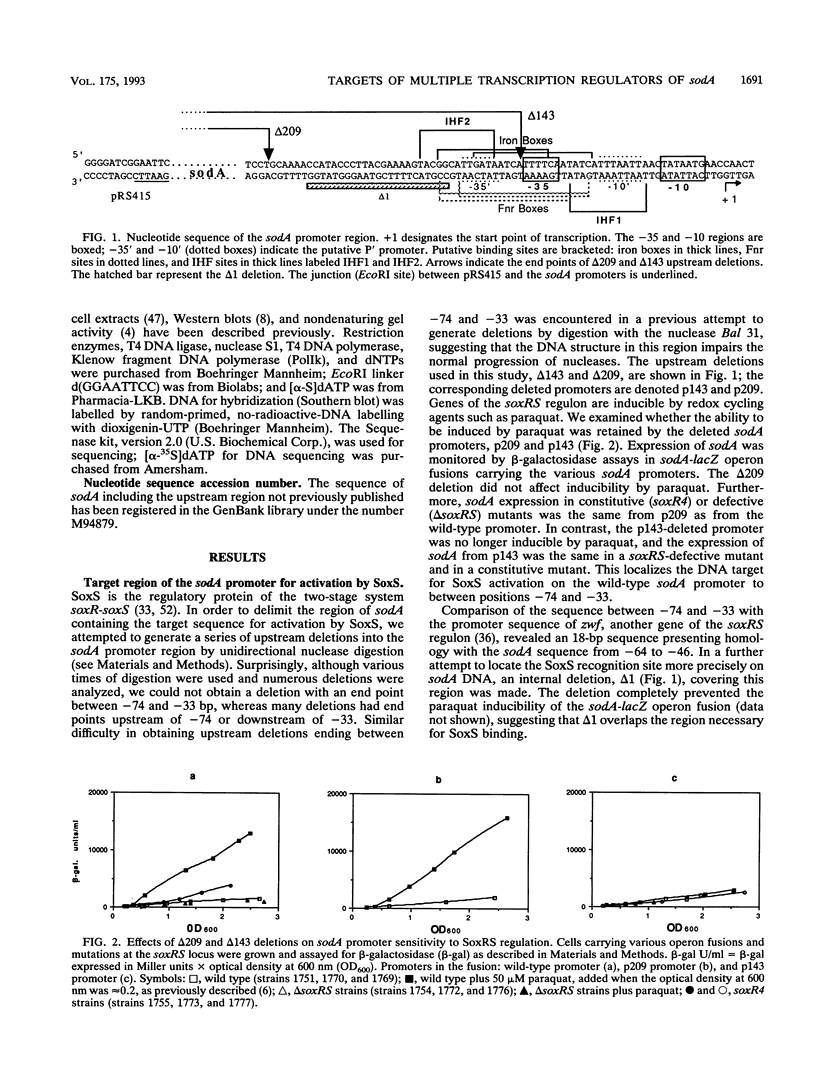
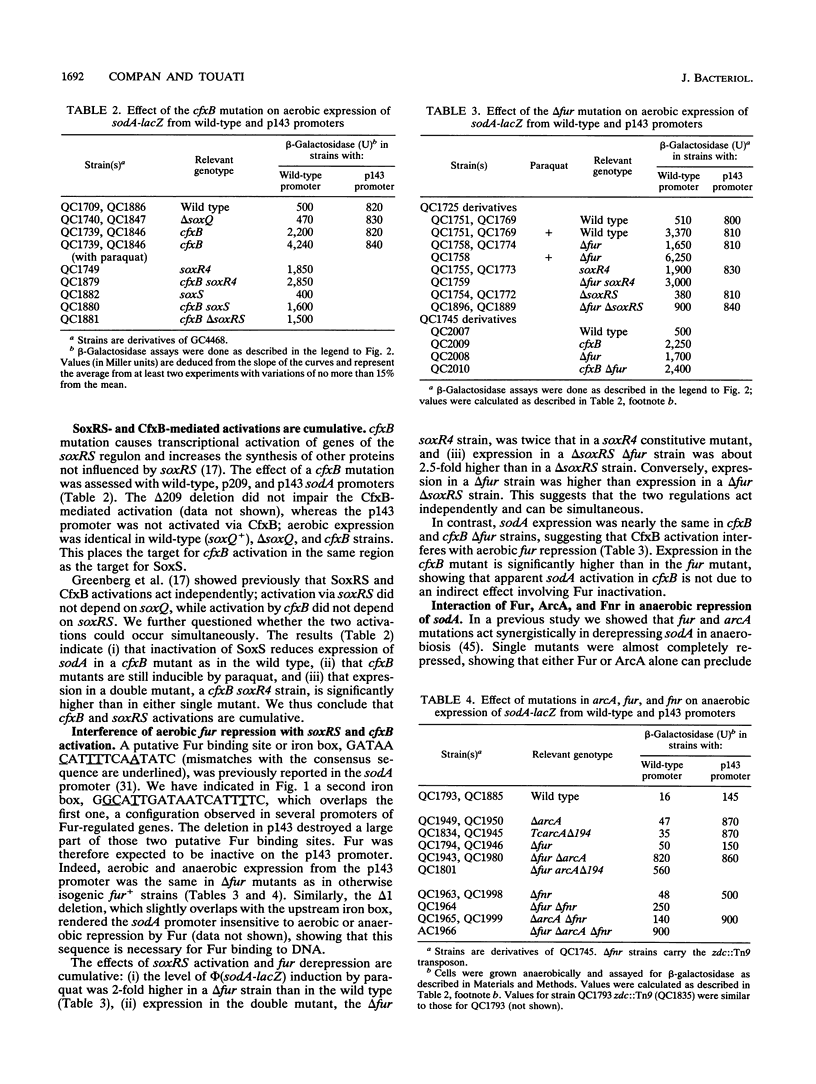
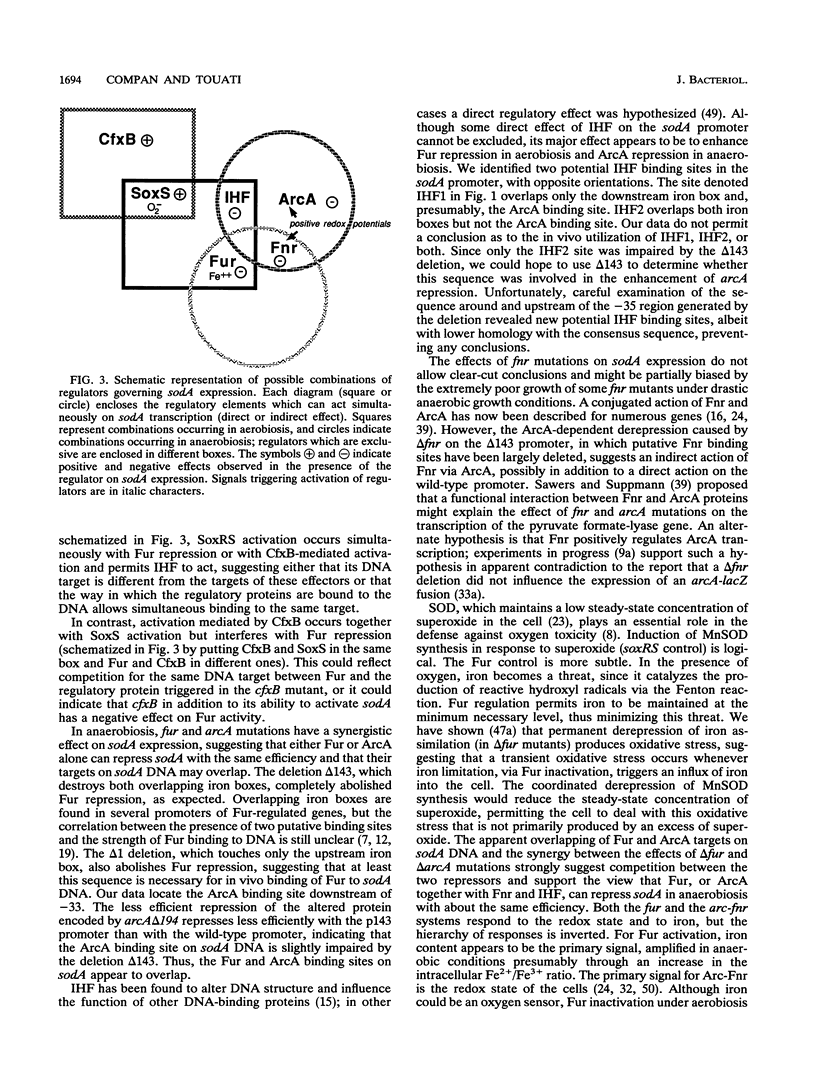
Transcription of the sodA gene of Escherichia coli, which encodes manganese superoxide dismutase, is governed by six global regulators: the product of the soxRS locus (superoxide response) and mutated alleles of the soxQ locus (such as cfxB) act as activators; the products of the fur (ferric uptake regulation), arcA (aerobic regulation control), and fnr (fumarate nitrate reductase) genes and the integration host factor (IHF) negatively regulate sodA. The action of these effectors on the sodA promoter was investigated by using chromosomal sodA-lacZ operon fusions with intact or deleted promoters, different environmental conditions, and strains carrying different combinations of null mutations in the effector genes. The data allow us to assign target regions in the sodA promoter for activation by SoxRS and CfxB and for repression by Fur and ArcA. In aerobiosis, activation of sodA transcription by SoxRS was compatible with CfxB activation or Fur repression, whereas cfxB and fur controls were mutually exclusive. Repression by Fnr appeared, at least in part, to be ArcA dependent. IHF enhanced aerobic Fur repression, and in the absence of Fur, it enhanced anaerobic repression by ArcA. The DNA targets for Fur (encompassing the -35 region) and ArcA (from and downstream of the -35 region) appear to overlap, suggesting that Fur and ArcA repressions are mutually exclusive. Fur (in response to the iron pool) or ArcA, acting with Fnr and IHF (in response to the redox state of the cells), can block anaerobic sodA-lacZ expression with about equivalent efficiencies. The possible biological significance of this result is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr A method for unidirectional deletion mutagenesis with application to nucleotide sequencing and preparation of gene fusions. Gene. 1986;49(1):119–128. doi: 10.1016/0378-1119(86)90391-4. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beyer W. F., Jr, Fridovich I. In vivo competition between iron and manganese for occupancy of the active site region of the manganese-superoxide dismutase of Escherichia coli. J Biol Chem. 1991 Jan 5;266(1):303–308. [PubMed] [Google Scholar]
- Beyer W., Imlay J., Fridovich I. Superoxide dismutases. Prog Nucleic Acid Res Mol Biol. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- Brickman T. J., Ozenberger B. A., McIntosh M. A. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J Mol Biol. 1990 Apr 20;212(4):669–682. doi: 10.1016/0022-2836(90)90229-F. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Bonnefoy-Orth V., Ratouchniak J., Pascal M. C. Operon fusions in the nitrate reductase operon and study of the control gene nir R in Escherichia coli. Mol Gen Genet. 1981;182(3):477–479. doi: 10.1007/BF00293938. [DOI] [PubMed] [Google Scholar]
- Cotter P. A., Chepuri V., Gennis R. B., Gunsalus R. P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990 Nov;172(11):6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Drury L. S., Buxton R. S. Identification and sequencing of the Escherichia coli cet gene which codes for an inner membrane protein, mutation of which causes tolerance to colicin E2. Mol Microbiol. 1988 Jan;2(1):109–119. doi: 10.1111/j.1365-2958.1988.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Fu H. A., Iuchi S., Lin E. C. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet. 1991 Apr;226(1-2):209–213. doi: 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Chou J. H., Monach P. A., Demple B. Activation of oxidative stress genes by mutations at the soxQ/cfxB/marA locus of Escherichia coli. J Bacteriol. 1991 Jul;173(14):4433–4439. doi: 10.1128/jb.173.14.4433-4439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. W., Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of fur protein to the promoters. J Bacteriol. 1989 Feb;171(2):1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Hassan H. M., Sun H. C. Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3217–3221. doi: 10.1073/pnas.89.8.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991 Apr 15;266(11):6957–6965. [PubMed] [Google Scholar]
- Iuchi S., Chepuri V., Fu H. A., Gennis R. B., Lin E. C. Requirement for terminal cytochromes in generation of the aerobic signal for the arc regulatory system in Escherichia coli: study utilizing deletions and lac fusions of cyo and cyd. J Bacteriol. 1990 Oct;172(10):6020–6025. doi: 10.1128/jb.172.10.6020-6025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Adaptation of Escherichia coli to respiratory conditions: regulation of gene expression. Cell. 1991 Jul 12;66(1):5–7. doi: 10.1016/0092-8674(91)90130-q. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I. Primary structure of the himA gene of Escherichia coli: homology with DNA-binding protein HU and association with the phenylalanyl-tRNA synthetase operon. Cold Spring Harb Symp Quant Biol. 1984;49:691–698. doi: 10.1101/sqb.1984.049.01.078. [DOI] [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Anaerobic biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J Biol Chem. 1984 Oct 25;259(20):12821–12825. [PubMed] [Google Scholar]
- Niederhoffer E. C., Naranjo C. M., Bradley K. L., Fee J. A. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J Bacteriol. 1990 Apr;172(4):1930–1938. doi: 10.1128/jb.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus F., Hantke K., Unden G. Iron content and FNR-dependent gene regulation in Escherichia coli. FEMS Microbiol Lett. 1991 Dec 1;68(3):319–323. doi: 10.1016/0378-1097(91)90376-l. [DOI] [PubMed] [Google Scholar]
- Nunoshiba T., Hidalgo E., Amábile Cuevas C. F., Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992 Oct;174(19):6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Anaerobic biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. Effects of diazenedicarboxylic acid bis(N,N'-dimethylamide) (diamide). J Biol Chem. 1990 Dec 15;265(35):21966–21970. [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Transcriptional and maturational effects of manganese and iron on the biosynthesis of manganese-superoxide dismutase in Escherichia coli. J Biol Chem. 1992 May 5;267(13):9140–9145. [PubMed] [Google Scholar]
- Rowley D. L., Wolf R. E., Jr Molecular characterization of the Escherichia coli K-12 zwf gene encoding glucose 6-phosphate dehydrogenase. J Bacteriol. 1991 Feb;173(3):968–977. doi: 10.1128/jb.173.3.968-977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C. B., Thaler D. S., Dahlquist F. W. Chromosomal transformation of Escherichia coli recD strains with linearized plasmids. J Bacteriol. 1989 May;171(5):2609–2613. doi: 10.1128/jb.171.5.2609-2613.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G., Suppmann B. Anaerobic induction of pyruvate formate-lyase gene expression is mediated by the ArcA and FNR proteins. J Bacteriol. 1992 Jun;174(11):3474–3478. doi: 10.1128/jb.174.11.3474-3478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., Green J., Guest J. R. FNR activates and represses transcription in vitro. Proc Biol Sci. 1991 Sep 23;245(1314):219–226. doi: 10.1098/rspb.1991.0113. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Adaptive responses to oxygen limitation in Escherichia coli. Trends Biochem Sci. 1991 Aug;16(8):310–314. doi: 10.1016/0968-0004(91)90125-f. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Inactivation of the FNR protein of Escherichia coli by targeted mutagenesis in the N-terminal region. Mol Microbiol. 1988 Nov;2(6):701–707. doi: 10.1111/j.1365-2958.1988.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardat B., Touati D. Two global regulators repress the anaerobic expression of MnSOD in Escherichia coli::Fur (ferric uptake regulation) and Arc (aerobic respiration control). Mol Microbiol. 1991 Feb;5(2):455–465. doi: 10.1111/j.1365-2958.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983 Sep;155(3):1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J Bacteriol. 1988 Jun;170(6):2511–2520. doi: 10.1128/jb.170.6.2511-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaneva I. R., Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990 Aug;172(8):4197–4205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui P., Huang L., Freundlich M. Integration host factor binds specifically to multiple sites in the ompB promoter of Escherichia coli and inhibits transcription. J Bacteriol. 1991 Sep;173(18):5800–5807. doi: 10.1128/jb.173.18.5800-5807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Trageser M., Duchêne A. Effect of positive redox potentials (greater than +400 mV) on the expression of anaerobic respiratory enzymes in Escherichia coli. Mol Microbiol. 1990 Feb;4(2):315–319. doi: 10.1111/j.1365-2958.1990.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon of Escherichia coli. J Bacteriol. 1991 May;173(9):2864–2871. doi: 10.1128/jb.173.9.2864-2871.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J Bacteriol. 1992 Jun;174(12):3915–3920. doi: 10.1128/jb.174.12.3915-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


