Abstract
The acetogen Clostridium thermoautotrophicum was cultivated under CO-dependent chemolithotrophic conditions. CO-dependent growth profiles and energetics indicated that supplemental CO2 was fundamental to efficient growth at the expense of CO. Overall product stoichiometry approximated 6.5CO----CH3CO2H + 3.5CO2 + 0.6 cell C + 0.5 unrecovered C. Initial CO/CO2 ratios of 2 to 4 yielded optimal doubling times and cell yields. Maximal YCO values approximated 2.5 g of cell dry weight per mol of CO consumed; YH2 was considerably lower than YCO. Cross-transfer growth experiments and protein profiles indicated differential expression of genes between CO and methanol cultures.
Full text
PDF
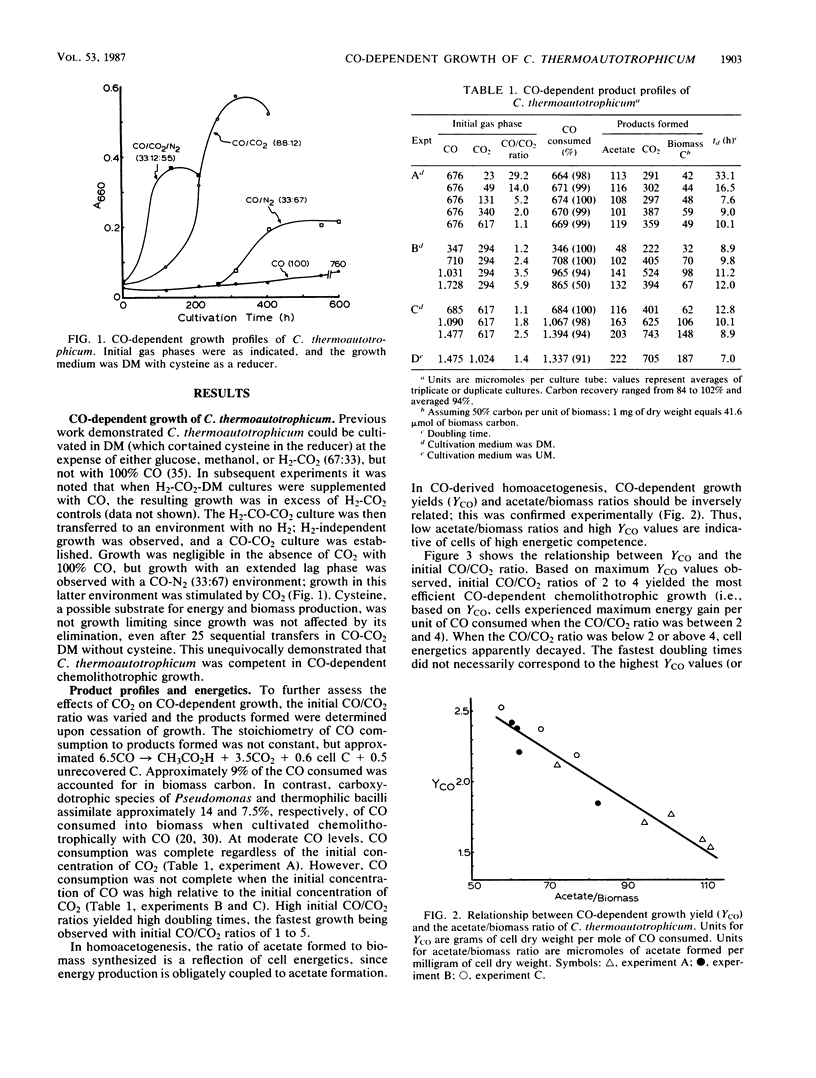
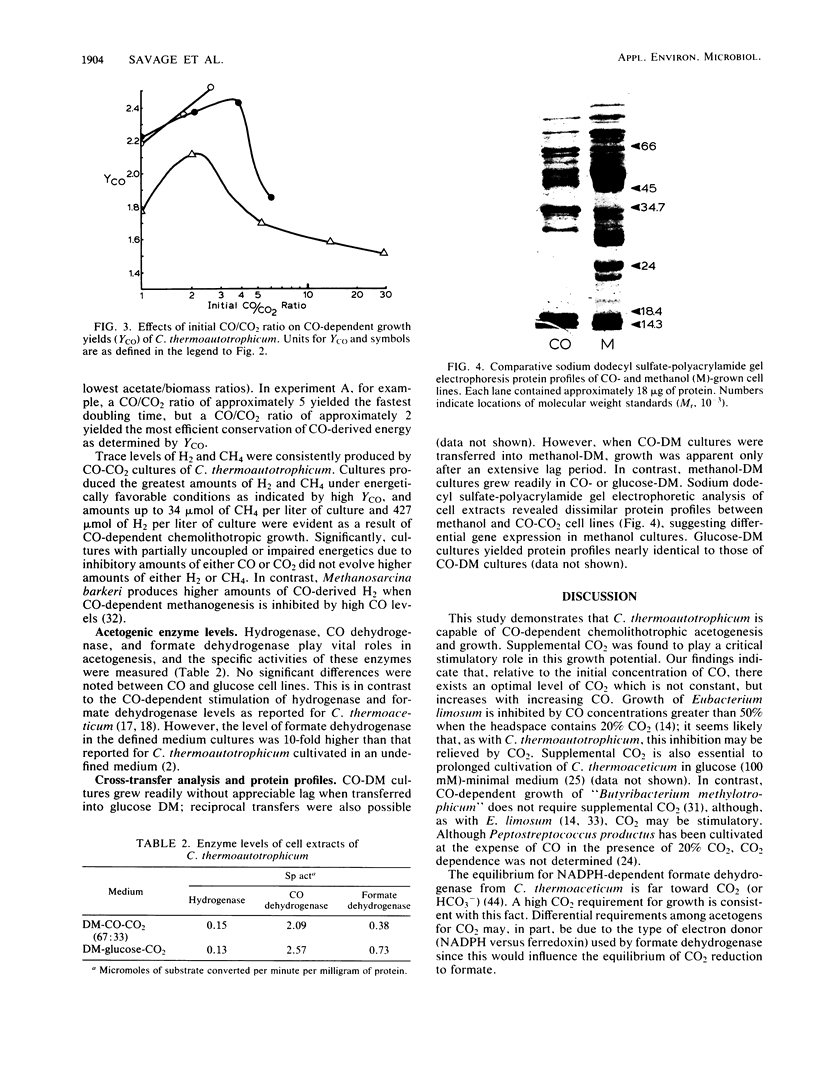


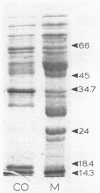
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark J. E., Ragsdale S. W., Ljungdahl L. G., Wiegel J. Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol. 1982 Jul;151(1):507–509. doi: 10.1128/jb.151.1.507-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J Biol Chem. 1980 Aug 10;255(15):7174–7180. [PubMed] [Google Scholar]
- Eikmanns B., Fuchs G., Thauer R. K. Formation of carbon monoxide from CO2 and H2 by Methanobacterium thermoautotrophicum. Eur J Biochem. 1985 Jan 2;146(1):149–154. doi: 10.1111/j.1432-1033.1985.tb08631.x. [DOI] [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Bryant M. P. Growth of Eubacterium limosum with Carbon Monoxide as the Energy Source. Appl Environ Microbiol. 1982 Jan;43(1):70–74. doi: 10.1128/aem.43.1.70-74.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Drake H. L. Effects of cultivation gas phase on hydrogenase of the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Oct;160(1):466–469. doi: 10.1128/jb.160.1.466-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Formate dehydrogenase, a selenium--tungsten enzyme from Clostridium thermoaceticum. Methods Enzymol. 1978;53:360–372. doi: 10.1016/s0076-6879(78)53042-5. [DOI] [PubMed] [Google Scholar]
- Lorowitz W. H., Bryant M. P. Peptostreptococcus productus strain that grows rapidly with CO as the energy source. Appl Environ Microbiol. 1984 May;47(5):961–964. doi: 10.1128/aem.47.5.961-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Drake H. L. Development of a minimally defined medium for the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Aug;159(2):700–703. doi: 10.1128/jb.159.2.700-703.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L. H., Zeikus J. G. Metabolism of H2-CO2, methanol, and glucose by Butyribacterium methylotrophicum. J Bacteriol. 1983 Mar;153(3):1415–1423. doi: 10.1128/jb.153.3.1415-1423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. R., Misra A., Drake H. L. Dissimilation of Carbon Monoxide to Acetic Acid by Glucose-Limited Cultures of Clostridium thermoaceticum. Appl Environ Microbiol. 1985 Jun;49(6):1412–1417. doi: 10.1128/aem.49.6.1412-1417.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- O'Brien J. M., Wolkin R. H., Moench T. T., Morgan J. B., Zeikus J. G. Association of hydrogen metabolism with unitrophic or mixotrophic growth of Methanosarcina barkeri on carbon monoxide. J Bacteriol. 1984 Apr;158(1):373–375. doi: 10.1128/jb.158.1.373-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage M. D., Drake H. L. Adaptation of the acetogen Clostridium thermoautotrophicum to minimal medium. J Bacteriol. 1986 Jan;165(1):315–318. doi: 10.1128/jb.165.1.315-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharak Genthner B. R., Bryant M. P. Additional characteristics of one-carbon-compound utilization by Eubacterium limosum and Acetobacterium woodii. Appl Environ Microbiol. 1987 Mar;53(3):471–476. doi: 10.1128/aem.53.3.471-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I., Saiki T., Liu S. M., Ljungdahl L. G. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem. 1983 Feb 10;258(3):1826–1832. [PubMed] [Google Scholar]