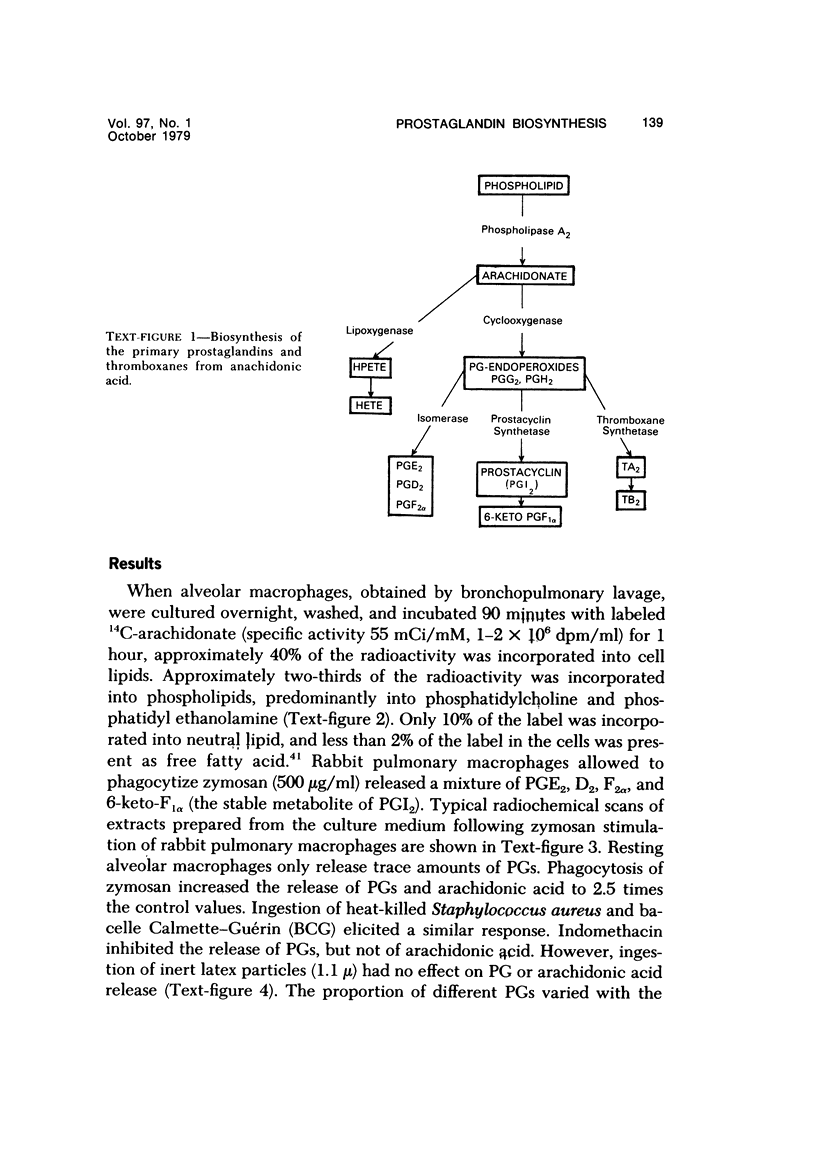
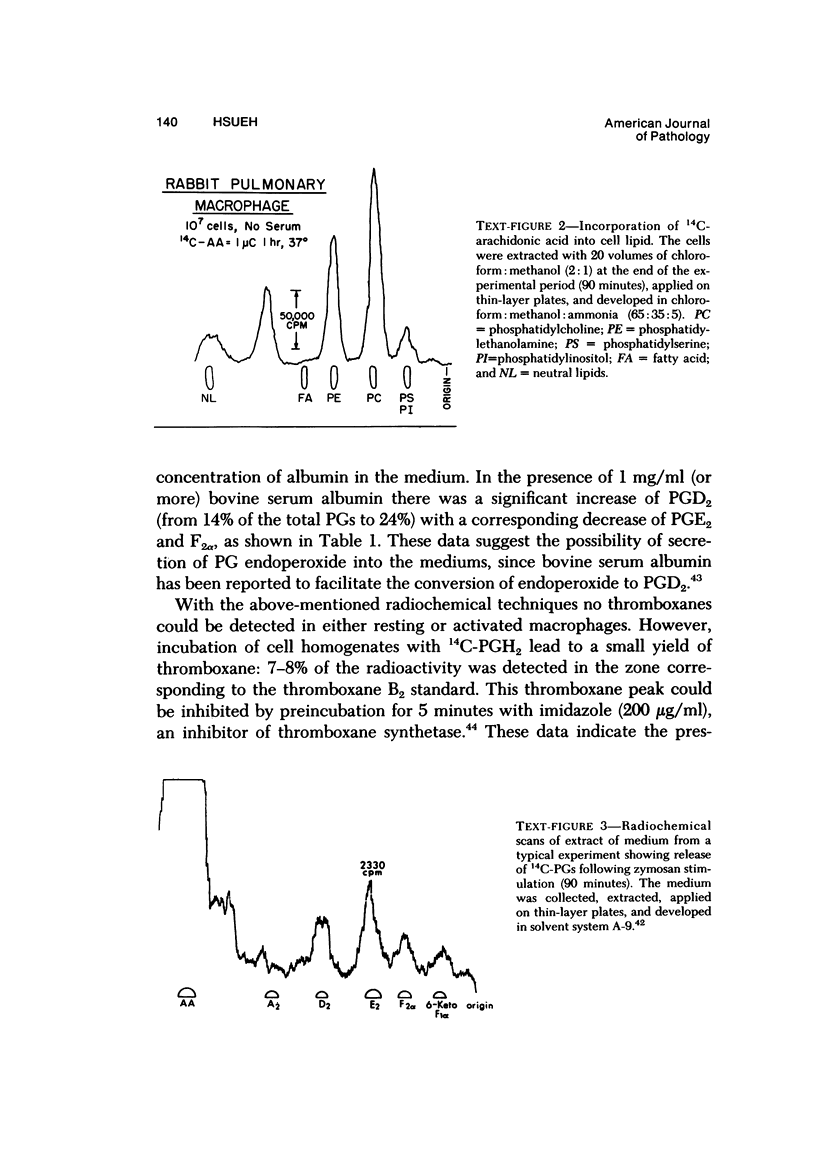
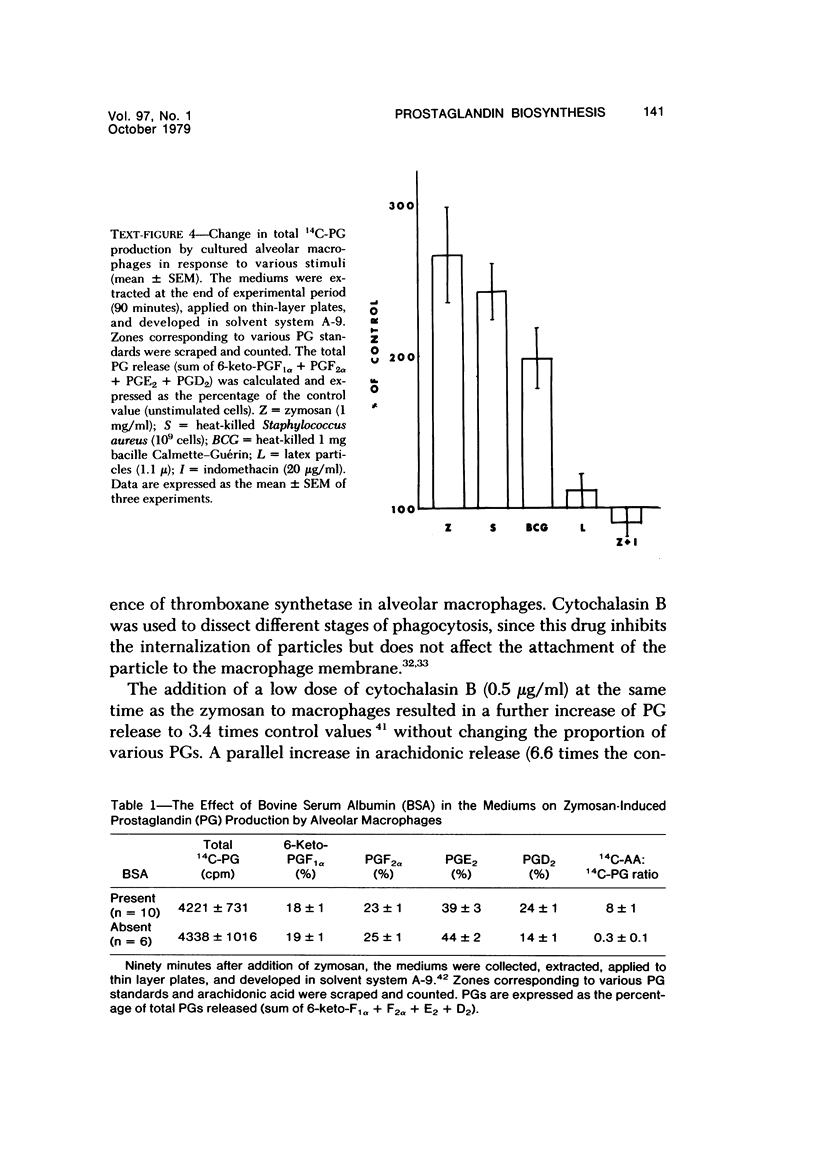
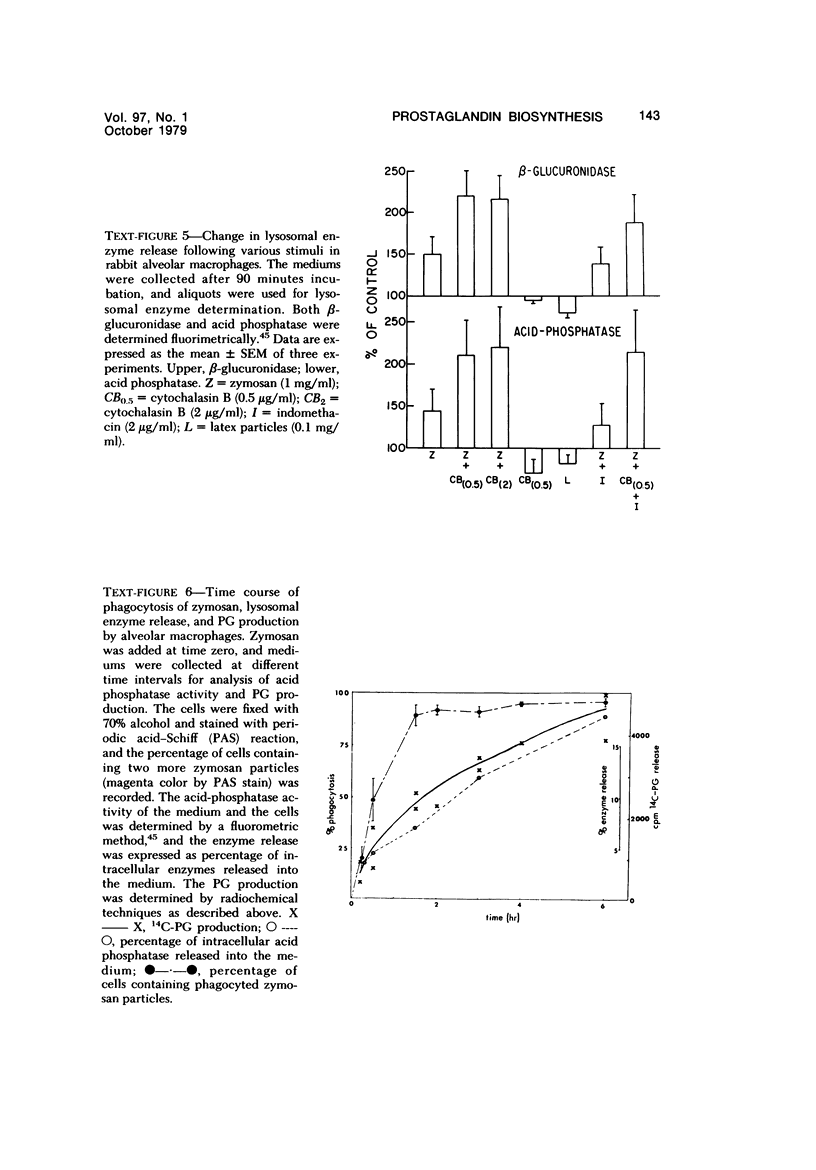
Abstract
Cultured rabbit alveolar macrophages, prelabeled with 14C-arachidonic acid (AA), released into the medium a trace amount of labeled prostaglandins (PG) as well as their precursor, AA. Phagocytosis of zymosan, heat-killed Staphylococcus, or bacille Calmette-Guérin (BCG) increased the AA and PG release to 2--2.5 times control values. The released PGs consisted of PGE2, D2, F2 alpha, and 6-keto F1 alpha. Phagocytosis of latex particles had no effect on PG release. Indomethacin inhibited release of PGs but did not affect AA release at low doses. Analysis of the cellular lipids showed that zymosan decreased the radioactive label in phosphatidylcholine (PC), but not in other phospholipids or neutral lipids, suggesting that PC is the main source of AA for PG synthesis in pulmonary macrophages. Cytochalasin B (CB) at phagocytosis-inhibiting doses or below, markedly increased PG synthesis by zymosan-treated macrophages. These data suggest that PG release is not dependent on engulfment of the particles. Phagocytosis of zymosan (but not latex) also resulted in the release of two lysosomal enzymes, acid phosphatase and beta-glucuronidase, which appeared temporally associated with the release of PGs (but not to phagocytosis). Furthermore, CB augmented the zymosan-stimulated release of these enzymes at the same doses stimulating PG synthesis. However, indomethacin, at a dose completely inhibiting PG synthesis, failed to block lysosomal enzyme release. Thus, the coincidental release of PGs and lysosomal enzymes is not the result of a regulatory role of PGs in the release of lysosomal enzymes, but probably is the result of a common pathway of stimulation. (Am J Pathol 97:137--148, 1979).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Henson P. M. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Farzad A., Penneys N. S., Ghaffar A., Ziboh V. A., Schlossberg J. PGE2 and PGF2alpha biosynthesis in stimulated and nonstimulated peritoneal preparations containing macrophages. Prostaglandins. 1977 Nov;14(5):829–837. doi: 10.1016/0090-6980(77)90300-8. [DOI] [PubMed] [Google Scholar]
- Feldman J. D., Tubergen D. G., Pollock E. M., Unanue E. R. Distribution of a macrophage-specific antigen. Cell Immunol. 1972 Oct;5(2):325–337. doi: 10.1016/0008-8749(72)90058-5. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. The importance of phospholipase-A2 in prostaglandin biosynthesis. Biochem Pharmacol. 1976 Feb 1;25(3):285–291. doi: 10.1016/0006-2952(76)90216-1. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. Production of colony-stimulating factor by human macrophages. Lancet. 1972 Dec 30;2(7792):1397–1399. doi: 10.1016/s0140-6736(72)92966-2. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Gordon S., Werb Z. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc Natl Acad Sci U S A. 1976 Mar;73(3):872–876. doi: 10.1073/pnas.73.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Fredholm B. B. Isomerization of prostaglandin H2 into prostaglandin D2 in the presence of serum albumin. Biochim Biophys Acta. 1976 Apr 22;431(1):189–183. doi: 10.1016/0005-2760(76)90273-3. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandins in human seminal plasma. Prostaglandins and related factors 46. J Biol Chem. 1966 Jan 25;241(2):257–263. [PubMed] [Google Scholar]
- Higgs G. A., Bunting S., Moncada S., Vane J. R. Polymorphonuclear leukocytes produce thromboxane A2-like activity during phagocytosis. Prostaglandins. 1976 Nov;12(5):749–757. doi: 10.1016/0090-6980(76)90050-2. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., McCall E., Youlten L. J. A chemotactic role for prostaglandins released from polymorphonuclear leucocytes during phagocytosis. Br J Pharmacol. 1975 Apr;53(4):539–546. doi: 10.1111/j.1476-5381.1975.tb07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh W., Isakson P. C., Needleman P. Hormone selective lipase activation in the isolated rabbit heart. Prostaglandins. 1977 Jun;13(6):1073–1091. doi: 10.1016/0090-6980(77)90135-6. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Raz A., Denny S. E., Wyche A., Needleman P. Hormonal stimulation of arachidonate release from isolated perfused organs. Relationship to prostaglandin biosynthesis. Prostaglandins. 1977 Nov;14(5):853–871. doi: 10.1016/0090-6980(77)90302-1. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. S., Broxmeyer H. E., Moore M. A. Limitation of excessive myelopoiesis by the intrinsic modulation of macrophage-derived prostaglandin E. Science. 1978 Feb 3;199(4328):552–555. doi: 10.1126/science.304600. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Kincade P. W., Moore M. A. Regulation of B-lymphocyte clonal proliferation by stimulatory and inhibitory macrophage-derived factors. J Exp Med. 1977 Nov 1;146(5):1420–1435. doi: 10.1084/jem.146.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Bunting S., Mullane K., Thorogood P., Vane J. R., Raz A., Needleman P. Imidazole: a selective inhibitor of thromboxane synthetase. Prostaglandins. 1977 Apr;13(4):611–618. doi: 10.1016/0090-6980(77)90232-5. [DOI] [PubMed] [Google Scholar]
- Northover B. J. Effect of indomethacin and related drugs on the calcium ion-dependent secretion of lysosomal and other enzymes by neutrophil polymorphonuclear leucocytes in vitro. Br J Pharmacol. 1977 Feb;59(2):253–259. doi: 10.1111/j.1476-5381.1977.tb07487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P., Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971 Apr 30;180:363–385. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Tolone G., Bonasera L., Brai M., Tolone C. Prostaglandin production by human polymorphnuclear leucocytes during phagocytosis in vitro. Experientia. 1977 Jul 15;33(7):961–962. doi: 10.1007/BF01951305. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Calderon J., Kiely J. M., Stadecker M. J. Regulation of immunity and inflammation by mediators from macrophages. Am J Pathol. 1976 Nov;85(2):465–478. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Peskar B. A., Wrogemann K., Rietschel E. T., Staudinger H., Fischer H. Prostaglandin and thromboxane synthesis in a pure macrophage population and the inhibition, by E-type prostaglandins, of chemiluminescence. FEBS Lett. 1978 May 1;89(1):136–140. doi: 10.1016/0014-5793(78)80539-0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Lysosome. N Engl J Med. 1965 Nov 11;273(20):1084–contd. doi: 10.1056/NEJM196511112732006. [DOI] [PubMed] [Google Scholar]
- White R., Lin H. S., Kuhn C., 3rd Elastase secretion by peritoneal exudative and alveolar macrophages. J Exp Med. 1977 Sep 1;146(3):802–808. doi: 10.1084/jem.146.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]
- Zimecki M., Webb D. R., Jr The role of prostaglandins in the control of the immune response to an autologous red blood cell antigen (Hb). Clin Immunol Immunopathol. 1977 Nov;8(3):420–429. doi: 10.1016/0090-1229(77)90006-x. [DOI] [PubMed] [Google Scholar]


