Abstract
Lung slices from adult rats incubated in [methyl-3H]-choline chloride formed [3H]-disaturated phosphatidylcholine ( [3H]-DSPC) which was used as an index of lung surfactant. The slices were perifused after 3 h incubation in [methyl-3H]-choline chloride and the overflow of [3H]-DSPC, as a rate coefficient, was used as a measure of surfactant secretion. The basal overflow of [3H]-DSPC rapidly declined over the first 30 min of perifusion and then declined slowly. Salbutamol induced a prolonged, and sometimes delayed, increase in [3H]-DSPC overflow, which was reduced by (+/-)-propranolol. Potassium chloride produced an immediate, and usually transient, increase in [3H]-DSPC overflow which was not modified by atropine or (+/-)-propranolol. Adenosine 5'-triphosphate, but not phenylephrine, also increased [3H]-DSPC overflow. This method can measure the magnitude and time-course of lung surfactant secretion induced by drugs.
Full text
PDF
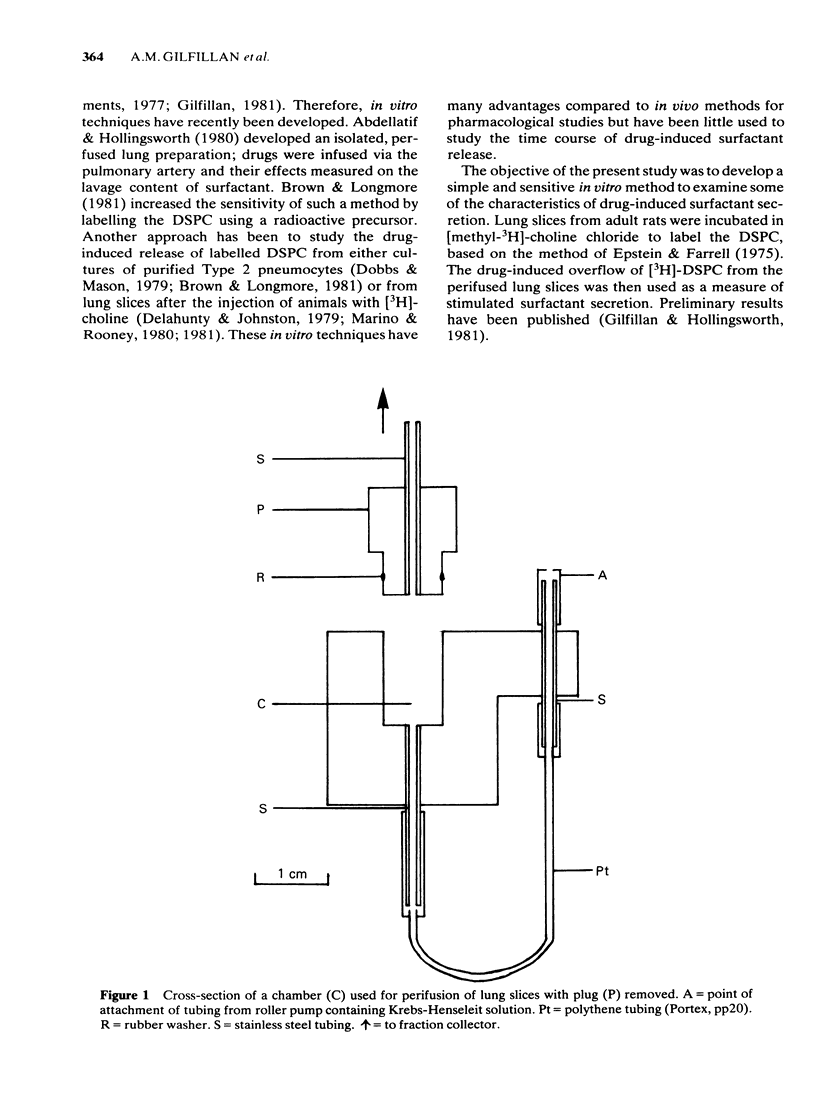


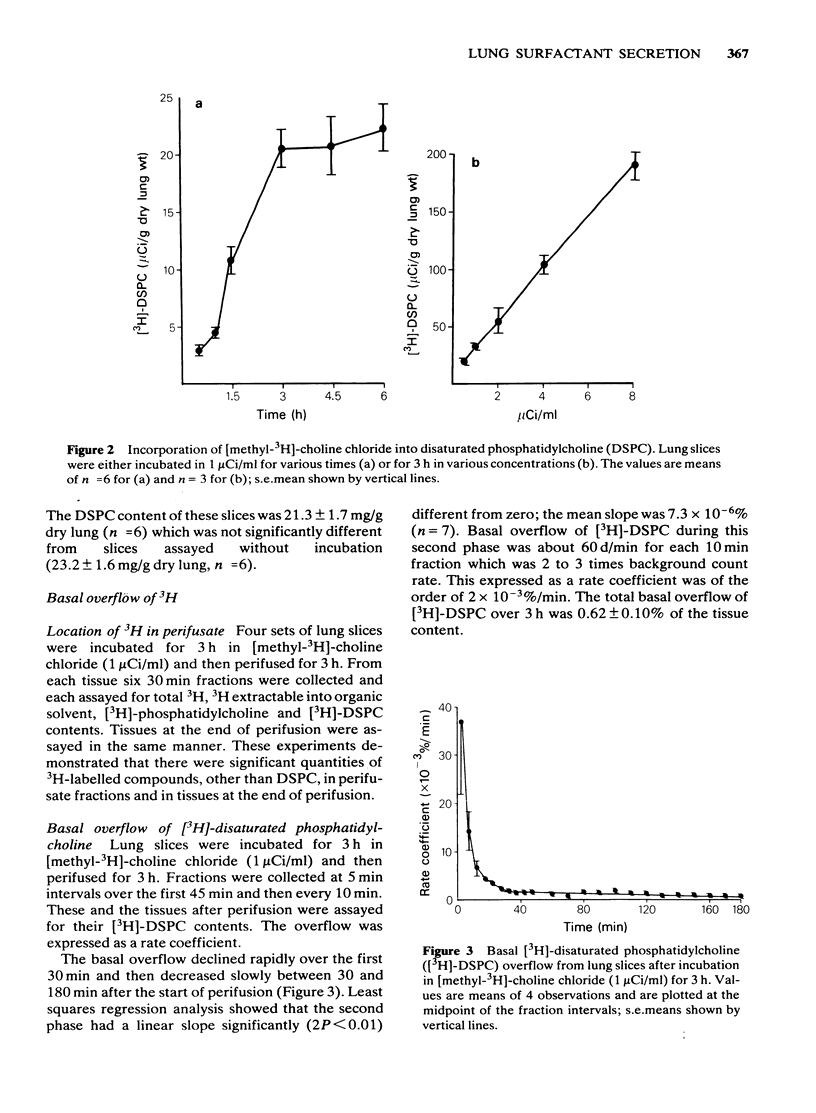
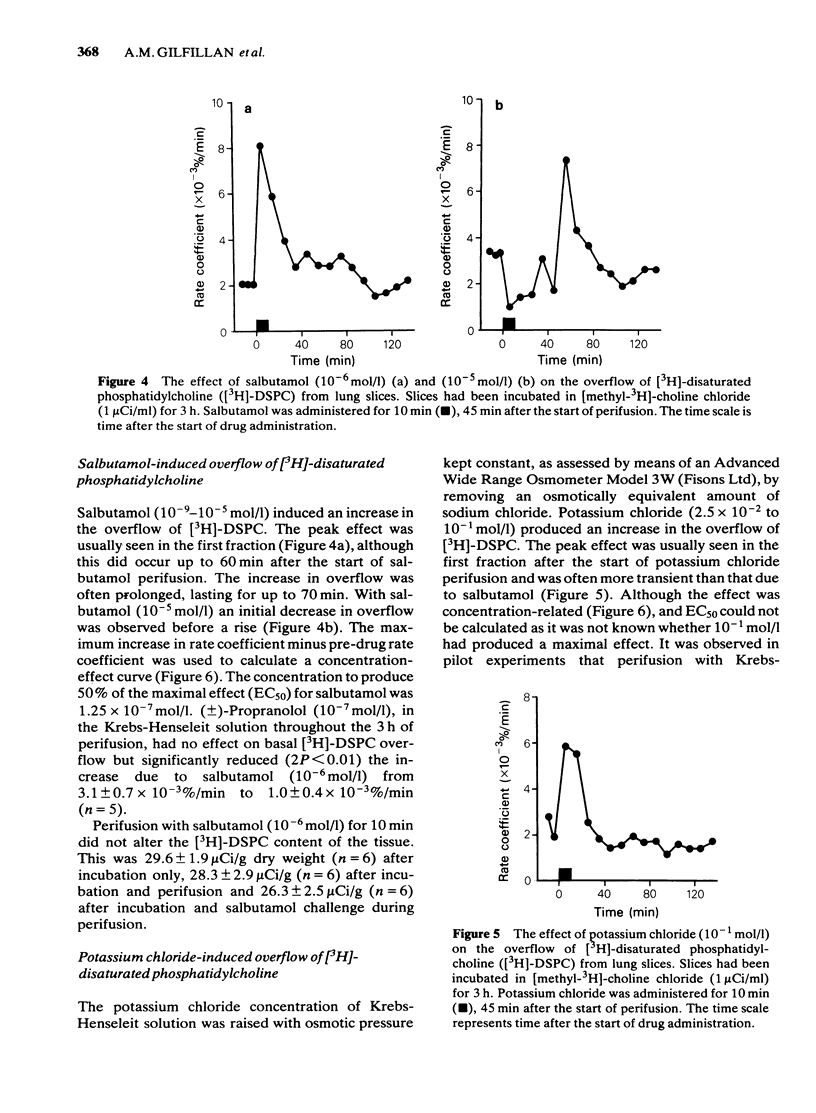
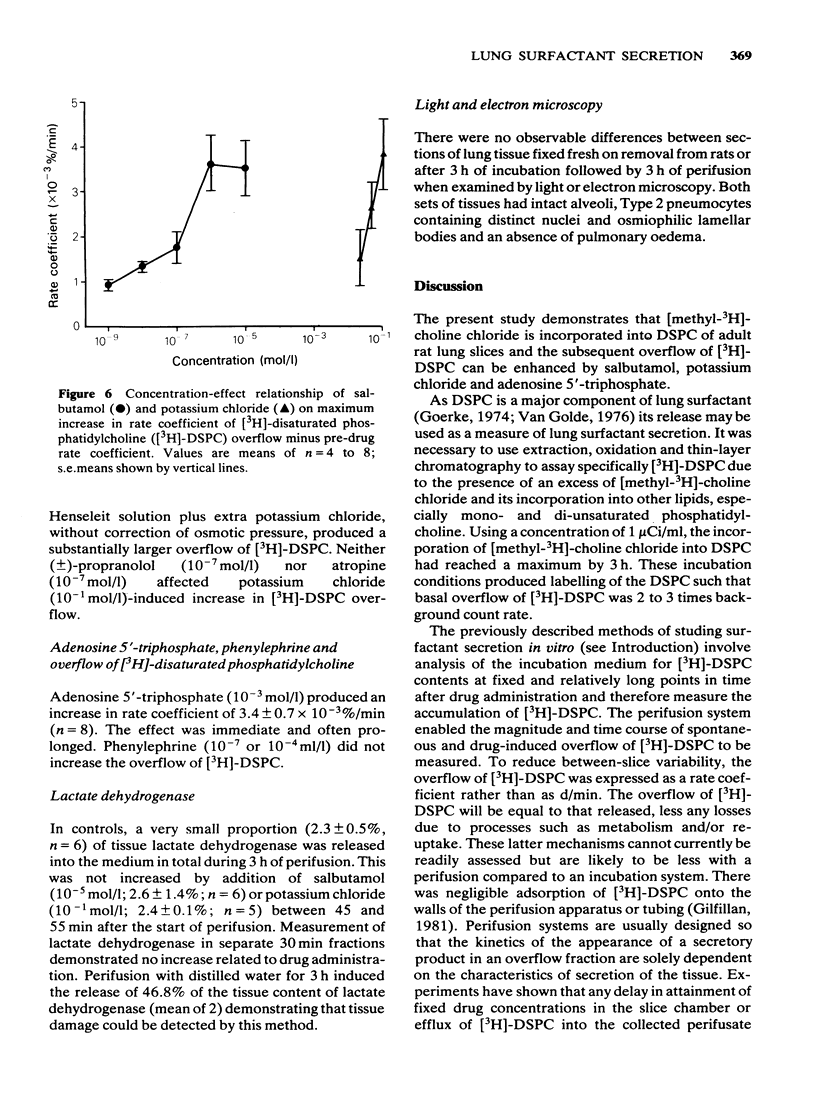


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdellatif M. M., Hollingsworth M. Effect of oxotremorine and epinephrine on lung surfactant secretion in neonatal rabbits. Pediatr Res. 1980 Aug;14(8):916–920. doi: 10.1203/00006450-198008000-00004. [DOI] [PubMed] [Google Scholar]
- Ahmed A., Chiswick M. L. Origin of osmophilic inclusion bodies in type II pneumocytes. J Pathol. 1974 Jul;113(3):161–163. doi: 10.1002/path.1711130305. [DOI] [PubMed] [Google Scholar]
- Brown L. A., Longmore W. J. Adrenergic and cholinergic regulation of lung surfactant secretion in the isolated perfused rat lung and in the alveolar type II cell in culture. J Biol Chem. 1981 Jan 10;256(1):66–72. [PubMed] [Google Scholar]
- Case R. M. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978 May;53(2):211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Collet A. J. In vivo incorporation of choline- 3 H, leucine- 3 H and galactose- 3 H in alveolar type II pneumocytes in relation to surfactant synthesis. A quantitative radoautographic study in mouse by electron microscopy. Anat Rec. 1972 Nov;174(3):289–310. doi: 10.1002/ar.1091740303. [DOI] [PubMed] [Google Scholar]
- Delahunty T. J., Johnston J. M. Neurohumoral control of pulmonary surfactant secretion. Lung. 1979;157(1):45–51. doi: 10.1007/BF02713593. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Mason R. J. Pulmonary alveolar type II cells isolated from rats. Release of phosphatidylcholine in response to beta-adrenergic stimulation. J Clin Invest. 1979 Mar;63(3):378–387. doi: 10.1172/JCI109313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. F., Farrell P. M. The choline incorporation pathway: primary mechanism for de novo lecithin synthesis in fetal primate lung. Pediatr Res. 1975 Aug;9(8):658–665. doi: 10.1203/00006450-197508000-00009. [DOI] [PubMed] [Google Scholar]
- Gilfillan A. M., Harkes A., Hollingsworth M. Secretion of lung surfactant following delivery after uterine section. J Dev Physiol. 1980 Feb-Apr;2(1-2):101–110. [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M. Mechanical responses of rat isolated uterine horns to transmural stimulation. Br J Pharmacol. 1975 Sep;55(1):41–46. doi: 10.1111/j.1476-5381.1975.tb07607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack D. An introduction to salbutamol and other modern beta-adrenoreceptor stimulants. Postgrad Med J. 1971 Mar;47(Suppl):8–11. [PubMed] [Google Scholar]
- Lacy P. E. Endocrine secretory mechanisms. A review. Am J Pathol. 1975 Apr;79(1):170–188. [PMC free article] [PubMed] [Google Scholar]
- Marino P. A., Rooney S. A. Surfactant secretion in a newborn rabbit lung slice model. Biochim Biophys Acta. 1980 Dec 5;620(3):509–519. doi: 10.1016/0005-2760(80)90143-5. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Rooney S. A. The effect of labor on surfactant secretion in newborn rabbit lung slices. Biochim Biophys Acta. 1981 May 22;664(2):389–396. doi: 10.1016/0005-2760(81)90061-8. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Oyarzún M. J., Clements J. A. Ventilatory and cholinergic control of pulmonary surfactant in the rabbit. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jul;43(1):39–45. doi: 10.1152/jappl.1977.43.1.39. [DOI] [PubMed] [Google Scholar]
- Smith B. T., Bogues W. G. Effects of drugs and hormones on lung maturation in experimental animal and man. Pharmacol Ther. 1980;9(1):51–74. doi: 10.1016/0163-7258(80)90016-9. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M. Metabolism of phospholipids in the lung. Am Rev Respir Dis. 1976 Nov;114(5):977–1000. doi: 10.1164/arrd.1976.114.5.977. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Electrical correlates of secretion in endocrine and exocrine cells. Fed Proc. 1981 Feb;40(2):128–134. [PubMed] [Google Scholar]


