Abstract
Prion diseases are fatal neurodegenerative disorders that are caused by the conversion of a normal host-encoded protein, PrPC, to an abnormal, disease-causing form, PrPSc. This paper reports that cyclodextrins have the ability to reduce the pathogenic isoform of the prion protein PrPSc to undetectable levels in scrapie-infected neuroblastoma cells. Beta-cyclodextrin removed PrPSc from the cells at a concentration of 500 μM following 2 weeks of treatment. Structure activity studies revealed that antiprion activity was dependent on the size of the cyclodextrin. The half-maximal inhibitory concentration (IC50) for beta-cyclodextrin was 75 μM, whereas α-cyclodextrin, which possessed less antiprion activity, had an IC50 of 750 μM. This report presents cyclodextrins as a new class of antiprion compound. For decades, the pharmaceutical industry has successfully used cyclodextrins for their complex-forming ability; this ability is due to the structural orientation of the glucopyranose units, which generate a hydrophobic cavity that can facilitate the encapsulation of hydrophobic moieties. Consequently, cyclodextrins could be ideal candidates for the treatment of prion diseases.
Transmissible spongiform encephalopathies (TSEs), also known as prion diseases, are a group of fatal neurodegenerative disorders that affect humans and animals and are unique in that they can be sporadic, inherited, or of transmissible origin (49). The TSEs affecting humans include kuru, Gerstmann-Straussler Scheinker syndrome, fatal familial insomnia, and Creutzfeldt-Jakob disease. Among the TSEs affecting other animals are natural scrapie of sheep and goats and bovine spongiform encephalopathy of cattle. Central to the control of the development of all TSEs is the prion protein, which in humans is the product of a single gene located on chromosome 20 (48). The prion protein exists in at least two conformational forms with distinct physiochemical properties. The normal cellular form of the prion protein (PrPC) is expressed at high levels in neuronal cells (6, 59). It is a glycosyl-phosphatidylinositol (GPI)-anchored cell surface protein (59), and during TSE disease, it is converted to an abnormal, pathological form known as PrPSc. Unlike PrPC, PrPSc is detergent insoluble and partially proteinase resistant (31). The tendency of PrPSc to aggregate in the brain leads to its characteristic neuropathological features, such as spongiform degeneration of the brain.
PrPC and PrPSc have the same amino acid sequence but differ dramatically in conformation. PrPC consists of 42% α-helix and 3% β-sheet, while PrPSc possesses a much higher β-sheet content of 43% and a lower α-helix content of 30% (41, 52). The conversion of PrPC to the pathogenic isoform is a critical event. It is believed that the interaction of endogenous PrPC substrate with the pathogenic PrPSc template causes PrPC to unfold and refold as the β-sheet-rich isoform PrPSc. This step starts a chain reaction, where each newly converted PrPSc molecule interacts with more PrPC molecules, fueling the formation of PrPSc (17, 48).
Lipid rafts, which are found in the plasma membrane, are also known as detergent-resistant microdomains (DRMs) (9, 38, 63) and are enriched in cholesterol and sphingolipids. The formation of rafts is dependent on cholesterol (51), which is believed to function as a spacer between hydrocarbon chains of sphingolipids, thereby holding the raft assembly together (56). The extraction of cholesterol from membranes leads to the dissociation of proteins from rafts (15, 25, 53). These rafts are important in many cell processes, such as membrane sorting and trafficking and signal transduction (23). As has been reported for other GPI-anchored proteins, both PrPC and PrPSc have been found to be associated with DRMs, and DRMs have been hypothesized to be involved in both the function of PrPC (34) and the conversion of PrPC to the pathogenic isoform (37, 38, 63). Moreover, in addition to the GPI anchor, PrP presents a sphingolipid binding domain that allows the interaction of the protein with the polar head groups of the sphingolipids (28, 64). A number of studies have provided evidence that in cell culture, conversion requires association with rafts (22, 63, 68), which may explain why cholesterol extraction modifies PrPSc formation in cell culture (63).
To date, there is no known cure for TSEs, as standard approaches to treating the diseases have proved ineffective. A range of compounds have been tested in the search for treatments for TSEs, and these compounds have been directed against targets such as preventing a rise in calcium, preventing apoptosis, directly interfering with conversion, and altering prion trafficking and dominant negative inhibition (2, 11, 12, 14, 19, 24, 29, 30, 35, 43-45, 46, 47, 54). Over the last several years, a wide variety of compounds have been tested for their effect in the treatment of TSEs, but unfortunately, none have been successful at completely eliminating the disease when given either immediately before, during, or after disease onset. Limitations on success have also related to the toxicity of antiprion compounds to humans and/or an inability to cross the blood-brain barrier (BBB), where most of the PrPSc accumulates.
Prion diseases share neuropathological characteristics with other neurodegenerative diseases, such as Parkinson's disease and Alzheimer's disease (AD). These characteristics include intracellular and extracellular aggregates of proteinaceous fibrils resulting from irregular protein-protein interactions (65). In a search for a new antiprion compound, β-cyclodextrin (β-CD), which reduces the toxic effects of an AD-associated protein (β-amyloid [Aβ] [amino acids 1 to 40]) in cell culture (10), was tested here. The formation of β-sheet fibrils is a critical event in AD, and β-CD, by attenuating fibrillization of the toxic peptide, appears to inhibit the toxic effects of Aβ (10). The CDs are macrocyclic, nonreducing maltooligosaccharides made from α-1,4-linked glucose units (58). α-, β-, and γ-CDs, composed of 6, 7, and 8 glucosyl units, respectively, are the parent CDs (61) and have attracted interest as natural complexing agents and as vehicles to increase drug delivery, bioavailability, and solubility (18, 58, 66).
In this report, the ability of noncytotoxic concentrations of β-CD and methyl-β-CD (Mβ-CD) to clear PrPSc from ScN2a cells following 2 weeks of treatment is demonstrated. Of the naturally occurring CDs, β-CD, with a half-maximal inhibitory concentration (IC50) of 75 μM, was much more efficient at clearing infection than was α-CD or γ-CD, with IC50s of 750 μM and 1,150 μM, respectively. The antiprion activity of β-CDs was dependent not only on the ring size of the CD but also on the cyclic nature of the molecule. The antiprion activity of the CDs tested related to their ability to sequester and move molecules. β-CD modified the location of both PrPC and PrPSc, but it did so differently, separating the isoforms into different lipid domains. Such separations have the potential to restrict the conversion process. β-CD also bound to PrPC and interfered with abnormal prion conversion. These data indicate that the antiprion action of β-CDs is derived from a combination of mechanisms.
MATERIALS AND METHODS
Reagents and antibodies.
α-, β-, and γ-CDs; Mβ-CD; maltohexaose (G6); maltoheptaose (G7); and water-soluble cholesterol complex were purchased from Sigma-Aldrich. Cell culture reagents, such as OptiMEM, fetal bovine serum, glutamine, and penicillin-streptomycin, were Gibco products supplied by Biosciences. Secondary antibody anti-mouse peroxidase conjugate and cholera toxin B subunit type Inaba 569B peroxidase conjugate were purchased from Calbiochem. Epoxy-activated Sepharose 6B was obtained from Amersham Pharmacia. Pefabloc was purchased from Boehringer Manheim, and G418 was purchased from Promega; all other reagents were purchased from Sigma-Aldrich. The primary antibody 3F4 (1:10,000 dilution) used in this study is characterized as recognizing the peptide epitope comprising amino acids 109 to 112 of human PrP and was purchased from Signet Laboratories. The primary antibody 8H4 used in this study is characterized as recognizing the peptide epitope comprising amino acids 175 to 185 of PrP and was described previously (27).
Cell culture and effect of compounds on PrPC, PrPSc, and cholesterol levels.
Neuroblastoma cells (N2a) transfected with mouse PrP (N2a58) and N2a58 cells infected with the 22L scrapie strain (N2a22L), as reported previously (39), were obtained from S. Lehmann (Montpelier, France). To obtain cells producing high levels of PrPSc, the N2a22L cell line was subcloned and is referred to as N2a22L20 in this study. 3F4-tagged mouse PrPC (MoPrPC) cDNA in the pcDNA3 vector was obtained from S. Lehmann (Montpelier, France). N2a cells were transfected with the cDNA using FuGENE transfection reagent according to the manufacturer's instructions. Stably transfected cells were screened and maintained using G418 resistance. The N2a22L, N2a22L20, N2a58, and 3F4MoPrPC N2a cells were cultured in OptiMEM supplemented with 10% fetal bovine serum, penicillin-streptomycin, 2 mM glutamine, and 300 μg/ml Geneticin (G418 sulfate) in a humidified atmosphere.
On passage, cells were treated with the compounds indicated in the text at the concentrations and times indicated unless otherwise stated. All compounds were solublized in Millipore water and were sterilized by filtering through a 0.2-μm filter prior to their being added to the medium. Cells were lysed in cold lysis buffer (0.5% [wt/vol] sodium deoxycholate, 0.5% [vol/vol] Triton X-100, 150 mM NaCl, and 50 mM Tris, pH 7.5) and analyzed for PrPC or PrPSc. The total protein concentration was measured using the bicinchoninic acid protein assay kit (Sigma).
Total cholesterol was measured by the method of Mizoguchi et al. (32). Twenty-five microliters of sample and 150 μl reagent solution [0.05 M MES (morpholineethanesulfonic acid) buffer, pH 6.1, 0.5 U ml−1 cholesterol oxidase, 4 U ml−1 cholesterol esterase, 0.2 mM 4-aminoantipyrine, 1 mM N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline sodium salt, and 4 U ml−1 horseradish peroxidase] were added and incubated at 37°C for 15 min, and cholesterol was measured at 600 nm.
Western blotting and densitometry.
For PrPC analysis, lysates were adjusted to 18 μg of protein in gel loading buffer and boiled for 5 min prior to being loaded onto 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, followed by Western blotting. For PrPSc analysis, lysates were prepared to 200 μg for N2a22L cells and 50 μg for N2a22L20 cells and then digested with 16 μg of proteinase K (pK)/mg of protein at 37°C for 15 min. One millimolar Pefabloc was then added, and samples were incubated on ice for 5 min, followed by centrifugation at 14,000 rpm for 45 min. The resulting pellets were then resuspended in loading buffer, boiled for 5 min, and loaded onto 12% SDS-PAGE, followed by Western blotting using standard techniques. PrPC and PrPSc were detected by incubating immunoblots with antibody 8H4 (3F4-tagged MoPrPC was detected with antibody 3F4), followed by a horseradish peroxidase secondary antibody, and developed by enhanced chemiluminescence. Densitometry was performed with Bio-Rad Imager analysis software.
Sucrose density gradients.
N2a22L20 cells from 3-by-60-mm dishes were lysed in 400 μl of ice-cold sucrose gradient (SG) lysis buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA and 1% Triton X-100) on ice for 30 min. Lysates were then spun at 4,850 rpm for 10 min, and the 400-μl supernatant was adjusted to 40% (wt/vol) sucrose prepared in SG lysis buffer without detergent. One milliliter 25% (wt/vol) sucrose and 0.5 ml 5% (wt/vol) sucrose, both in SG lysis buffer without detergent, were then added. The gradient was then spun at 37,000 rpm for 18 h at 4°C. Fractions (10 × 230 μl) were collected from the top of the tube and processed for PrPC, PrPSc, and ganglioside GM1, a marker of DRM, as follows.
For PrPC detection, 100 μl of each fraction was diluted with 300 μl ΤΝ (10 mM Tris, pH 7.8, and 150 mM NaCl) and then made to 1% (wt/vol) Sarkosyl (to precipitate PrPSc) and incubated on ice for 30 min. Fractions were then spun at 45,000 rpm for 2 h at 4°C, and the supernatants were methanol (MeOH) precipitated. The precipitated pellet was resuspended in gel loading buffer, and PrPC was visualized by using 12% SDS-PAGE and Western blotting with 8H4 antibody. For PrPSc detection, 120 μl from each protein fraction was obtained and protein was determined using the bicinchoninic acid protein assay kit and treated with 16 μg pK/mg protein for 15 min at 37°C, followed by centrifugation at 70,000 rpm for 45 min. PrPSc was visualized by using SDS-PAGE and Western blotting with 8H4 antibody. For GM1 detection, 10 μl from each fraction was dotted onto a nitrocellulose membrane, which was then blocked in 3% bovine serum albumin for 3 h and incubated with cholera toxin B (1:4,000 dilution) for 1 h. Following four 10-min washes in TTBS (0.1 M Tris, 0.154 NaCl, pH 7.5, containing 0.3% [vol/vol] Tween 20), blots were developed by enhanced chemiluminescence.
Triton X-100 solubility assay.
Cells were washed twice with ice-cold phosphate-buffered saline and were then left on ice for 15 min. The cells were then scraped into ice-cold buffer containing 1% Triton X-100, 150 mM NaCl, and 10 mM Tris-HCl, pH 7.8, and left on ice for 15 min. The lysate was then spun at 39,000 rpm in the ultracentrifuge Sorvall Discovery N12SE Micro Ultra at 2°C for 30 min. The pellet (insoluble protein) was resuspended in 1× loading buffer, and the supernatant (soluble protein) was methanol precipitated prior to centrifugation at 14,000 rpm for 10 min. The resulting pellet was resuspended in 1× loading buffer, and PrPC in the soluble and insoluble fractions was analyzed by 12% SDS-PAGE and immunoblotting with antibody 8H4.
Affinity chromatography and PrP binding.
Freeze-dried epoxy-activated Sepharose 6B was reconstituted in water and activated with 0.1 M NaOH according to the manufacturer's instructions (Phamacia). The gel was incubated with or without 0.02 M β-CD in 0.1 M NaOH for 16 h at 40°C with shaking. The gel was then washed in sequence with 0.1 M NaOH, distilled water, 0.1 M NaOH, and finally 0.1 M acetate buffer, pH 4.0. Both the control gel (without β-CD) and the β-CD-complexed gel were blocked with 0.2 M glycine, pH 8.0, for 4 h at 40°C. The gel was then washed with 0.1 M NaOH, followed by 0.1 M acetate buffer, pH 4.0.
For the binding of PrP to affinity gels containing β-CD, gel made as described above with β-CD incorporated was washed three times with 0.5 M HEPES, pH 7.5, and was resuspended in this buffer. Eight hundred micrograms of N2a cells transfected with 3F4 MoPrPC lysed in cold lysis buffer (0.5% [wt/vol] sodium deoxycholate, 0.5% [vol/vol] Triton X-100, 150 mM NaCl, and 50 mM Tris, pH 7.5) was then added to 800 μg of gel and mixed by inversion over a 15-min period. The mixture of gel and lysate was spun down at 1,000 rpm for 1 min, and the supernatant was removed and MeOH precipitated (S1). The gel was then washed four times with 500 μl of 0.5 M HEPES, pH 7.5, with a 15-min mixing period between each wash. A total of 500 μl of 0.5 M HEPES, pH 7.5, containing 500 μM β-CD was then added, and the solution was mixed as before for 15 min and then spun at 1,000 rpm for 1 min. The supernatant was removed and MeOH precipitated (eluent 1 [E1]). A total of 500 μl of 0.5 M HEPES, pH 7.5, containing 1 mM β-CD, was then added to the gel, and the solution was mixed as before for 15 min. It was then spun, and the supernatant was removed and MeOH precipitated (E2). The remaining 800 μl of gel was boiled in 400 μl of 1× loading buffer for 10 min. The mixture was spun at 1,000 rpm for 1 min, and the supernatant was taken and analyzed for PrPC (E3).
Conversion reaction.
Two-by-60-mm dishes of N2a22L20 and 3F4 MoPrPC N2a cells rinsed twice with ice-cold phosphate-buffer saline were scraped into 300 μl of ice-cold conversion buffer (0.1 M MES, pH 6.2, and 1 μM CaCl2) and subjected to two freeze-thaw cycles and vortexed. The suspension was then incubated at 37°C for 72 h as indicated in the text. Two volumes of ice-cold lysis buffer (0.5% [wt/vol] sodium deoxycholate, 0.5% [vol/vol] Triton X-100, 150 mM NaCl, and 50 mM Tris, pH 7.5, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, and 2 mM EDTA) was added, and samples were MeOH precipitated. The pellet was resuspended in 50 μl of ice-cold lysis buffer, and samples were then assayed for insolubility by centrifuging them at 70,000 rpm for 40 min at 4°C. The pellet (insoluble fraction) was resuspended in loading buffer. The supernatant (soluble fraction) was precipitated in MeOH. All samples were analyzed by SDS-12% PAGE and immunoblotting with antibody 3F4.
RESULTS
β-CD clears 22L-infected neuroblastoma cells of infection.
Prion diseases and AD are protein conformational disorders, and in both cases, cholesterol levels are thought to affect the disease process. For AD, it was identified that β-CD altered the toxic effects of the Aβ peptide associated with AD (10). Consequently, as β-CD is known to modify cholesterol content, its effect on PrPSc levels in cell culture models was tested here. The scrapie-infected cell line employed for this study was N2a infected with 22L (39). A subclone possessing six times the PrPSc level was also employed (N2a22L20).
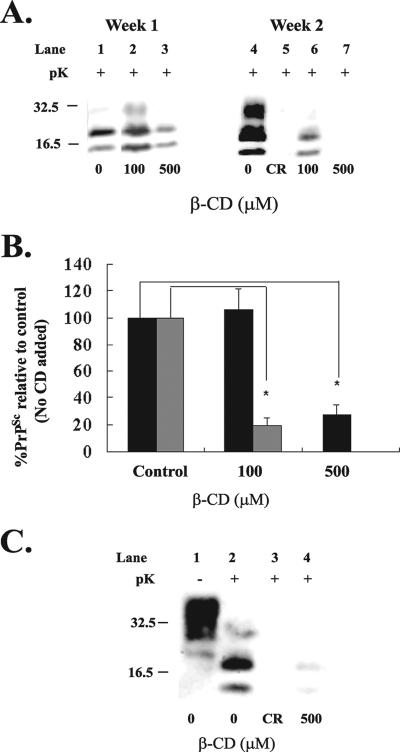
To examine the effect of β-CD on scrapie, β-CD was added to the N2a22L cell line at 100 μM and 500 μM at the time of passage and the cells were cultured in the presence of the compound for periods of 1 and 2 weeks. Cells were then lysed (Fig. 1A) and analyzed for PrPSc content by immunoblotting with 8H4 antibody. After 1 week of treatment, 500 μM β-CD reduced PrPSc to 30% of the control level (Fig. 1A, lane 3, and B). Following 2 weeks of treatment, 100 μM β-CD reduced PrPSc levels to 20% (Fig. 1A, lane 6, and B) and complete clearance was achieved with 500 μM (Fig. 1A, lane 7, and B). A total of 500 μM β-CD also cleared PrPSc after 2 weeks of treatment in N2a22L20, which possessed six times more PrPSc than N2a22L did (Fig. 1C, lane 4). Clearance was measured as the disappearance of the proteinase-resistant core of PrP, amino acids 27 to 30, relative to the amount in the untreated control, which was taken as 100%, and clearance was comparable to that of the known antiprion compound Congo red (Fig. 1A, lane 5, and C, lane 3).
FIG. 1.
β-CD reduces PrPSc to undetectable levels in the 22L-infected scrapie cell line. (A) β-CD at concentrations of 100 μM and 500 μM was added to N2a22L cells at the time of passage, and the cells were then passaged in the presence of the drug for 1 and 2 weeks. (B) Representative bands shown in panel A were quantified by densitometry and expressed as a percentage of the control (untreated). Black bars represent week 1, and gray bars represent week 2. *, P < 0.005 (Student's t test). Error bars indicate standard deviations. (C) β-CD at a concentration of 500 μM was added to N2a22L20 cells at the time of passage, and the cells were passaged in the presence of the drug for 2 weeks. (A and C) Cells were lysed, and the lysate was then analyzed for PrPSc by 12% SDS-PAGE and immunoblotting with 8H4 antibody. Cell lysates were left untreated (−pK) or digested with pK (+pK) for the detection of PrPSc. Where indicated, as a drug control for PrPSc clearance, Congo red (CR) was added to the cells at 5 μg/ml for 2 weeks. Results are representative of three independent experiments. Molecular mass markers in kilodaltons are shown on the left of the panels.
The concentrations of β-CD used in this study were noncytotoxic as demonstrated by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] cell viability assay (data not shown). To control for the possibility that clearance may have been due to a stimulation of pK activity by β-CD, β-CD was added to scrapie-infected cell lysate before the addition of pK and PrPSc detection was carried out as normal. Results from this experiment revealed that β-CD had no effect on pK activity (data not shown).
CDs have higher antiprion activity than their linear counterparts.
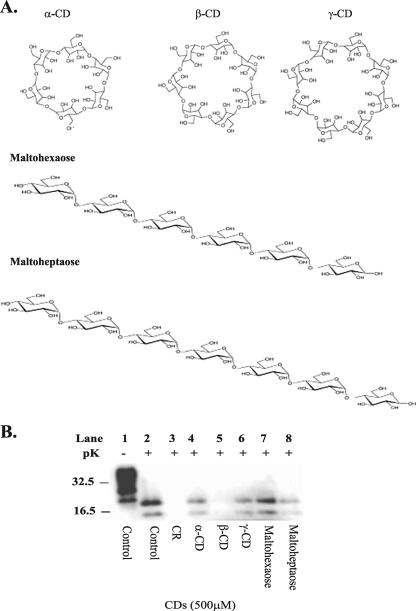
The effect of other members of the natural CD family, α-, β-, and γ-CDs (Fig. 2A), were examined for potential antiprion activity. The N2a22L cell line was treated with 500 μM of α-, β-, and γ-CD for a period of 2 weeks, after which cells were analyzed for PrPSc levels by immunoblotting with 8H4 antibody (Fig. 2B, lanes 4 to 6). Of the CDs tested here, only β-CD (Fig. 2B, lane 5) completely cured infection, whereas α-CD and γ-CD demonstrated less antiprion activity (Fig. 2B, lanes 4 and 6). The structure-antiprion activity relationship of CDs was examined further by comparing their antiprion activity with that of their linear counterparts. The linear counterparts for β-CD and α-CD were maltoheptaose (G7) and maltohexaose (G6), respectively (Fig. 2A). The corresponding linear sugar for γ-CD, maltooctaose, was not commercially available. Once again, the compounds were added at 500 μM to the cells at the time of passage and the cells were cultured in this manner for a period of 2 weeks, after which the cells were lysed and analyzed for PrPSc levels. As G6 and G7 displayed less antiprion activity than α- and β-CD, respectively, the importance of the cyclic nature of the CDs for enhancing antiprion activity is demonstrated. G7 showed some ability to reduce PrPSc levels, but it was not able to reduce infection like β-CD. CDs differ in size with α-CD possessing 6, β-CD possessing 7, and γ-CD possessing 8 glucopyranose units (Fig. 2A), leading to differences in the cavity sizes of the compounds (8 Å for γ-CD, 6 Å for β-CD, and 4.5 Å for α-CD) (66). These data indicate that the 6-Å size of β-CD is important for antiprion activity, and this indication is further emphasized by comparing the IC50 values of β-CD, α-CD, and γ-CD for clearing infection in N2a22L.
FIG. 2.
Structure activity studies. (A) Structures of α-, β-, and γ-CD and their corresponding linear sugars. (B) α-, β-, and γ-CD and the linear sugars maltohexaose and maltoheptaose were added to the N2a22L cells at a concentration of 500 μM at the time of passage, and the cells were then passaged in the presence of the drugs for 2 weeks. The cells were lysed, and the lysate was then analyzed for PrPSc by 12% SDS-PAGE and immunoblotting with 8H4 antibody. Cell lysates were left untreated (−pK) or were digested with pK (+pK) for the detection of PrPSc. Where indicated as a drug control for PrPSc clearance, Congo red (CR) was added to the cells at 5 μg/ml for 2 weeks. Results are representative of three independent experiments. Molecular mass markers in kilodaltons are shown on the left of the panel.
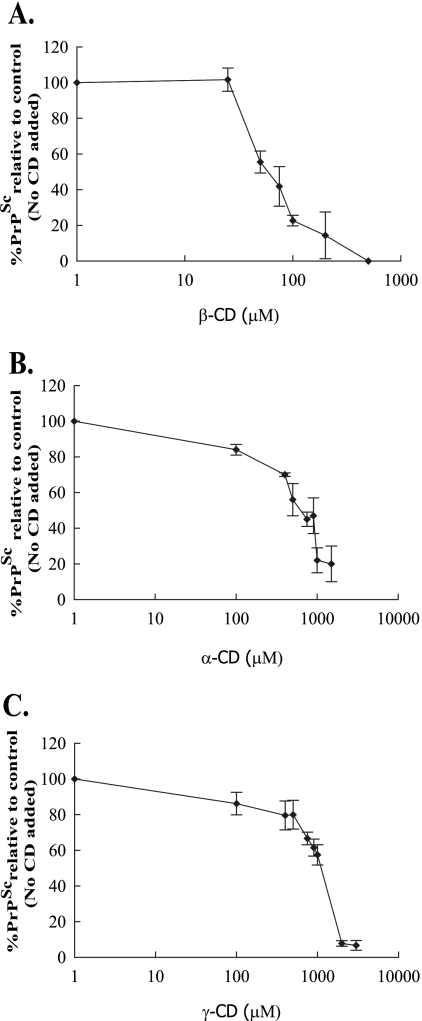
IC50s were determined for β-, α-, and γ-CD by adding a range of concentrations of the compounds to the cells at the time of passage over a period of 2 weeks, after which the cells were lysed and analyzed for PrPSc. β-CD with an IC50 of 75 μM (Fig. 3A) was 10 times more efficient at clearing infection than was α-CD, which had an IC50 of 750 μM (Fig. 3B). γ-CD had an IC50 of 1,150 μM (Fig. 3C).
FIG. 3.
IC50 determination for α-, β-, and γ-CD. Various concentrations of α-, β-, and γ-CD were added to the N2a22L cells at the time of passage, and the cells were then passaged in the presence of the compounds for 2 weeks. Cells were then lysed, and the lysate was then analyzed by 12% SDS-PAGE and immunoblotting with 8H4 antibody for the effect of β-CD (A), α-CD (B), and γ-CD (C) on PrPSc levels. Representative bands from immunoblots were quantified by densitometry and expressed as a percentage of the control (untreated). Results are representative of three independent experiments. Error bars indicate standard deviations.
β-CD does not act through altering PrPC levels or through an acidic compartment.
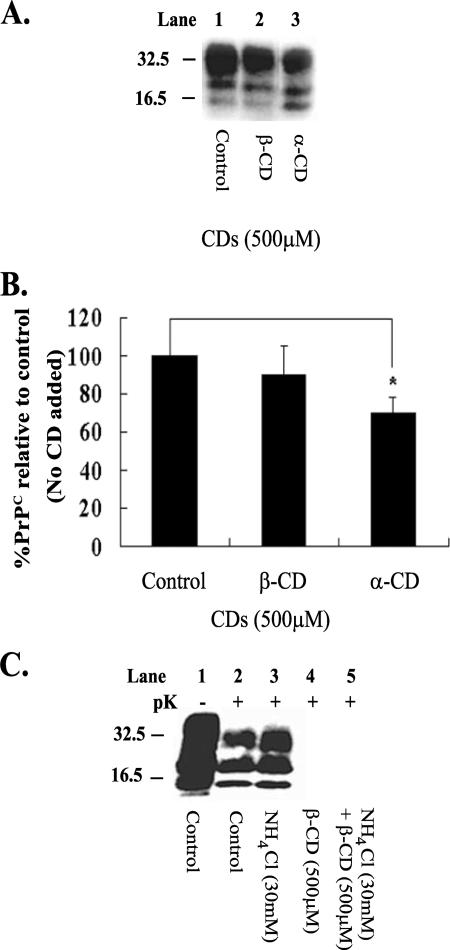
The conversion of PrPC to pathogenic PrPSc is believed to be a central event leading to the disease state in TSEs, and based on this belief, we reasoned that β-CD could be working by reducing substrate levels (PrPC) for conversion. To test this hypothesis, noninfected N2a cells (N2a58 cell line) were treated with both β- and α-CD for a period of 2 weeks to investigate whether the compounds had an effect on PrPC levels (Fig. 4A and B). Cells were then lysed and analyzed for PrPC levels by immunoblotting with antibody 8H4. β-CD did not have a significant effect on PrPC levels compared to the control (Fig. 4A, lane 2, and B). On the other hand, α-CD, which is 10 times less efficient than β-CD as an antiprion compound, lowered PrPC levels by 30% (Fig. 4A, lane 3, and B).
FIG. 4.
β-CD does not act through altering PrPC levels or through an acidic compartment. (A) β- and α-CD were added to noninfected N2a cells at 500 μM each time the cells were passaged, and the cells were then lysed at 2 weeks. The lysate was prepared to 18 μg of protein, as described in Materials and Methods, before SDS-PAGE and Western blotting, and PrPC was detected with 8H4 antibody. (B) Representative bands shown in panel A were quantified by densitometry and expressed as a percentage of the control (untreated). Error bars indicate standard deviations. (C) The compounds NH4Cl (30 mM) and β-CD (500 μM) were added separately at the time of passage to N2a22L cells for 2 weeks (lanes 3 and 4, respectively). In addition, 30 mM NH4Cl was added to the N2a22L cells at the time of passage and β-CD (500 μM) was added an hour later. The cells were passaged in this manner for a period of 2 weeks (lane 5). The cells were lysed, and the lysate was then analyzed for PrPSc by 12% SDS-PAGE and immunoblotting with 8H4 antibody. Cell lysates were left untreated (−pK) or were digested with pK (+pK) for the detection of PrPSc. *, P < 0.05 (Student's t test). Results are representative of three independent experiments. Molecular mass markers in kilodaltons are shown on the left of the panels.
The possibility that all known antiprion compounds are trafficked through endosomes or lysosomes has been suggested previously (20, 60, 62). Consequently, the effect of NH4Cl on the antiprion activity of β-CD was investigated. NH4Cl is a lysomotropic agent that alkanizes endosomes and lysosomes without influencing PrPSc levels (60). N2a22L cells were treated as before, with 500 μM β-CD for 2 weeks. The cells were also treated with NH4Cl (30 mM) and a combination of NH4Cl and β-CD for the 2-week period, and the cells were then lysed and analyzed for PrPSc content. On its own, NH4Cl did not clear PrPSc (Fig. 4C, lane 3), and it was unable to block the antiprion activity of β-CD (Fig. 4C, compare lanes 4 and 5), indicating that β-CD may not be acting through lysosomes.
Effect of β-CD on PrPC, PrPSc and GM1 association with DRMs.
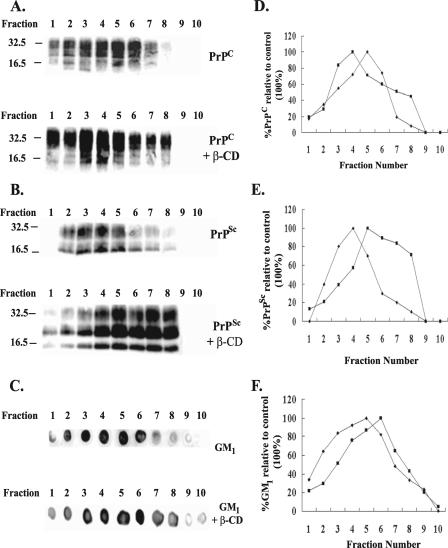
CDs are powerful extractors of cholesterol, but of the CDs, β-CD had the highest capacity to sequester lipids (16, 40, 57). The accumulation of proteins in rafts is altered or abolished upon cholesterol depletion (46). This holds true for PrPC, and altering cholesterol levels interferes with PrPSc production in cell culture (63). So, to investigate how β-CD acts against PrPSc, its effect on the floatation properties of PrPC and PrPSc was determined. Like most GPI-anchored proteins, PrPC and PrPSc are insoluble in cold Triton X-100 (9, 38, 63). This is due to their association with membrane domains or rafts that are enriched in cholesterol, sphingolipids, and glycosphingolipids; these domains are also referred to as DRMs (4, 8). The association of PrPC with such domains is linked with the conversion to PrPSc (37, 38, 63). The solubility of DRMs is thought to be associated with the way their lipid content affects their rigidity and, consequently, the inability of cold Triton X-100 to penetrate and solubilize (9). Consequently, PrPC and PrPSc float in buoyant fractions in density gradients and float in similar fractions to the raft resident, cell surface ganglioside GM1 (38).
To examine for the effect of β-CD on floatation properties, floatation gradients were carried out with the N2a22L20 cell line. Cells were lysed after 3 days of growth, as for SGs, and the gradients were applied for 18 h at 37,000 rpm. Fractions were then processed for PrPC, PrPSc, and GM1 content as described in Materials and Methods and analyzed by immunoblotting with 8H4 antibody for PrPC and PrPSc and by dot blot analysis with CTXB for GM1. PrPC was separated from PrPSc by Sarkosyl treatment. Naslavsky et al. (38) reported that this methodology does not affect PrPC floatation properties. In the absence of β-CD, as expected, PrPC, PrPSc and the raft marker, GM1, floated in light fractions (Fig. 5). To examine for the effect of β-CD on the floatation characteristics, N2a22L20 cells were treated with β-CD (500 μM) for 3 days, and floatation gradients were carried out as before. In keeping with the hypothesis that the removal of cholesterol increases the solubility of raft domains and consequently decreases the floatation of the associated GPI-anchored proteins, treatment with β-CD caused PrPSc and the GM1 marker to float in heavier fractions. PrPSc peaked in fraction 4 prior to the addition of β-CD and between fractions 5 to 8 afterwards (Fig. 5B, compare upper panel, lane 4, and lower panel, lanes 5 to 8, and E). The GM1 marker similarly shifted from fraction 5 to 6 under β-CD treatment (Fig. 5C, compare lane 5, upper panel, and lane 6, lower panel, and F). Unexpectedly, however, upon treatment with β-CD, PrPC floated in lighter fractions (fractions 3 and 4) (Fig. 5A, lower panel, lanes 3 and 4, and D) to the control (fraction 5) (Fig. 5A, upper panel, lane 5, and D). It would appear that β-CD acts through modification of the lipid raft domain, but surprisingly, the two isoforms reacted differently, with PrPC floating in more insoluble fractions than PrPSc. This separation of the localization of the isoforms could have the potential to restrict conversion. However, although the major portions of PrPC and PrPSc have been separated, an overlap in gradients that could allow for a low level of conversion to occur does exist.
FIG. 5.
Effect of β-CD on PrPC, PrPSc, and GM1 association with DRMs. The N2a22L20 cell line was either treated with 500 μM β-CD at the time of passage (PrPC, PrPSc, and GM1, each with β-CD) or left untreated (PrPC, PrPSc, and GM1), and the cells were grown for 72 h. The cells were then lysed as for density gradients and adjusted to 40% sucrose, and a density gradient was applied and spun at 37,000 rpm for 18 h at 4°C. Fractions (10 × 230 μl) were collected from the top of the tube and processed for PrPC, PrPSc, and GM1 detection as described in Materials and Methods. Fraction 1 is the top of the gradient and fraction 10 is the bottom. Fractions for PrPC (A) and PrPSc (B) detection were prepared as described in Materials and Methods and analyzed by 12% SDS-PAGE and immunoblotting with 8H4 antibody. Fractions for GM1 detection (C) were blotted onto a nitrocellulose membrane and visualized using cholera toxin B conjugated to horseradish peroxidase. (D, E, and F) Representative bands from panels A (D) and B (E) and dots from panel C (F) were quantified by densitometry and were expressed as a percentage of the control (the fraction presenting with the highest signal [100%]). Results are representative of three independent experiments. Molecular mass markers in kilodaltons are shown on the left of the panels. Control results are plotted as closed diamonds, and β-CD results are plotted as closed squares.
Reintroduction of cholesterol encapsulated in Mβ-CD does not reverse PrPSc clearance but facilitates it.
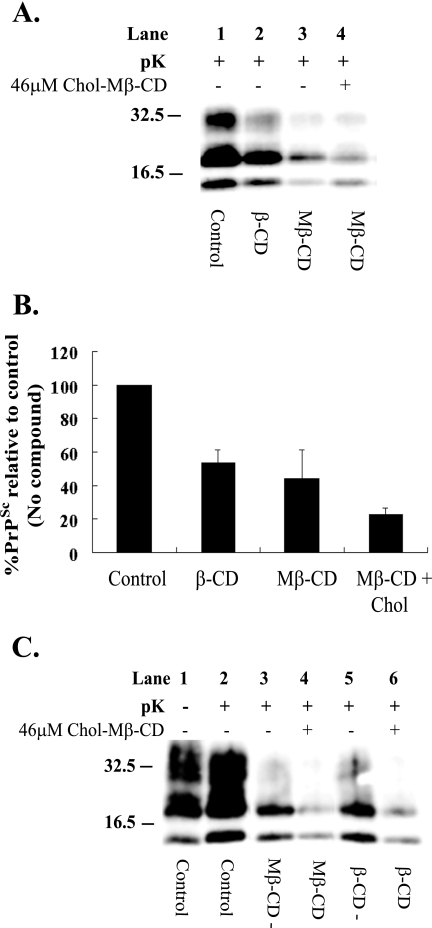
Cholesterol depletion by Mβ-CD has previously been shown to inhibit Aβ formation in neurons, when it was combined with lovastatin (57) and when, on its own, it redistributed the N-methyl-d-aspartic acid receptor subunit, postsynaptic density protein, from Triton X-100 insoluble to soluble fractions (1). In both reports, the CD effect was reversed by reintroducing cholesterol in a cholesterol-Mβ-CD complex (chol-Mβ-CD). As β-CD altered the floatation properties of PrPC and PrPSc, the ability of chol-Mβ-CD to reverse the antiprion activity of β-CD was investigated. In addition, to control for an effect of the Mβ-CD, which is the cholesterol vector, the effect of Mβ-CD on PrPSc levels was determined. N2a22L20 cells were cultured with both β-CD and Mβ-CD, each at 500 μM, for 1 week. Cells were then lysed and analyzed for PrPSc content by immunoblotting with antibody 8H4 (Fig. 6A). As expected, after 1 week, β-CD lowered PrPSc levels to 50% of the control (Fig. 6A, lane 2, and B). Mβ-CD presented with marginally higher antiprion activity, lowering PrPSc levels to 40% (Fig. 6A, lane 3, and B). The IC50 for Mβ-CD was determined to be 60 μM after 2 weeks of treatment of N2a22L20 cells (data not shown). To investigate the ability of chol-Mβ-CD to interfere with the antiprion activity of Mβ-CD, N2a22L20 cells were grown for 1 week in the presence of Mβ-CD (500 μM) in combination with chol-Mβ-CD (46 μM, a concentration chosen from toxicity assays, but also used by others for this purpose [1]) and cells were analyzed for PrPSc (Fig. 6A, lane 4, and B). The presence of cholesterol enhanced, rather than inhibited, the clearance of PrPSc, as levels fell to 20%, indicating that the replenishment with cholesterol could not inhibit the antiprion activity of Mβ-CD.
FIG. 6.
Reintroduction of cholesterol encapsulated in Mβ-CD does not reverse PrPSc clearance, but rather facilitates it. (A) β-CD and Mβ-CD at a concentration of 500 μM were added to N2a22L20 cells at the time of passage, and the cells were then passaged in the presence of the drugs for 1 week (lanes 2 and 3, respectively). N2a22L20 cells were also treated with a combination of Mβ-CD (500 μM) and chol-Mβ-CD (46 μM), and the cells were then passaged in their presence for 1 week (lane 4). (B) Representative bands shown in panel A were quantified by densitometry and were expressed as a percentage of the control (untreated). Error bars indicate standard deviations. (C) N2a22L20 cells were treated with Mβ-CD or β-CD at a concentration of 500 μM at the time of passage, and the cells were passaged in the presence of the drugs for 1 week and then passaged in the absence of the drugs for 3 days (lanes 3 and 5) or passaged in the presence of chol-Mβ-CD (46 μM) alone for 3 days (lanes 4 and 6). (A and C) Cells were lysed, and the lysate was then analyzed for PrPSc by 12% SDS-PAGE and immunoblotting with 8H4 antibody. Cell lysates were left untreated (−pK) or digested with pK (+pK) for the detection of PrPSc. Results are representative of three independent experiments. Molecular mass markers in kilodaltons are shown on the left of the panels.
To further demonstrate that chol-Mβ-CD could not inhibit the antiprion activity, N2a22L20 cells were treated with Mβ-CD or β-CD (each at 500 μM) for 1 week. The compounds were then removed, and the cells were allowed to grow in their absence for 72 h or they were replaced by chol-Mβ-CD (46 μM) for the 72-h period. Cells were then lysed and analyzed for PrPSc levels by immunoblotting with antibody 8H4 (Fig. 6C). Passage of the cells for 1 week in the presence of Mβ-CD and then in its absence for 3 days lowered PrPSc levels to 35% (Fig. 6C, lane 3), which was equivalent to the level of PrPSc reduction observed after 7 days of treatment (compare Fig. 6A, lane 3, with C, lane 3). This result indicates that PrPSc clearance does not continue on drug removal. However, when the cells were grown for 3 days with chol-Mβ-CD (46 μM), following the 1-week treatment with Mβ-CD, PrPSc clearance continued to 20% of the control (Fig. 6C, lane 4), reaffirming that the reintroduction of cholesterol for 3 days did not reverse the antiprion activity. The same pattern was seen when cells were grown in the presence of β-CD for 1 week, followed by 3 days in the absence or presence of chol-Mβ-CD (Fig. 6C, lanes 5 and 6, respectively).
CDs affect cholesterol levels but do not move PrPC to soluble Triton X-100 domains.
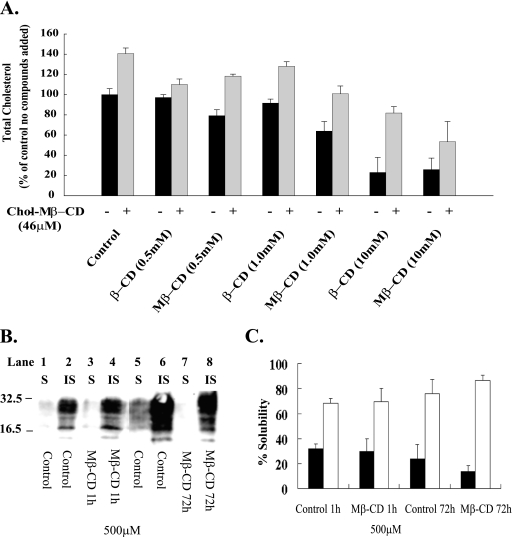
CDs are known for their ability to complex with and sequester cholesterol. To examine this ability in N2a22L20 cells, cells were incubated for 1 h in the presence of increasing concentrations of either β-CD or Mβ-CD and their effects on total cholesterol levels were determined. The ability of chol-Mβ-CD to reintroduce cholesterol was also examined. After the hour of treatment with the CDs, the medium was replaced, and cells were incubated for a further hour in the presence of chol-Mβ-CD (Fig. 7A). Both CDs lowered cholesterol levels; however, Mβ-CD was more efficient. At 0.5, 1, and 10 mM, β-CD lowered levels to 97, 91, and 23% of those of the control, whereas Mβ-CD lowered levels to 79, 63, and 25% (Fig. 7A). Results are in line with those reported previously (1). Chol-Mβ-CD introduced 40% extra cholesterol into control cells and reintroduced cholesterol into CD-treated cells. Cells that had been treated with 0.5, 1, and 10 mM β-CD had levels increased by chol-Mβ-CD to 110, 128, and 81.8%, respectively, of those of the untreated control. For cells that had been treated with 0.5, 1, and 10 mM Mβ-CD, the levels were brought to 118, 100, and 53%, respectively, relative to levels of the untreated control (Fig. 7A).
FIG. 7.
Effect of CDs on cholesterol levels and Triton X-100 insolubility of PrPC. (A) N2a22L20 cells were incubated with 0, 0.5, 1, and 10 mM β-CD or Mβ-CD for 1 h, the medium was removed, and cells were treated (+) or not treated (−) with chol-Mβ-CD (46 μM) for 1 h. Cholesterol levels were then determined. Black bars represent cells treated with CDs as indicated, and gray bars represent cells treated with CDs, followed by treatment with chol-Mβ-CD. Error bars indicate standard deviations. (B) N2a cells were grown to confluence, and the medium was then replaced with fresh medium containing 500 μM Mβ-CD for a period of 1 h or cells were left untreated (lanes 1 to 4). N2a cells were also treated with 500 μM Mβ-CD at the time of passage for a period of 72 h or cells were left untreated (lanes 5 to 8). The cells were then lysed, the lysate was prepared for the TX-100 solubility assay, and then soluble (S) and insoluble (IS) fractions were separated. Samples were then analyzed for PrPC by 12% SDS-PAGE and immunoblotting with 8H4 antibody. (C) Representative bands shown in panel B were quantified by densitometry and were expressed as percent soluble and percent insoluble. Black bars represent the percentage of soluble PrPC, and white bars represent the percentage of insoluble PrPC. Results are representative of at least three independent experiments. Molecular mass markers in kilodaltons are shown on the left of the panels.
β-CD and Mβ-CD are known to complex cholesterol and have been employed by others to modify cholesterol levels (16). In light of the ability of chol-Mβ-CD to introduce cholesterol into the cell, if the CDs were working solely by removing cholesterol, it was surprising that chol-Mβ-CD could not reverse the antiprion effect in the experiment for Fig. 6. In the past, lovastatin (63) and squalastatin (5), compounds working by reducing cholesterol levels, have moved PrPC from cholesterol-rich lipid rafts, which are insoluble in cold Triton X-100, to domains that are soluble. To reconfirm our density gradient data that PrPC was present in more insoluble domains after β-CD treatment, the effect of Mβ-CD on PrPC location was examined (Fig. 7B and C). As for β-CD, Mβ-CD moved PrPC to more insoluble domains (data not shown). This effect of CDs was reconfirmed by a simple solubility assay. Confluent N2a cells were grown in the presence and absence of 500 μM Mβ-CD for 1 h, and the solubility of PrPC in cold Triton X-100 was determined. The majority of PrPC was insoluble, and treatment with Mβ-CD did not redistribute PrPC to soluble domains (Fig. 7B, lanes 3 and 4, and C). Cells were also grown from the time of passage in the presence and absence of Mβ-CD for 72 h, and again PrPC was maintained in the insoluble fraction (Fig. 7B, lanes 7 and 8, and C) and became slightly more insoluble.
β-CD binding to PrPC.
With CDs moving PrPC to domains of lower density in sucrose density experiments, an effect that chol-Mβ-CD could not inhibit, it is possible that CDs (through cholesterol movement) could facilitate the generation of some altered density compartments to which PrPC, and not PrPSc, is directed. Alternatively, PrPC may be directed by these compounds to naturally existing high-lipid domains as a consequence of drug presence. Due to their hydrophobic cores, CDs possess the ability to encapsulate hydrophobic moieties. If CDs bound PrP, this binding could aid in the antiprion mechanism. Consequently, the ability of β-CD to bind PrPC was determined.
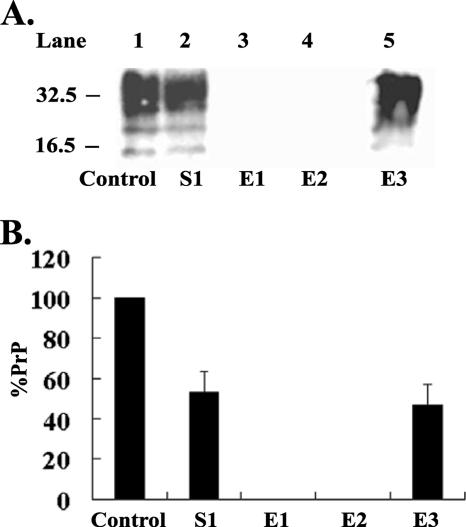
β-CD was bound to epoxy-activated Sepharose 6B as described in Materials and Methods. N2a cells expressing 3F4-tagged MoPrPC were lysed in cold lysis buffer (0.5% [wt/vol] sodium deoxycholate, 0.5% [vol/vol] Triton X-100, 150 mM NaCl and 50 mM Tris, pH 7.5). The lysate was then added to gel containing β-CD that had been equilibrated to pH 7.5 with 0.5 M HEPES buffer, and the binding process was carried out as described in Materials and Methods. Samples were analyzed by 12% SDS-PAGE and immunoblotting with 3F4 antibody. When binding was carried out with β-CD incorporated into the gel, approximately 50% of the 3F4-tagged PrPC bound (Fig. 8A, lane 2, and B) and no PrPC was eluted off in subsequent washes with 0.5 M HEPES buffer, pH 7.5, alone (data not shown). Depending on the strength of binding, it is sometimes possible to elute a bound protein with the complexing agent (β-CD) in solution. However, neither 500 μM β-CD nor 1 mM β-CD in solution could elute PrPC from the gel (Fig. 8A, lanes 3 and 4, and B). Some protein-to-ligand complexes require more stringent methods for elution; therefore, the gel with PrPC bound was boiled in loading buffer, and 12% of the eluate was analyzed for PrPC content. A 100% elution of bound PrPC occurred under these conditions (Fig. 8A, lane 5, and B). On the other hand, when binding was carried out without β-CD incorporated into gel, PrPC did not bind (data not shown).
FIG. 8.
PrPC binding to Sepharose gel containing β-CD. (A) Eight hundred micrograms of lysate from N2a cells expressing 3F4-tagged MoPrPC was loaded onto epoxy-activated Sepharose 6B gels prepared with β-CD. The gels were processed as described previously. The following were analyzed for PrPC: 2.25% (18 μg) of cell lysate (control), equivalent level (2.25%) of protein in unbound material (S1), 100% β-CD eluents (E1 and E2), and 12% of final eluent (E3). PrPC in these fractions was analyzed by 12% SDS-PAGE and immunoblotting with 3F4 antibody. (B) Representative bands shown in panel A were quantified by densitometry, and PrP levels were adjusted to the level that would be present if 100% samples were loaded. The percentage of PrP was then plotted relative to the control (the level of PrPC prior to binding to the gel). Results are representative of three independent experiments. Molecular mass markers in kilodaltons are shown on the left of the panels. Error bars indicate standard deviations.
CDs inhibit an in vitro conversion of PrP.
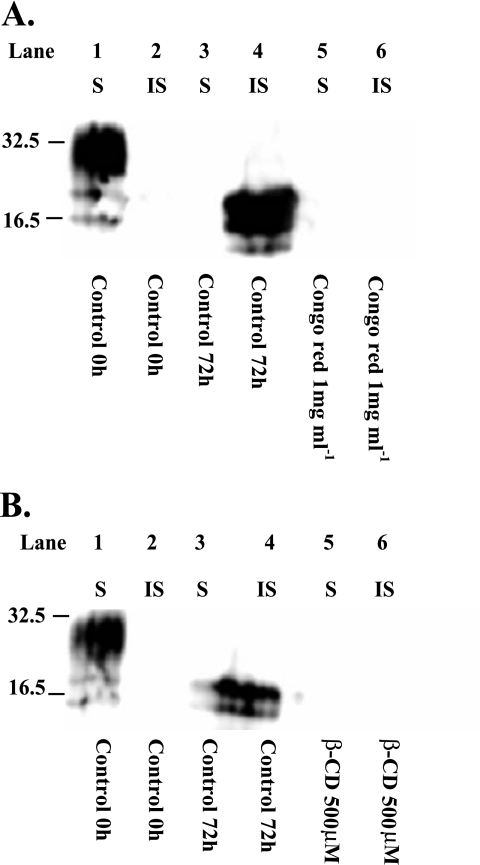
As CDs have been reported to modify protein conformation (50) and as PrPC bound to β-CD, the ability of β-CD to interfere with an in vitro conversion assay, based on the abnormal insolubility property of PrPSc, was determined. Cell lysate from N2a22L20 cells and N2a cells expressing 3F4-tagged PrPC were mixed and incubated for up to 72 h as described above, and the conversion of 3F4-tagged PrPC to insoluble 3F4-tagged PrPC was determined. After 72 h, PrPC was converted into insoluble 3F4-tagged PrP (Fig. 9A, lane 4, and B, lane 4) (this was not seen in the absence of N2a22L20 lysate [data not shown]). When the assay was carried out in the presence of Congo red (which is known to interfere with in vitro conversion) and β-CD (500 μM), the in vitro conversion was inhibited (Fig. 9A, lane 6, and B, lane 6, respectively).
FIG. 9.
β-CD blocks the conversion of PrPC to PrPSc. (A and B) Samples were incubated as described in Materials and Methods for 0 h (lanes 1 and 2) or 72 h at 37°C (lanes 3 and 4). Samples were also incubated in the presence of Congo red (1 mg/ml) for 72 h (panel A, lanes 5 and 6) or β-CD (500 μM) (panel B, lanes 5 and 6). After incubation, soluble (S) and insoluble (IS) PrP fractions were separated and levels were then analyzed by 12% SDS-PAGE and immunoblotting with 3F4 antibody.
DISCUSSION
CDs, a family of cyclic maltooligosaccharides, have been used for decades as complexing agents for humans. The glucose units of these compounds are linked by α-1-4 bonds, and due to the structural orientation of the glucopyranose units, a hydrophobic cavity, which can facilitate the encapsulation of hydrophobic moieties, is generated (58, 61). This report demonstrates that CDs also possess antiprion activity, which appeared to be due to their ability to sequester compounds.
The modulation of cholesterol levels with Mβ-CD on its own has been shown to alter the susceptibility of cells to Aβ toxicity in PC12 cells (3) and, when Mβ-CD is combined with lovastatin, to modify Aβ production in hippocampal cells (57). In this report, the capacity of β-CD to clear PrPSc from infected cell culture is demonstrated. The antiprion activity of β-CD was time dependent, with PrPSc clearance in N2a22L cells occurring in 2 weeks with 500 μM β-CD. Structural studies revealed the cyclic nature of the CDs to be important to the antiprion activity, and this is reflected in the IC50 for β-CD (75 μM), which was 10 times lower than that for α-CD (750 μM). The linear form of β-CD also reduced PrPSc levels, but as it could not clear PrPSc, we infer that cyclicization of the molecule is significant for activity.
The antiprion action of β-CD did not appear to relate to a reduction in the substrate PrPC within the cell for conversion. However, Mβ-CD (which is also reported here, for the first time, to have antiprion activity) has recently been reported to facilitate the shedding of PrPC from neuronal cells (42). This shedding of PrPC could have the potential to prevent conversion by reducing the access of PrPSc to PrPC. This possibility would be in line with the report stating that the release of PrP from the plasma membrane by phosphatidylinositol-specific phospholipase C (13) prevents PrPSc formation. Additionally, although it has been reported that known antiprion compounds pass through endosomes and lysosomes and act at these compartments to interfere with PrPSc production (60, 62), β-CD does not appear to act through these compartments, as the lysomotropic agent NH4Cl could not inhibit the action of β-CD.
It is more likely that the antiprion activity of CDs relates to their ability to sequester and move molecules. CDs are known for their ability to remove cholesterol from cell membranes (40, 57), and as cholesterol levels affect PrPSc production (63), it is possible that their action may be through an effect on the lipid raft environment where both PrPC and PrPSc reside. Rafts are composed of sphingolipids and cholesterol, and the latter is thought to act as a spacer between the sphingolipids, keeping the lipid raft domain together (55). These domains house a number of protein networks, including an array of GPI-anchored proteins, and the ability of the domains to concentrate proteins/molecules may facilitate protein-to-protein interactions, including the conversion of PrPC to PrPSc (38, 63). Cholesterol extraction with Mβ-CD has been reported to dissociate proteins from rafts and to move proteins from Triton X-100 insoluble to soluble compartments (46). The modulation of the raft environment has also been shown by others to interfere with the PrP conversion. However, reports on this are conflicting. Lovastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-co-enzyme A reductase, which reduces cholesterol levels, reduced prion infection in cell culture (63). Likewise, squalestatin, an inhibitor of cholesterol production, also cured prion-infected neurons (5). Both squalestatin and lovastatin moved PrPC from Triton X-100 insoluble fractions to soluble fractions on extraction at 4°C (5, 63). Mβ-CD did not do this. However, the depletion of sphingolipid with fumonisin B actually increased the formation of PrPSc in neuroblastoma cells and had no effect on the floatation properties of either PrPC or PrPSc (37), suggesting that the modulation of cholesterol and sphingolipids have different effects on the conversion.
Of the natural CDs, β-CD presented with the highest antiprion activity. This result was not surprising, as the ability of CDs to extract cholesterol is highly dependent on CD size, with β-CD being more effective than either α- or γ-CD (33). However, in light of the report of fumonisin B, β-CD's antiprion action, rather than proprion action, was unexpected. CDs are powerful cholesterol effluxors, but whereas α-CD preferentially sequesters phospholipids, β-CD preferentially extracts shingomyelin (33). Of course β-CD and fumonisin B are most probably acting very differently; however, Naslavsky et al. (37) could not rule out whether the effect of fumonisin B on PrPSc related to the level of sphingolipids in rafts or the head group identity.
Unlike fumonisin B, β-CD affected the floatation properties of both PrPC and PrPSc. Prior to treatment with β-CD, PrPC, PrPSc, and the DRM marker GM1 all floated in buoyant fractions (Fig. 5A to F), which is in agreement with the results of other reports (38). However, on β-CD treatment, PrPC and PrPSc were resolved into separate fractions (Fig. 5). PrPSc moved to the less buoyant fractions along with the GM1 marker. This result could be expected as an effect of cholesterol extraction on the location of GPI-anchored proteins, but PrPC moved to more buoyant fractions. This physical separation of PrPC and PrPSc could be sufficient to hinder the conversion process. However, a slight overlap in gradients still existed after treatment, and this overlap could still have allowed some conversion. In keeping with these data, Mβ-CD, which had higher antiprion activity than that of β-CD and had the highest ability to remove cellular cholesterol, failed to move PrPC to domains that were Triton X-100 soluble, but a higher percentage of PrPC was located in domains that were Triton X-100 insoluble after treatment. On extraction of neuroblastoma cells at 37°C prior to density floatation gradients, Naslavsky et al. (38) identified that in certain cell lines and in scrapie brain homogenate, PrPC and PrPSc could be resolved into different fractions, indicating that the two isoforms reside in rafts of different contents. This separation was seen only in certain clones, and the researchers proposed that the lipid content of cellular membranes differed between cell types. It is possible that β-CD affects the lipid content of the rafts hosting PrPC differently than those hosting PrPSc, with PrPC residing in domains of higher lipid content after β-CD treatment. Cholesterol can transfer between different lipid vesicles, and CDs have been reported, by binding cholesterol in water-soluble complexes, to donate cholesterol to cellular membranes (16, 26) and in these locations PrPC would reside.
As CDs have the capacity to sequester (33) and move hydrophobic molecules, the possibility that CDs act via a combination of mechanisms cannot be ruled out. β-CD binds PrPC in affinity chromatography, and although this binding would not be specific to PrPC, as CDs can bind to a range of proteins (21), this binding may play a part in its action. CDs have also been reported to modify protein conformation (18). CDs interfere with insulin self-association (67) and the toxicity of Aβ (10). Qin et al. (50) demonstrated the ability of β-CD to inhibit the Aβ peptide 12-28 β-sheet conformational change. Interestingly, in keeping with our antiprion data that are specific for β-CD, only β-CD, and not α-CD or γ-CD, possessed the ability to inhibit the structural conversion of Aβ. It could be hypothesized that β-CD may interact with the hydrophobic central core of PrP; such an interaction could lead to the ability of β-CD to interfere with the abnormal conversion.
The successfulness of antiprion compounds is dependent on a number of factors, including their ability to pass the BBB. CDs have been used for a long time to increase the solubility of compounds and to aid drug delivery. They are biocompatible, show resistance to human enzymes, have low toxicity in humans, and do not elicit an immune response (18). One millimolar of β-CD has been reported to pass the BBB without affecting the tight junctions; a tight junction breakdown has only been observed to start at 5 mM β-CD (33). Consequently, β-CD's antiprion IC50 of 75 μM falls well below the toxic level reported for this compound. CDs are a new class of antiprion compounds, and their usage has the potential to open up a number of avenues in the search for novel therapeutics in TSEs.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Abulrob, A., J. S. Tauskela, G. Mealing, E. Brunette, K. Faid, and D. Stanimirovic. 2005. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-D-aspartate receptor redistribution. J. Neurochem. 92:1477-1486. [DOI] [PubMed] [Google Scholar]
- 2.Adjou, K. T., R. Demaimay, J. P. Deslys, C. I. Lasmezas, V. Beringue, S. Demart, F. Lamoury, M. Seman, and D. Dormont. 1999. MS-8209, a water-soluble amphotericin B derivative, affects both scrapie agent replication and PrPres accumulation in Syrian hamster scrapie. J. Gen. Virol. 80:1079-1085. [DOI] [PubMed] [Google Scholar]
- 3.Arispe, N., and M. Doh. 2002. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AbetaP (1-40) and (1-42) peptides. FASEB J. 16:1526-1536. [DOI] [PubMed] [Google Scholar]
- 4.Barenholz, Y. 2004. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell. Biochem. 37:167-215. [DOI] [PubMed] [Google Scholar]
- 5.Bate, C., M. Salmona, L. Diomede, and A. Williams. 2004. Squalestatin cures prion-infected neurons and protects against prion neurotoxicity. J. Biol. Chem. 279:14983-14990. [DOI] [PubMed] [Google Scholar]
- 6.Bendheim, P. E., H. R. Brown, R. D. Rudelli, L. J. Scala, N. L. Goller, G. Y. Wen, R. J. Kascsak, N. R. Cashman, and D. C. Bolton. 1992. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology 42:149-156. [DOI] [PubMed] [Google Scholar]
- 7.Boussif, O., F. Lezoualc'h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix, and J. P. Behr. 1995. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 92:7297-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, D. A., and E. London. 1997. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 240:1-7. [DOI] [PubMed] [Google Scholar]
- 9.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri, P., N. J. Haskins, and D. R. Howlett. 1994. Beta-cyclodextrin interacts with the Alzheimer amyloid beta-A4 peptide. FEBS Lett. 341:256-258. [DOI] [PubMed] [Google Scholar]
- 11.Caughey, B., D. Ernst, and R. E. Race. 1993. Congo red inhibition of scrapie agent replication. J. Virol. 67:6270-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey, B., and R. E. Race. 1992. Potent inhibition of scrapie-associated PrP accumulation by Congo red. J. Neurochem. 59:768-771. [DOI] [PubMed] [Google Scholar]
- 13.Caughey, B., and G. J. Raymond. 1991. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J. Biol. Chem. 266:18217-18223. [PubMed] [Google Scholar]
- 14.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95:12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerneus, D. P., E. Ueffing, G. Posthuma, G. J. Strous, and A. van der Ende. 1993. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. Role of cholesterol. J. Biol. Chem. 268:3150-3155. [PubMed] [Google Scholar]
- 16.Christian, A. E., M. P. Haynes, M. C. Phillips, and G. H. Rothblat. 1997. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38:2264-2272. [PubMed] [Google Scholar]
- 17.Cohen, F. E., and S. B. Prusiner. 1998. Pathologic conformations of prion proteins. Annu. Rev. Biochem. 67:793-819. [DOI] [PubMed] [Google Scholar]
- 18.Davis, M. E., and M. E. Brewster. 2004. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3:1023-1035. [DOI] [PubMed] [Google Scholar]
- 19.Gilch, S., K. F. Winklhofer, M. H. Groschup, M. Nunziante, R. Lucassen, C. Spielhaupter, W. Muranyi, D. Riesner, J. Tatzelt, and H. M. Schatzl. 2001. Intracellular re-routing of prion protein prevents propagation of PrP(Sc) and delays onset of prion disease. EMBO J. 20:3957-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haensler, J., and F. C. Szoka, Jr. 1993. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem. 4:372-379. [DOI] [PubMed] [Google Scholar]
- 21.Ishimura, K., K. Fukunaga, T. Irie, K. Uekama, T. Ohta, and H. Nakamura. 1997. Application of a beta-cyclodextrin sulfate-immobilized precolumn to selective on-line enrichment and separation of heparin-binding proteins by column-switching high-performance liquid chromatography. J. Chromatogr. 769:209-214. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko, K., M. Vey, M. Scott, S. Pilkuhn, F. E. Cohen, and S. B. Prusiner. 1997. COOH-terminal sequence of the cellular prion protein directs subcellular trafficking and controls conversion into the scrapie isoform. Proc. Natl. Acad. Sci. USA 94:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolesnick, R. 2002. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Investig. 110:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladogana, A., P. Casaccia, L. Ingrosso, M. Cibati, M. Salvatore, Y. G. Xi, C. Masullo, and M. Pocchiari. 1992. Sulphate polyanions prolong the incubation period of scrapie-infected hamsters. J. Gen. Virol. 73:661-665. [DOI] [PubMed] [Google Scholar]
- 25.Ledesma, M. D., K. Simons, and C. G. Dotti. 1998. Neuronal polarity: essential role of protein-lipid complexes in axonal sorting. Proc. Natl. Acad. Sci. USA 95:3966-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leventis, R., and J. R. Silvius. 2001. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys. J. 81:2257-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, R., T. Liu, B. S. Wong, T. Pan, M. Morillas, W. Swietnicki, K. O'Rourke, P. Gambetti, W. K. Surewicz, and M. S. Sy. 2000. Identification of an epitope in the C terminus of normal prion protein whose expression is modulated by binding events in the N terminus. J. Mol. Biol. 301:567-573. [DOI] [PubMed] [Google Scholar]
- 28.Mahfoud, R., N. Garmy, M. Maresca, N. Yahi, A. Puigserver, and J. Fantini. 2002. Identification of a common sphingolipid-binding domain in Alzheimer, prion, and HIV-1 proteins. J. Biol. Chem. 277:11292-11296. [DOI] [PubMed] [Google Scholar]
- 29.Mangé, A., N. Nishida, O. Milhavet, H. E. McMahon, D. Casanova, and S. Lehmann. 2000. Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected cultures. J. Virol. 74:3135-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May, B. C., A. T. Fafarman, S. B. Hong, M. Rogers, L. W. Deady, S. B. Prusiner, and F. E. Cohen. 2003. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc. Natl. Acad. Sci. USA 100:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer, R. K., M. P. McKinley, K. A. Bowman, M. B. Braunfeld, R. A. Barry, and S. B. Prusiner. 1986. Separation and properties of cellular and scrapie prion proteins. Proc. Natl. Acad. Sci. USA 83:2310-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizoguchi, T., T. Edano, and T. Koshi. 2004. A method of direct measurement for the enzymatic determination of cholesteryl esters. J. Lipid Res. 45:396-401. [DOI] [PubMed] [Google Scholar]
- 33.Monnaert, V., S. Tilloy, H. Bricout, L. Fenart, R. Cecchelli, and E. Monflier. 2004. Behavior of alpha-, beta-, and gamma-cyclodextrins and their derivatives on an in vitro model of blood-brain barrier. J. Pharmacol. Exp. Ther. 310:745-751. [DOI] [PubMed] [Google Scholar]
- 34.Mouillet-Richard, S., M. Ermonval, C. Chebassier, J. L. Laplanche, S. Lehmann, J. M. Launay, and O. Kellermann. 2000. Signal transduction through prion protein. Science 289:1925-1928. [DOI] [PubMed] [Google Scholar]
- 35.Müller, W. E., J. L. Laplanche, H. Ushijima, and H. C. Schroder. 2000. Novel approaches in diagnosis and therapy of Creutzfeldt-Jakob disease. Mech. Ageing Dev. 116:193-218. [DOI] [PubMed] [Google Scholar]
- 36.Müller, W. E., U. Scheffer, S. Perovic, J. Forrest, and H. C. Schroder. 1997. Interaction of prion protein mRNA with CBP35 and other cellular proteins: possible implications for prion replication and age-dependent changes. Arch. Gerontol. Geriatr. 25:41-58. [DOI] [PubMed] [Google Scholar]
- 37.Naslavsky, N., H. Shmeeda, G. Friedlander, A. Yanai, A. H. Futerman, Y. Barenholz, and A. Taraboulos. 1999. Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. J. Biol. Chem. 274:20763-20771. [DOI] [PubMed] [Google Scholar]
- 38.Naslavsky, N., R. Stein, A. Yanai, G. Friedlander, and A. Taraboulos. 1997. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J. Biol. Chem. 272:6324-6331. [DOI] [PubMed] [Google Scholar]
- 39.Nishida, N., D. A. Harris, D. Vilette, H. Laude, Y. Frobert, J. Grassi, D. Casanova, O. Milhavet, and S. Lehmann. 2000. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 74:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtani, Y., T. Irie, K. Uekama, K. Fukunaga, and J. Pitha. 1989. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186:17-22. [DOI] [PubMed] [Google Scholar]
- 41.Pan, K. M., M. Baldwin, J. Nguyen, M. Gasset, A. Serban, D. Groth, I. Mehlhorn, Z. Huang, R. J. Fletterick, F. E. Cohen, et al. 1993. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 90:10962-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkin, E. T., N. T. Watt, A. J. Turner, and N. M. Hooper. 2004. Dual mechanisms for shedding of the cellular prion protein. J. Biol. Chem. 279:11170-11178. [DOI] [PubMed] [Google Scholar]
- 43.Peretz, D., R. A. Williamson, K. Kaneko, J. Vergara, E. Leclerc, G. Schmitt-Ulms, I. R. Mehlhorn, G. Legname, M. R. Wormald, P. M. Rudd, R. A. Dwek, D. R. Burton, and S. B. Prusiner. 2001. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412:739-743. [DOI] [PubMed] [Google Scholar]
- 44.Perrier, V., K. Kaneko, J. Safar, J. Vergara, P. Tremblay, S. J. DeArmond, F. E. Cohen, S. B. Prusiner, and A. C. Wallace. 2002. Dominant-negative inhibition of prion replication in transgenic mice. Proc. Natl. Acad. Sci. USA 99:13079-13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrier, V., A. C. Wallace, K. Kaneko, J. Safar, S. B. Prusiner, and F. E. Cohen. 2000. Mimicking dominant negative inhibition of prion replication through structure-based drug design. Proc. Natl. Acad. Sci. USA 97:6073-6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pralle, A., P. Keller, E. L. Florin, K. Simons, and J. K. Horber. 2000. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148:997-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503-1506. [DOI] [PubMed] [Google Scholar]
- 48.Prusiner, S. B. 1991. Molecular biology of prion diseases. Science 252:1515-1522. [DOI] [PubMed] [Google Scholar]
- 49.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin, X. R., H. Abe, and H. Nakanishi. 2002. NMR and CD studies on the interaction of Alzheimer beta-amyloid peptide (12-28) with beta-cyclodextrin. Biochem. Biophys. Res. Commun. 297:1011-1015. [DOI] [PubMed] [Google Scholar]
- 51.Rothberg, K. G., Y. S. Ying, B. A. Kamen, and R. G. Anderson. 1990. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J. Cell Biol. 111:2931-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Safar, J., P. P. Roller, D. C. Gajdusek, and C. J. Gibbs, Jr. 1993. Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J. Biol. Chem. 268:20276-20284. [PubMed] [Google Scholar]
- 53.Scheiffele, P., M. G. Roth, and K. Simons. 1997. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 16:5501-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schröder, H. C., and W. E. Muller. 2002. Neuroprotective effect of flupirtine in prion disease. Drugs Today 38:49-58. [DOI] [PubMed] [Google Scholar]
- 55.Simons, K., and R. Ehehalt. 2002. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 110:597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 57.Simons, M., P. Keller, B. De Strooper, K. Beyreuther, C. G. Dotti, and K. Simons. 1998. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. USA 95:6460-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh, M., R. Sharma, and U. C. Banerjee. 2002. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 20:341-359. [DOI] [PubMed] [Google Scholar]
- 59.Stahl, N., D. R. Borchelt, K. Hsiao, and S. B. Prusiner. 1987. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51:229-240. [DOI] [PubMed] [Google Scholar]
- 60.Supattapone, S., H. O. Nguyen, F. E. Cohen, S. B. Prusiner, and M. R. Scott. 1999. Elimination of prions by branched polyamines and implications for therapeutics. Proc. Natl. Acad. Sci. USA 96:14529-14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szejtli, J. 1998. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98:1743-1754. [DOI] [PubMed] [Google Scholar]
- 62.Taraboulos, A., A. J. Raeber, D. R. Borchelt, D. Serban, and S. B. Prusiner. 1992. Synthesis and trafficking of prion proteins in cultured cells. Mol. Biol. Cell 3:851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taraboulos, A., M. Scott, A. Semenov, D. Avrahami, L. Laszlo, and S. B. Prusiner. 1995. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 129:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor, D. R., and N. M. Hooper. 2006. The prion protein and lipid rafts. Mol. Membr. Biol. 23:89-99. [DOI] [PubMed] [Google Scholar]
- 65.Trojanowski, J. Q., and V. M. Lee. 2000. “Fatal attractions” of proteins. A comprehensive hypothetical mechanism underlying Alzheimer's disease and other neurodegenerative disorders. Ann. N. Y. Acad. Sci. 924:62-67. [DOI] [PubMed] [Google Scholar]
- 66.Uekama, K. 2004. Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. (Tokyo) 52:900-915. [DOI] [PubMed] [Google Scholar]
- 67.Uekama, K., F. Hirayama, and T. Irie. 1998. Cyclodextrin drug carrier systems. Chem. Rev. 98:2045-2076. [DOI] [PubMed] [Google Scholar]
- 68.Vey, M., S. Pilkuhn, H. Wille, R. Nixon, S. J. DeArmond, E. J. Smart, R. G. Anderson, A. Taraboulos, and S. B. Prusiner. 1996. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl. Acad. Sci. USA 93:14945-14949. [DOI] [PMC free article] [PubMed] [Google Scholar]