Abstract
Rationale: Arhgef1 is an intracellular protein, expressed by hematopoietic cells, that regulates signaling by both G protein–coupled receptors and RhoA, and, consequently, is required for appropriate migration and adhesion of diverse leukocyte populations.
Objectives: To evaluate a possible contribution for Arhgef1 in the development of airway inflammation and airway hyperreactivity.
Methods: Arhgef1-deficient (Arhgef1−/−) and wild-type (WT) mice were sensitized and airway challenged, followed by measurement of airway responsiveness to inhaled methacholine. Inflammation was assessed by several parameters that included flow cytometric analysis and histology. Arhgef1-deficient recipients were reconstituted with WT T lymphocytes before sensitization and challenge, and again measured for airway responsiveness and inflammation. Cytokine production in response to specific antigen was measured in cultures of isolated leukocytes from lung and spleen and compared with the levels generated in lung and spleen explant cultures.
Measurements and Main Results: Arhgef1−/− mice display significantly reduced airway hyperreactivity, Th2 cytokine production, and lung inflammation, despite intact systemic immunity. After airway challenge of Arhgef1−/− mice, antigen-specific T cells were present in mutant lungs, but were found to interact with CD11c+ cells at a significantly reduced frequency. Adoptive transfer of WT T cells into Arhgef1−/− mice restored airway hyperreactivity and inflammation.
Conclusions: These data demonstrate that T cells depend on Arhgef1 to promote lung inflammation. Moreover, a deficiency in Arhgef1 results in reduced T cell–CD11c+ antigen-presenting cell interaction, and likely underscores the inability of Arhgef1−/− mice to mount an adaptive immune response to airway challenge.
Keywords: airway hyperreactivity, cytokines, lung inflammation, T cells
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Arhgef1 is an intracellular protein that has been shown to regulate migration and adhesion through its regulation of G protein–coupled receptor signaling and RhoA activation.
What This Study Adds to the Field
T lymphocytes require Arhgef1 for airway hyperreactivity and inflammation. In the absence of Arhgef1, T cells interact with antigen-presenting cells in the lungs at a reduced frequency.
Asthma is a disease characterized by reversible airway hyperresponsiveness (AHR) and inflammation of the airways. Mouse models of airway hyperreactivity share many features with this human disease that include reversible airway hyperreactivity, goblet cell metaplasia with subsequent enhanced mucus production, increased presence of CD4+ T cells and levels of Th2 cytokines, and eosinophilic inflammation (1). Both T cells and dendritic cells have been shown to participate in asthma, and are present in the lungs of both individuals with asthma and mouse models of airway hyperreactivity (1–3). Importantly, the physical interaction between dendritic cells and T cells within the lung has been shown to promote the development of both airway hyperreactivity and inflammation (4, 5).
In an inflammatory response, leukocytes are recruited to the lung by chemoattractant signaling through cognate G protein–coupled receptors (GPCRs). Specifically, signaling by Gαi-associated chemoattractant receptors leads to activation of Rac or Cdc42 Rho family member GTPases at the leading edge of the cell, and formation of lamellipodia or filopodia, respectively, that attach via integrin-mediated adhesion to supporting substrate (6, 7). In contrast, GPCRs signaling through Gα12/13-associated subunits leads to activation of RhoA at the trailing edge and actin–myosin filament contraction (8). In motile cells, such as lymphocytes and neutrophils, integrin adhesion at the leading and trailing edge must be concomitantly regulated to permit forward movement, integrin endocytosis, and recycling (9). Thus, cell migration is accomplished through Gα-associated receptor signaling pathways that coordinately activate Rho family GTPases and regulate integrin adhesion.
In hematopoietically restricted cells, the Arhgef1 protein, also known as Lsc, acts as an effector of Gα12/13-associated GPCR signaling to activate RhoA (10, 11). Arhgef1 harbors a regulator of G-protein signaling (RGS) domain that associates with Gα12/13 subunits and accelerates Gα GTPase activity (11, 12). In addition, as a consequence of the interaction between the Arhgef1 regulator of G-protein signaling domain and Gα13, Arhgef1 RhoGEF activity is stimulated, and leads to RhoA activation (10, 13). In accord, neutrophils and lymphocytes deficient in Arhgef1 display migration and adhesion defects (14, 15), and Arhgef1−/− marginal zone B cells have been shown not to efficiently resolve integrin-mediated adhesion during migration on integrin ligands (15).
Arhgef1 is expressed by all hematopoietic cells examined (16–19), and we anticipated that additional Arhgef1−/− leukocyte populations might similarly manifest migration defects. Because the migration of leukocytes to and within the lungs is important in allergen-induced airway hyperreactivity and inflammation, we exploited a mouse model of airway inflammation to determine if Arhgef1 participates in the development of allergic lung inflammation. Here, we show that Arhgef1−/− mice do not develop airway hyperreactivity or associated eosinophil lung inflammation despite an intact primary systemic immune response. However, transfer of wild-type (WT) T cells to Arhgef1−/− mice restores the allergic lung disease phenotype, demonstrating that T cells require Arghef1 to facilitate airway inflammation and hyperreactivity. Moreover, Arhgef1−/− T cells and antigen-presenting cells are found to interact at significantly reduced frequencies in lung tissue, and we suggest likely accounts for this defect.
METHODS
Animals
C57BL/6 mice were maintained at the National Jewish Biological Resource Center. Arhgef1−/− animals were generated as described (15). Ly5.2/CR mice (NCI, Rockville, MD) were used for adoptive transfer studies. Animals were used between 8 and 12 weeks of age, and manipulations were performed in accordance with the institutional animal care and use committee.
Ovalbumin Sensitization and Challenge
WT and Arhgef1−/− mice were sensitized by intraperitoneal injection of 20 μg of ovalbumin (OVA) (grade V; Sigma-Aldrich, St. Louis, MO) emulsified in 2.25 mg of alum (Imject Alum; Pierce, Rockford, IL) on Days 0 and 14 of the sensitization protocol. Mice were challenged by inhalation with OVA (1% in saline) aerosols produced by ultrasonic nebulizer (particle size, 1–5 μm; Omron, Kyoto, Japan) for 20 minutes on Days 28–30. On Day 32, airway function was measured.
Assessment of Airway Function
Airway function was assessed as described previously (20). Briefly, mice were anesthetized, tracheostomized, and then ventilated, and lung resistance (Rl) and dynamic compliance (Cdyn) measured after exposure to increasing doses of inhaled methacholine (MCh), as previously described (21).
Bronchoalveolar Lavage
Lungs were lavaged with Hanks' balanced salt solution, counted, and stained with Leukostat (Fisher Diagnostics, Pittsburgh, PA), as previously described (22).
Determination of OVA-specific IgE
Blood was collected on Days 0, 14, and 27 of the OVA sensitization protocol and OVA-specific antibody levels measured by ELISA, as described previously (22).
Lung Leukocyte Isolation and Culture
Lungs and spleen were harvested from either naive animals or 48 hours after the last airway challenge. Leukocytes were isolated from perfused lungs after treatment with collagenase types II and IV (Sigma-Aldrich) and dispase II (Roche, Basel, Switzerland), as previously described (23), and splenocytes were isolated from spleens of the same mice. Leukocytes pooled from three mice were resuspended at 107/ml and distributed in a 96-well plate at 200 μl/well in RPMI 1640 media (Mediatech, Herndon, VA), and cultured for 24 hours in media, OVA (100 μg/ml), or anti-CD3 (145–2C11) and anti-CD28 (37.51) monoclonal antibodies (1 μg/ml each).
Proliferation Assay
Isolated lung and spleen leukocytes were cultured in RPMI media with 15 mM HEPES with or without 100 μg/ml OVA for 5 or 3 days, respectively, and as previously described (24).
Organ Explant Cultures
Organ explant cultures were established as previously described (25). Briefly, each set of lungs from four animals were cut into 16 equally sized fragments (64 total), 4 randomly chosen fragments used for each condition, and distributed into 24-well plates. In this manner, we ensured that each condition had fragments that contained similar structural composition. Spleens harvested from the same mice were cut into four equally sized fragments, and one randomly selected fragment was used for each condition. Cultures were incubated in AIM V serum-free medium (GIBCO/Invitrogen, Grand Island, NY) for 24 hours. Cultures were treated with OVA or anti-CD3/CD28, as described previously here. Supernatants were collected at 24 hours and assayed by ELISA for cytokines, and tissue was then dried and weighed. Data are expressed as pg cytokine/mg dry tissue weight.
Cytokine ELISAs
Levels of IL-4, IL-5, IL-10, IFN-γ (BD Pharmingen, San Diego, CA), and IL-13 (R&D Systems, Minneapolis, MN) in the bronchoalveolar lavage (BAL) and cell culture supernatants were measured by ELISA, as previously described (22).
Lung Leukocyte Staining
Isolated lung leukocytes were resuspended in phosphate-buffered saline (PBS), 2% bovine serum albumin, and 0.1% sodium azide, and incubated with antibodies against CD3ε (145–2C11), CD4 (L3T4), CD69 (H1.2F3) (eBiosciences, San Diego, CA), CD8a (53-6.7), CD11b (M1/70), CD11c (HL3), CD80 (B7.1, 16–10A1), and CD86 (B7.2, GL1), (BD Pharmingen). Commercial antibodies were directly conjugated to fluorochromes. Ly5.2 (CD45.2, 104-2.1) was purified from hybridoma and fluorescein isothiocyanate conjugated. Data was collected with a FACSCalibur (BD Pharmingen). All flow-cytometric data were analyzed with FlowJo 4.4.3 software (Tree Star, Inc., Ashland, OR).
Adoptive Transfer
Unstimulated T cells were purified by negative selection from spleens from donor mice using immunomagnetic cell sorting (MACS; Miltenyi Biotec, Auburn, CA). Specifically, cells were incubated with biotin-conjugated antibodies against CD45R (B220), CD11b (Mac1), CD49b (DX5), and Ter-119, then incubated with antibiotin microbeads, and separated using an AutoMacs magnetic cell sorter (Miltenyi Biotec). Cells were transferred via intravenous injection of 1 × 107 cells in 150 μl of sterile 1× PBS. Purified T cells from WT and Arhgef1−/− mice were more than 86% CD3+ T cells. At the time of transfer, recipient mice were immunized with OVA, and the presence of donor cells was assessed in peripheral blood 2 days later. Recipient mice were sensitized again with OVA on Day 14, and mice were airway challenged on Days 28–30, as described previously here. On Day 32, airway function was assessed and lungs cells isolated.
OVA-pulsed Dendritic Cell Transfer
OVA-pulsed dendritic cells were transferred as previously described (26). Briefly, bone marrow was isolated and cultured with granulocyte-macrophage colony–stimulating factor and IL-4 for 7 days, pulsed with OVA on Day 8, and instilled in the tracheas of recipient mice. Six days after transfer, mice were exposed to aerosolized OVA (1% in saline) for 20 minutes per day for three consecutive days. Forty-eight hours after the last challenge, AHR was assessed and BAL fluid was collected.
Histology
Lungs were inflated with OCT compound (Sakura Finetek, Torrance, CA) and frozen on dry ice. Tissue sections, 5-μm thick, were blocked with avidin and biotin block (Vector Laboratories, Burlingame, CA), stained with antibodies against CD3ε (KT3), and CD11c (HL3). Secondary antibodies were SA-Cy3 and F(ab′)2 donkey anti-rat Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA). A total of four sections from each set of lungs from 4 individual mice, with an average of four fields per view on 10× magnification, were evaluated for the number of T cells, dendritic cells, and T cells in contact with dendritic cells. Images were collected and analyzed using the Marianas/Zeiss 200M inverted microscope with SlideBook software (Intelligent Imaging Innovations, Inc., Denver, CO). Hematoxylin and eosin and periodic acid Schiff (PAS) staining were performed as previously described (22). The number of mucus-containing cells (PAS positive) per millimeter of basement membrane was determined in a blinded fashion with a Nikon microscope (Nikon Corp., Melville, NY), as described previously (22, 27).
Statistical Analysis
Values for all measurements are expressed as mean (± SEM). Significance between individual groups was determined by an unpaired, two-tailed Student's t test, unless indicated. Significance levels were set at a p value of 0.05 or less.
RESULTS
Arhgef1−/− Mice Display Significantly Reduced Antigen-induced AHR
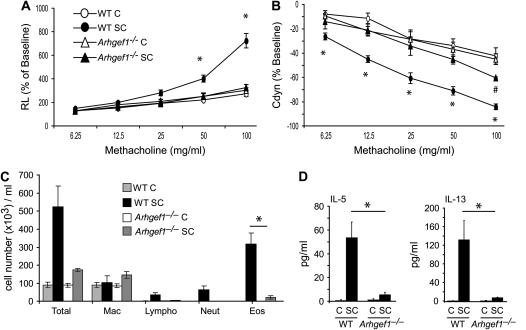
To investigate whether Arhgef1 participates in antigen-mediated lung inflammation, we evaluated Arhgef1−/− mice using a well characterized model of AHR and lung inflammation (21). Specifically, we examined the change in Rl and Cdyn of WT and Arhgef1−/− mice in response to increasing doses of inhaled MCh after sensitization and three challenges with OVA (Figures 1A and 1B). WT mice immunized intraperitoneally with OVA and subsequently challenged with aerosolized OVA developed substantial alterations in lung function as measured by significant increases in Rl (Figure 1A) and decreases in Cdyn (Figure 1B). These changes are not observed in WT animals that are challenged but not sensitized to OVA. In contrast, Arhgef1−/− mice sensitized and challenged with OVA did not develop a significant increase in Rl in response to MCh, behaving similar to challenged-only Arhgef1−/− and WT mice (Figure 1A). Furthermore, changes in Cdyn exhibited by sensitized and challenged Arhgef1−/− mice were similar to challenged-only animals at all MCh concentrations. These data show that the Arhgef1−/− mice did not develop a significant AHR response after a sensitization and challenge with OVA.
Figure 1.
Airway function and inflammation is impaired in Arhgef1−/− mice in response to ovalbumin (OVA) systemic sensitization and subsequent airway challenge. (A) Lung resistance (Rl) and (B) dynamic compliance of wild-type (WT) and Arhgef1−/− mice in response to increasing concentrations of aerosolized methacholine after sensitization and challenge (SC) or challenge only (C). Symbols represent the geometric mean and error bars show SEM. WT C, n = 7; WT SC, n = 10; Arhgef1−/− C, n = 7; Arhgef1−/− SC, n = 4). *p < 0.05 for WT SC compared with Arhgef1−/− SC; #p < 0.05 for Arhgef1−/− SC compared with challenged only. (C) Leukocyte numbers and (D) cytokine levels in bronchoalveolar lavage (BAL) from sensitized and challenged or challenged-only WT and Arhgef1−/− mice. *p < 0.05.
Minimal Lung Inflammation in Arhgef1−/− Mice after Sensitization and Challenge
Impaired lung function is often associated with inflammatory cell infiltrate; thus, we questioned whether the lack of AHR response in Arhgef1−/− mice was reflected by perturbations in inflammatory cell infiltration of the airway and lungs. To assess this, leukocytes were enumerated in BAL and enzymatic digests of lung from sensitized and challenged animals (Figures 1C and 2C). In WT mice, after sensitization and challenge, we detected more than a fivefold increase in the total number of leukocytes in BAL, which was composed primarily of eosinophils (Figure 1C). In contrast, sensitized and challenged Arhgef1−/− mice had negligible inflammatory cell infiltration, as indicated by the less than twofold increase in the number of total lung leukocytes, and that were predominantly macrophages.
We next examined WT and Arhgef1-deficient BAL for the presence of Th2 cytokines, which are normally elevated in allergic lung disease (28). After sensitization and challenge, WT BAL had a fourfold increase in the level of IL-4 (challenged only, 11.6 ± 6.4 pg/ml; sensitized and challenged, 47.5 ± 10.3 pg/ml) and significant increases in IL-5 and IL-13 Th2 cytokine levels compared with challenged-only WT mice (Figure 1D). BAL from sensitized and challenged Arhgef1−/− mice, in contrast, did not show increased levels of IL-4 (challenged only, 20.8 ± 5.5 pg/ml; sensitized and challenged, 19.2 ± 3.3 pg/ml), and only minimally increased levels of IL-5 and IL-13. All Th2 cytokines were similarly expressed in BAL in challenged-only Arhgef1−/− and WT mice. Thus, sensitization and challenge of Arhgef1−/− animals did not result in significant increases in BAL Th2 cytokine levels, and paralleled the lack of AHR and absence of inflammatory cell accumulation in the airways. Furthermore, while the levels of the Th1 cytokines IL-12 and IFN-γ decreased in BAL from WT sensitized and challenged mice, BAL from Arhgef1−/− mice did not show significant changes in the level of either of these cytokines after sensitization and challenge or with challenge alone (data not shown).
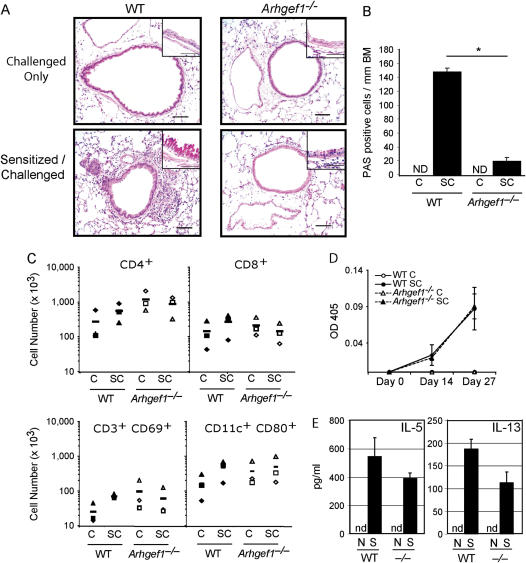
Consistent with numbers of inflammatory cells detected in BAL, histologic examination of WT lung sections showed inflammatory cell infiltrate in the perivascular and peribronchial areas only after sensitization and challenge (Figure 2A). In contrast, lungs from Arhgef1−/− mice that were sensitized and challenged with OVA displayed minimal inflammatory infiltration in the perivascular and peribronchial area, and were similar to challenged-only Arhgef1−/− mice. Furthermore, the large airways from sensitized and challenged WT mice contained sevenfold more PAS-positive cells per millimeter of basement membrane compared with similar areas from sensitized and challenged Arhgef1−/− mice (Figure 2A, insets, and Figure 2B), highlighting the absence of goblet cell metaplasia and mucus production in mutant animals. No PAS-positive cells were observed in the airways from challenged-only mice of either genotype.
Figure 2.
Sensitized Arhgef1−/− mice mount a normal systemic immune response, but display only minor infiltration in lung after airway challenge. (A) Hematoxylin and eosin–stained lung sections from sensitized and challenged wild-type (WT) and mutant mice. Original magnification of insets: ×100 of periodic acid Schiff (PAS)–stained sections. Bars = 50 μm. (B) Number of PAS-positive cells/mm of basement membrane (BM) were identified from paraffin sections of sensitized and challenged lungs. ND = none detected. *p < 0.05 (C) Number of WT and Arhgef1−/− cells in indicated lung leukocyte populations after sensitization and/or challenge. Leukocytes were isolated from enzymatic lung digests and individual populations identified by flow cytometry, as described in Methods. Filled symbols are WT and open symbols are Arhgef1−/−. CD4 and CD8 T cells identified as CD3+CD4+ or CD3+CD8+, respectively. Symbols represent individual experiments and are the average number from three mice per experiment. (D) OVA-specific IgE levels were determined in serum collected on Days 0, 14, and 27 of the OVA protocol from WT and Arhgef1−/− mice. Open symbols represent challenged-only mice and filled symbols represent sensitized and challenged mice. (E) After sensitization, levels of IL-5 and IL-13 were quantified from supernatants taken from splenocyte cultures. N = naive; S = sensitized; nd = not detected.
These data show that, in contrast to WT mice, sensitization and challenge of Arhgef1−/− mice did not lead to inflammatory cell recruitment to the lungs or airways beyond that observed in challenged-only animals. Similarly, sensitized and challenged mutant mice did not develop significant goblet cell metaplasia or mucus production.
Arhgef1−/− Mice Harbor Elevated Numbers of Lung Leukocytes That Do Not Increase after Sensitization and Challenge
Given the importance of T cells and dendritic cells in the development of allergic lung disease (19–25), we enumerated total and activated CD3+ and CD11c+ cells in the lungs of WT and mutant lungs by flow cytometry. In the lungs of sensitized and challenged WT mice, the average number of each of the CD4+, CD8+, and CD69+ T-cell and CD80+ CD11c+ cell populations increased two- to threefold relative to challenged-only WT mice, although these increases did not reach statistical significance (Figure 2C). In contrast, and consistent with the histologic findings (Figure 2A), lungs from sensitized and challenged Arhgef1−/− mice did not show an increase in cell number of any leukocyte population when compared with challenged-only Arhgef1−/− lungs (Figure 2C).
Of interest, we found the number of T cells and CD80+ CD11c+ cells to be elevated in the lungs of naive (data not shown) and challenged-only Arhgef1−/− mice relative to WT mice (Figure 2C). Furthermore, increased numbers of cells expressing CD11b, CD11c, Gr-1, or B220 antigens were also observed in naive and challenged-only Arhgef1−/− lungs, although flow cytometric analyses demonstrated that they were present at similar proportions as WT (data not shown), indicating that all lung leukocyte cell types are increased in number.
Arhgef1−/− Mice Display an Intact Systemic Primary Immune Response
Although Arhgef1−/− animals do not appear to mount an inflammatory response in the lung upon airway challenge of sensitized animals, we have previously shown that Arhgef1-deficient mice mount normal primary IgG antibody responses against protein antigens (15). Because antigen-specific IgE production is a component of a Th2-dominated allergic lung response (29, 30), we measured antigen-specific IgE production by Arhgef1 mutants after sensitization. Arhgef1−/− mice were able to generate similar titers of OVA-specific IgE and IgG1 after immunization as compared with WT mice (Figure 2D and data not shown). Furthermore, titers of total serum IgE increased significantly after sensitization in both WT and Arhgef1−/− mice (data not shown). These experiments demonstrate that Arhgef1−/− mice are capable of developing a humoral systemic OVA response that includes antigen-specific IgG1 and IgE.
Further evidence for an intact adaptive immune response in mutant animals was revealed by the ability of splenic T cells to produce IL-5 and IL-13 in response to specific OVA antigen after sensitization. In these experiments, OVA treatment of splenocytes isolated from sensitized WT and Arhgef1−/− mice resulted in the production of both IL-5 and IL-13, but not when isolated from naive animals (Figure 2E).
Together, these experiments demonstrate that Arhgef1−/− T and B lymphocytes are functionally activated in a primary immune response to a foreign protein antigen, and that systemic adaptive immunity is intact in Arhgef1−/− mice.
Transfer of WT T Cells into Arhgef1−/− Mice Restores AHR and Lung Inflammation
Arhgef1 is expressed in all hematopoietic cells examined (16–19). Therefore, to identify the cell population(s) dependent on Arhgef1 for the development of allergic lung disease, we performed adoptive transfer experiments. Naive splenic T cells were isolated from Ly5.1+ congenic WT mice and 1 × 107 cells transferred intravenously into recipient Arhgef1−/− and WT mice, both of which express the Ly5.2 allotype. Recipient mice were immunized intraperitoneally with OVA at the same time as cell transfer, and again on Day 14, after which mice were challenged with aerosolized OVA on Days 28–30, and airway function assessed on Day 32. The presence of donor WT Ly5.1+ T cells in host Arhgef1−/− lungs after sensitization and challenge was confirmed by flow cytometric analysis (data not shown).
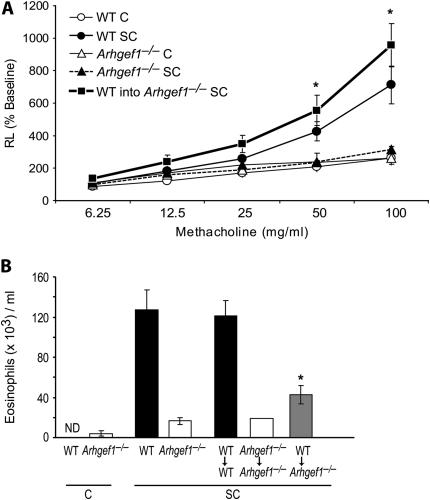
Results from these experiments revealed that transfer of WT T cells into sensitized and challenged Arhgef1−/− mice fully restores the development of AHR to levels observed in WT sensitized and challenged mice, as also determined by increased Rl and decreasing Cdyn in response to inhaled MCh (Figure 3A and data not shown). As control for the adoptive transfer, Arhgef1−/− T cells were adoptively transferred into Arhgef1−/− mice and WT T cells into WT animals, revealing that cell transfer, per se, did not impact AHR compared with the respective sensitized and challenged intact Arhgef1−/− and WT mice, respectively (data not shown). Importantly, the transfer of WT T cells into Arhgef1−/− mice also facilitated a significant increase in the number of eosinophils in the BAL of sensitized and challenged animals relative to intact Arhgef1−/− animals or Arhgef1−/− mice that had received mutant T cells (Figure 3B).
Figure 3.
Reconstitution of Arhgef1−/− mice with wild-type (WT) T cells fully restores the development of airway hyperresponsiveness and partially restores airway eosinophilic inflammation. (A) Change in lung resistance (Rl) after sensitization and/or airway challenge of intact WT and Arhgef1−/− mice and Arhgef1−/− animals reconstituted with naive WT splenic T cells before sensitization and challenge (squares). WT C, n = 3; WT SC, n = 4; Arhgef1−/− C, n = 3; Arhgef1−/− SC, n = 4; WT into Arhgef1−/− SC, n = 7. (B) Number of eosinophils recovered in BAL of intact WT and Arhgef1−/−mice after sensitization and/or challenge and in BAL of recipient WT and Arhgef1−/−mice in which indicated naive donor T cells were transferred before sensitization and challenge. *p < 0.05 by Student's t test when compared with Arhgef1−/− SC.
These data show that WT T cells can promote the development of AHR and associated eosinophil lung infiltration in Arhgef1−/− recipients after sensitization and challenge, and further demonstrate that Arhgef1 expression is required by T cells for this function.
Reduced Interactions between Arhgef1−/− CD3+ and CD11c+ Cells in Lung
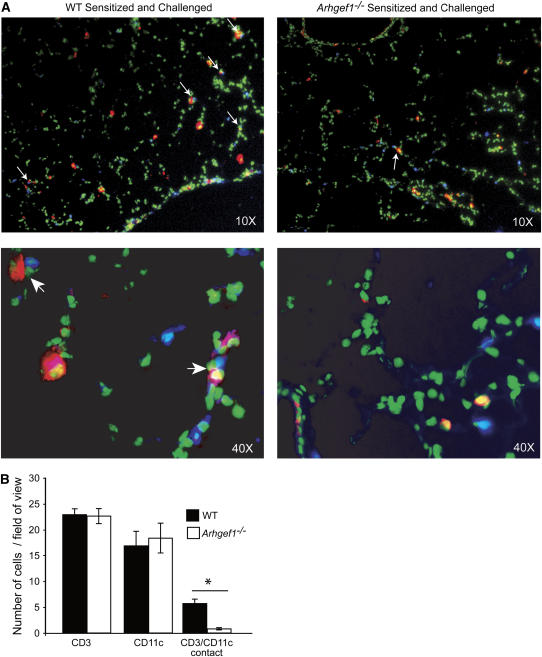
Dendritic cells are required for induction of AHR-associated inflammation, and have been shown to interact with CD4+ T cells in the peribronchial regions after sensitization and airway challenge (31). The ability of WT T cells to restore airway inflammation and AHR to Arhgef1-deficient hosts suggests that Arhgef1−/− dendritic cells are able to productively interact with WT T cells to facilitate a lung inflammatory response. Although we observe T cells in the lungs of Arhgef1−/− mice before and after challenge, these mutant cells are unable to promote airway inflammation. To assess whether Arhgef1−/− T cells and antigen-presenting cells are capable of interacting in situ after sensitization and challenge, a histologic analysis of lung tissue was performed and localization of CD3+ and CD11c+ cells determined. In lung sections from sensitized and challenged WT mice 48 hours after the final challenge, CD3+ T cells were readily observed immediately adjacent to CD11c+ cells in the peribronchial and perivascular regions of the lung (Figure 4A). Sections of the same areas from sensitized and challenged Arhgef1−/− lungs showed significantly fewer CD3+ T cells in immediate proximity to CD11c+ cells. Importantly, the number of CD3+ and CD11c+ cells per field of view was similar in the lungs of WT and Arhgef1−/− mice, but the number of CD3+ cells adjacent to CD11c+ cells was fivefold less in the Arhgef1−/− lungs when compared with WT lungs (Figure 4B).
Figure 4.
Reduced CD3+ and CD11c+ cell interaction in lung tissue of sensitized and challenged Arhgef1−/− mice. (A) Histologic lung sections stained with anti-CD3 (blue) and anti-CD11c (red). Green color is autofluorescence and is used to identify lung tissue. Top panels are ×10 original magnification and bottom panels ×40 original magnification. Arrows indicate CD3+/CD11c+ contacts. (B) Number of CD3+ T cells, CD11c+ cells, and CD3+-CD11c+ cell contacts per 10× field of view in sensitized and challenged wild-type (WT) and Arhgef1−/− mice. Average numbers were obtained from at least four fields of view per section from four different sections per set of lungs and from four individual mice. *p < 0.05 by Student's t test.
These data indicate that, in WT mice, T cells and CD11c+ antigen-presenting cells are often found adjacent to one another after airway challenge, whereas, after sensitization and challenge of Arhgef1−/− mice, despite a similar number of T cells and CD11c+ cells, the number of CD11c+ cells and T cells found adjacent to one another is significantly decreased.
Arhgef1-Deficient Dendritic Cells Are Competent to Facilitate AHR
Results from the above experiments suggested that dendritic cells from Arhgef1−/− mice are competent to present antigen and activate antigen-specific WT T cells, but that interactions between mutant T cells and dendritic cells after challenge of sensitized animals is notably decreased. To more directly evaluate Arhgef1−/− dendritic cell function, we investigated the ability of antigen-pulsed in vitro–derived dendritic cells to induce AHR after intratracheal instillation (26). Thus, bone marrow–derived dendritic cells from WT and Arhgef1−/− mice were pulsed with OVA, washed, and transferred intratracheally to WT mice. Six days after sensitization, recipient mice were then challenged on three consecutive days with aerosolized OVA and evaluated for AHR. These experiments showed that OVA-pulsed dendritic cells from WT and Arhgef1−/− mice were comparable in inducing increases in Rl and decreases in Cdyn and eosinophil-dominated influx into the airspace (data not shown; see Figure E1), confirming the functional competence of Arhgef1−/− dendritic cells in facilitating AHR and associated inflammation in the presence of WT T cells.
Antigen-specific Arhgef1−/− T Cells Are Present in Sensitized and Challenged Lungs and Spleens
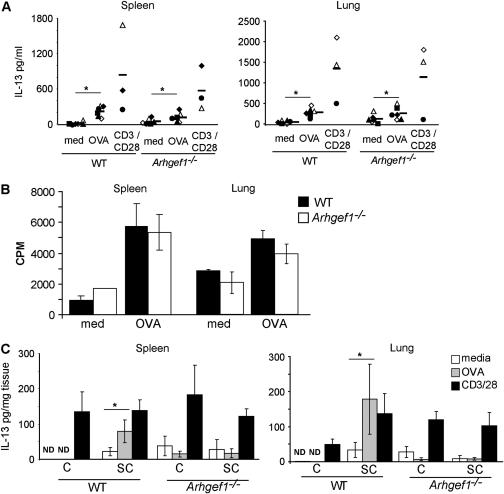
We considered that the lack of a response to an airway antigen challenge might result from the absence of antigen-specific T cells in the lungs of sensitized Arhgef1−/− mice. Alternatively, Arhgef1−/− antigen-specific T cells may be present, but unable to produce cytokines in response to antigen airway challenge. To investigate these possibilities, leukocytes were isolated from spleens and enzymatic lung digests from sensitized and challenged animals and cultured in the presence of media, OVA, or anti-CD3 plus anti-CD28 (CD3/CD28), and supernatants were analyzed for IL-13 production after 24 hours. As expected, leukocytes isolated from sensitized and challenged WT spleens and lungs produced considerable amounts of IL-13 in response to either OVA or anti-CD3/CD28 compared with media control (Figure 5A). Culture supernatants from Arhgef1−/− spleen and lung leukocytes stimulated with either OVA or anti-CD3/CD28 also produced substantial IL-13 and were comparable to those from WT mice. Similar findings were also observed in both control and mutant cultures with regard to IL-5 production (data not shown).
Figure 5.
Arhgef1-deficient antigen-specific Th2 cytokine production by isolated leukocytes but not explant organ cultures. (A) Amount of IL-13 produced in response to media, ovalbumin (OVA) (100 μg/ml), or anti-CD3 + anti-CD28 (both 1 μg/ml) (CD3/CD28) from cultures of isolated leukocytes from sensitized and challenged wild-type (WT) and Arhgef1−/− spleens (left panel) and lungs (right panel). Symbols represent the average response from individual experiments, and the bar is the mean of experiments. *p ⩽ 0.05 by Student's paired, two-tailed t test. (B) Proliferation of WT and Arhgef1−/− lung and spleen leukocytes in response to media or OVA (100 μg/ml). Data from lungs (right side) are representative of two experiments, and data from spleen (left side) represent three experiments. (C) IL-13 production from spleen (left panel) and lung (right panel) explant cultures treated with media, OVA, or anti-CD3/CD28 at same concentrations as described previously here. Values are expressed as pg of cytokine/mg of dry tissue and represent the mean ± SEM of three experiments. *p ⩽ 0.05 by Student's paired, two-tailed t test.
These data indicate that antigen-specific Arhgef1−/− T cells are generated and are present in the spleens and lungs of sensitized and challenged mutant animals 48 hours after the last challenge, and can be stimulated to produce Th2 cytokines.
As an alternate assessment for the presence of antigen-specific T cells in lungs, we measured proliferation in WT and Arhgef1−/− lung leukocyte cultures from sensitized and challenged animals after 5 days in culture with specific antigen. These data revealed that, similar to antigen-specific splenic T cells, OVA induced a similar amount of proliferation between WT and Arhgef1−/− cultures (Figure 5B), providing further support to our conclusion that antigen-specific T cells are present in the lungs of sensitized and challenged Arhgef1−/− and WT mice.
Arhgef1−/− Antigen-specific T Cells Do Not Produce Cytokines in Response to Antigen in Spleen or Lung Explant Cultures
Despite the presence of antigen-specific T cells and functional dendritic cells in the lungs and spleens of Arhgef1−/− mice, lung T cells do not appear to interact with CD11c+ cells, or to facilitate AHR. To better approximate the cellular migration and adhesion events occurring in vivo that are required for promoting Th2 cytokine production and inflammatory responses, we used an explant culture system with lung and spleen tissue from sensitized and/or challenged WT and mutant animals (25). Specifically, lungs and spleens were divided into fragments, distributed into tissue culture plates, and cultured for 24 hours with media alone, OVA, or anti-CD3/CD28, after which supernatants were examined for cytokine production. Importantly, lungs and spleens from sensitized and challenged WT and Arhgef1−/− mice harbor similar numbers and frequencies of T cells, CD11c+ cells, and other leukocyte cell types (Figure 2C and data not shown). To account for the different leukocyte composition in different regions of the lung, lungs from 4 mice were dissected into 16 equal-sized fragments each, mixed, and randomly distributed into cultures. Spleens were dissected into four fragments, and one fragment was randomly selected for each condition.
Consistent with the presence of antigen-specific WT splenic and pulmonary T cells, explant cultures of lung and spleen generated from sensitized and challenged WT mice produced significant levels of IL-13 in response to OVA antigen, but not when cultures were generated from challenged-only mice (Figure 5C). Furthermore, the level of IL-13 produced after OVA stimulation was not significantly different from that elicited from anti-CD3/CD28 treatment for both lung and spleen cultures (Figure 5C). In contrast to WT organ cultures and cultures of isolated Arhgef1−/− cells from spleen and lung (Figure 5A), treatment of sensitized and challenged Arhgef1−/− lung and spleen explant cultures with OVA did not lead to production of IL-13 above that obtained with media alone. Importantly, however, CD3/CD28 stimulation of mutant cultures produced similar levels of IL-13 compared with that of WT explant cultures (Figure 5C). IL-5 was also measured in lung and spleen explant culture supernatants and, similar to IL-13, was increased in WT cultures after either OVA or CD3/CD28 stimulation, whereas IL-5 only increased in Arhgef1−/− lung and spleen explant cultures after CD3/CD28 treatment (data not shown). Consistent with the increased number of T lymphocytes and dendritic cells in challenged-only lungs (Figure 2C), we also found challenged-only Arhgef1−/− cultures to produce similar amounts of IL-13 as sensitized and challenged mutant cultures after CD3/CD28 stimulation. These results show that, after antigen stimulation, cytokine production by antigen-specific Arhgef1−/− T cells within the lung and spleen explant cultures mirrored in vivo BAL cytokine levels, and was in contrast to the in vitro isolated lung and spleen leukocyte cultures. In other words, in the primary response to systemic antigenic challenge, antigen-specific effector Arhgef1−/− T cells are generated and are present in both lung and spleen. These antigen-specific lymphocytes can be further stimulated to produce cytokines in response to specific antigen when isolated and cultured together with antigen-presenting cells, but not when these cell types are together in explant tissue.
DISCUSSION
In the present study, we demonstrate that Arhgef1 contributes to the development of allergic lung inflammation, and specifically establish a T-cell requirement for Arhgef1 in promoting allergic lung disease and eosinophil recruitment to the airways. These data provide further evidence for the participation of heterotrimeric G proteins and RhoA in the regulation of airway inflammation (32, 33), and, considering that Arhgef1 regulates the activity of both classes of G proteins, suggest that Arhgef1 contributes to this regulation. We further demonstrate that, after airway challenge within the lung, Arhgef1−/− T cells interact in situ with functionally normal Arhgef1−/− antigen-presenting cells at a significantly reduced frequency, and this is reflected in the inability of antigen-specific Arhgef1−/− T cells to produce cytokines within tissue explants after antigen stimulation. In contrast, when antigen-specific T cells and antigen-presenting cells are isolated and cultured together, efficient activation of antigen-specific mutant T cells is observed.
T cells, and specifically Th2 responses, play a predominant role in the development of AHR and associated lung inflammation (34), and, in accord, we found an increased number of CD4+ and CD8+ T cells in WT lungs after sensitization and challenge. In contrast, the number of T cells found in lungs of sensitized and challenged Arhgef1−/− mice did not differ significantly from animals that were challenged only, and is consistent with diminished inflammatory cell infiltrate and Th2 cytokines present in BAL after airway challenge of sensitized Arhgef1−/− animals. However, Arhgef1-deficient mice harbor an increased number of all lung leukocyte cell types under both naive and inflammatory conditions, and our data indicate that antigen-specific T cells are included in this expanded population of lung leukocytes. Whether antigen-specific effector T cells are actively recruited during inflammation or are only present as a result of normal homeostatic trafficking was not addressed by these experiments. However, based on previous findings with Arhgef1-deficient marginal zone B cells and neutrophils, we speculate that mutant leukocytes accumulate in the lung as a result of impaired migration (14, 15).
Adaptive immunity is dependent on the activation of antigen-specific T cells by antigen-presenting dendritic cells and, in response to an initial antigen challenge in the airway, dendritic cells are thought to migrate to draining mediastinal lymph nodes to present captured antigen to appropriate T cells (35–37). However, with subsequent antigen challenge, antigen-presenting cell–T-cell interaction within lung tissue has been shown to facilitate Th2 cytokine production and eosinophil recruitment (38–40). In addition, studies in lymphotoxin-α–deficient mice, devoid of lymph nodes and Peyer's patches, demonstrate this in situ T cell–antigen-presenting cell interaction is able to promote lung inflammation (4, 5). Indeed, conditional depletion of pulmonary dendritic cells suggests these cells to be both necessary and sufficient for Th2 cell stimulation during ongoing airway inflammation and after initial antigen presentation (31). Our histologic analysis of lung tissue revealed that 48 hours after airway challenge, T cells were readily observed juxtaposed to CD11c+ antigen-presenting cells in WT lungs and similar to previous findings (38). In contrast, the frequency with which Arhgef1−/− T cells and antigen-presenting cells are observed to interact in mutant lungs is significantly reduced compared with WT.
The transfer of WT T cells to Arhgef1-deficient animals restored both antigen-induced AHR and inflammation, strongly suggesting that Arhgef1−/− dendritic cells are competent in capturing and presenting antigen to T cells. This was confirmed by demonstrating that antigen-pulsed bone marrow–derived Arhgef1−/− dendritic cells are able to promote AHR and leukocyte recruitment after intratracheal transfer into WT animals. Thus, in considering these data together, we suggest that the reduced interaction observed between T cells and CD11c+ cells in the lungs of mutant animals results from deficiency in T-cell function(s) in the absence of Arhgef1. However, we cannot exclude that Arhgef1−/− dendritic cell functional deficiencies exist, but are effectively compensated for by WT, but not mutant, T cells.
In response to systemic immunization with antigen, Arhgef1-deficient animals produced normal titers of antigen-specific IgE and IgG1, and generate antigen-specific splenic T cells (Figure 2), demonstrating that the primary adaptive immune response leads to the appropriate activation of naive antigen-specific B and T cells. In contrast to this, previously sensitized Arhgef1 mouse mutants do not display any evidence of pulmonary inflammation in response to a subsequent airway challenge. Nevertheless, we show that Arhgef1−/− dendritic cells are functional, and that antigen-specific T cells are present in the lungs and spleens of sensitized and challenged animals. When these cell populations are isolated and cultured in vitro together with specific antigen, T cells are induced to produce cytokines and proliferate. This suggests that sensitization and challenge of mutant animals generates functional antigen-specific T cells that are present in the spleen and lungs after airway challenge. However, consistent with the lack of an in vivo immune response to airway antigenic challenge, antigen-specific Arhgef1−/− T cells in spleen and lung organ fragment cultures generated from sensitized and challenged mutants are not activated in the presence of specific antigen. From these data, we concluded that Arhgef1−/− mice mount a normal primary systemic immune response to antigenic challenge that generates antigen-specific T cells capable of migrating to the lung, but that these cells are impaired in effector function when constrained in tissue in vivo or in vitro. Impaired T-cell function in the absence of Arhgef1 is not restricted to the lung, but is also a feature of antigen-specific T cells in the spleen, suggesting that Arhgef1 is not required for a primary systemic response, but is needed by T lymphocytes for efficient activation in subsequent antigenic challenges.
What are the functional deficiencies in effector T cells in the absence of Arhgef1? Arhgef1 is a hematopoietic-restricted intracellular signaling molecule that regulates both GPCR and RhoA signaling (10, 11). Loss of Arhgef1 has been shown to impair neutrophil and B lymphocyte migration (14, 15) and, in particular for marginal zone B cells, leads to aberrant resolution of integrin adhesion during migration (15). We have not yet satisfactorily established similar deficiencies in Arhgef1−/− T cells, but the concept of Arhgef1 playing a similar role in T cells, or a T-cell subset, is supported by the reduced frequency in which T cells and CD11c+ cells are found to interact in the lung and the inability of these cells to be activated in vivo or in vitro organ cultures where migration of these antigen-specific T cells on integrin ligands would be necessary. In contrast, in vitro cultures of isolated lung leukocytes (i.e, cell suspensions) from sensitized and challenged Arhgef1−/− animals demonstrate that mutant T cells can be stimulated to produce Th2 cytokines in an antigen-specific manner. These data demonstrate that antigen-specific Arhgef1−/− T cells are functional when isolated and cultured together with resident antigen-presenting cells, but not when these same cells are constrained by tissue, either in vivo or in vitro. Although speculative, we interpret these findings to suggest that aberrant effector T-cell migration within lung tissue underscores the reduced frequency with which Arhgef1−/− T cells interact with antigen-presenting cells and, consequently, precludes efficient antigen-specific T-cell activation. Furthermore, although we have previously suggested Arhgef1 activation of RhoA to be important in the regulation of integrin adhesion during migration, given the ability of Arhgef1 to also regulate GPCR signaling, Arhgef1−/− T cells may also be impaired in responding to dendritic cell–derived chemoattractants.
In summary, these data document an important role for Arhgef1 in the development of airway hyperreactivity and inflammation, and further demonstrate that effector T cells are dependent on Arhgef1 for promoting an inflammatory response. Moreover, we provide additional evidence that suggests that the inability of antigen-specific T cells to facilitate allergic lung disease is a consequence of inefficient interaction between the mutant T cells and functional CD11c+ cells in the Arhgef1−/− lung. Thus, Arhgef1, a regulator of Gα12/13 and RhoA signaling, plays an important role in regulating effector T-cell function required for the development of airway inflammation.
Supplementary Material
Acknowledgments
The authors thank Pamela Strauch and Anthony Joetham for technical help, and John Hartney and the rest of the Torres/Pelanda lab members for discussion.
Supported by the Sandler Program in Asthma Research (R.M.T.), Deutsche Forschungsgemeinschaft grant Ta 275/2-1 (C.T.), National Institutes of Health grants HL-36577 and HL-61005, and Environmental Protection Agency grant R825702 (E.W.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200702-270OC on April 26, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304. [DOI] [PubMed] [Google Scholar]
- 2.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham S, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis 1990;142:1407–1413. [DOI] [PubMed] [Google Scholar]
- 3.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma: an ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis 1989;140:1745–1753. [DOI] [PubMed] [Google Scholar]
- 4.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest 2002;110:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewska BU, Alvarez D, Vidric M, Goncharova S, Stampfli MR, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Generation of experimental allergic airways inflammation in the absence of draining lymph nodes. J Clin Invest 2001;108:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002;420:629–635. [DOI] [PubMed] [Google Scholar]
- 7.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003;302:1704–1709. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 2003;114:201–214. [DOI] [PubMed] [Google Scholar]
- 9.Cox EA, Huttenlocher A. Regulation of integrin-mediated adhesion during cell migration. Microsc Res Tech 1998;43:412–419. [DOI] [PubMed] [Google Scholar]
- 10.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 ρGEF by gα13. Science 1998;280:2112–2114. [DOI] [PubMed] [Google Scholar]
- 11.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. P115 ρGEF, a GTPase activating protein for Gα12 and Gα13. Science 1998;280:2109–2111. [DOI] [PubMed] [Google Scholar]
- 12.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem 2000;69:795–827. [DOI] [PubMed] [Google Scholar]
- 13.Glaven JA, Whitehead IP, Nomanbhoy T, Kay R, Cerione RA. Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the ρ GTP-binding protein. J Biol Chem 1996;271:27374–27381. [DOI] [PubMed] [Google Scholar]
- 14.Francis SA, Shen X, Young JB, Kaul P, Lerner DJ. Rho GEF Lsc is required for normal polarization, migration, and adhesion of formyl-peptide–stimulated neutrophils. Blood 2006;107:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubtsov A, Strauch P, Digiacomo A, Hu J, Pelanda R, Torres RM. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IGM T-dependent antibody response. Immunity 2005;23:527–538. [DOI] [PubMed] [Google Scholar]
- 16.Aasheim HC, Pedeutour F, Smeland EB. Characterization, expression and chromosomal localization of a human gene homologous to the mouse Lsc oncogene, with strongest expression in hematopoetic tissues. Oncogene 1997;14:1747–1752. [DOI] [PubMed] [Google Scholar]
- 17.Girkontaite I, Missy K, Sakk V, Harenberg A, Tedford K, Potzel T, Pfeffer K, Fischer KD. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat Immunol 2001;2:855–862. [DOI] [PubMed] [Google Scholar]
- 18.Hart MJ, Sharma S, elMasry N, Qiu RG, McCabe P, Polakis P, Bollag G. Identification of a novel guanine nucleotide exchange factor for the rho gtpase. J Biol Chem 1996;271:25452–25458. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead IP, Khosravi-Far R, Kirk H, Trigo-Gonzalez G, Der CJ, Kay R. Expression cloning of Lsc, a novel oncogene with structural similarities to the DBL family of guanine nucleotide exchange factors. J Biol Chem 1996;271:18643–18650. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell–deficient mice. J Exp Med 1997;186:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol 1988;64:2318–2323. [DOI] [PubMed] [Google Scholar]
- 22.Taube C, Duez C, Cui ZH, Takeda K, Rha YH, Park JW, Balhorn A, Donaldson DD, Dakhama A, Gelfand EW. The role of IL-13 in established allergic airway disease. J Immunol 2002;169:6482–6489. [DOI] [PubMed] [Google Scholar]
- 23.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O'Brien RL, Gelfand EW, et al. Different potentials of gamma delta t cell subsets in regulating airway responsiveness: V γ 1+ cells, but not V γ 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol 2004;172:2894–2902. [DOI] [PubMed] [Google Scholar]
- 24.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, Miyahara S, Balhorn A, Dakhama A, Gelfand EW. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol 2004;172:2549–2558. [DOI] [PubMed] [Google Scholar]
- 25.Proust B, Nahori MA, Ruffie C, Lefort J, Vargaftig BB. Persistence of bronchopulmonary hyper-reactivity and eosinophilic lung inflammation after anti-IL-5 or -IL-13 treatment in allergic BALB/c and IL-4Rα knockout mice. Clin Exp Allergy 2003;33:119–131. [DOI] [PubMed] [Google Scholar]
- 26.Koya T, Kodama T, Takeda K, Miyahara N, Yang ES, Taube C, Joetham A, Park JW, Dakhama A, Gelfand EW. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am J Respir Crit Care Med 2006;173:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, Takeda K, Loader J, Miyahara N, Kodama T, et al. Mast cells, Fc ɛ Ri, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol 2004;172:6398–6406. [DOI] [PubMed] [Google Scholar]
- 28.Wise JT, Baginski TJ, Mobley JL. An adoptive transfer model of allergic lung inflammation in mice is mediated by CD4+CD62LlowCD25+ T cells. J Immunol 1999;162:5592–5600. [PubMed] [Google Scholar]
- 29.Finkelman FD, Katona IM, Urban JF Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol 1988;141:2335–2341. [PubMed] [Google Scholar]
- 30.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (STAT6) deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med 1998;187:939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11C+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005;201:981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson EN, Druey KM. Heterotrimeric g protein signaling: role in asthma and allergic inflammation. J Allergy Clin Immunol 2002;109:592–602. [DOI] [PubMed] [Google Scholar]
- 33.Gosens R, Schaafsma D, Nelemans SA, Halayko AJ. ρ-Kinase as a drug target for the treatment of airway hyperresponsiveness in asthma. Mini Rev Med Chem 2006;6:339–348. [DOI] [PubMed] [Google Scholar]
- 34.Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Int Arch Allergy Immunol 2004;135:173–186. [DOI] [PubMed] [Google Scholar]
- 35.Holt PG, Upham JW. The role of dendritic cells in asthma. Curr Opin Allergy Clin Immunol 2004;4:39–44. [DOI] [PubMed] [Google Scholar]
- 36.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol 2006;176:3578–3584. [DOI] [PubMed] [Google Scholar]
- 37.Lambrecht BN. Allergen uptake and presentation by dendritic cells. Curr Opin Allergy Clin Immunol 2001;1:51–59. [DOI] [PubMed] [Google Scholar]
- 38.Byersdorfer CA, Chaplin DD. Visualization of early APC/T cell interactions in the mouse lung following intranasal challenge. J Immunol 2001;167:6756–6764. [DOI] [PubMed] [Google Scholar]
- 39.Hammad H, Lambrecht BN. Recent progress in the biology of airway dendritic cells and implications for understanding the regulation of asthmatic inflammation. J Allergy Clin Immunol 2006;118:331–336. [DOI] [PubMed] [Google Scholar]
- 40.Kuipers H, Lambrecht BN. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol 2004;16:702–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.