Abstract
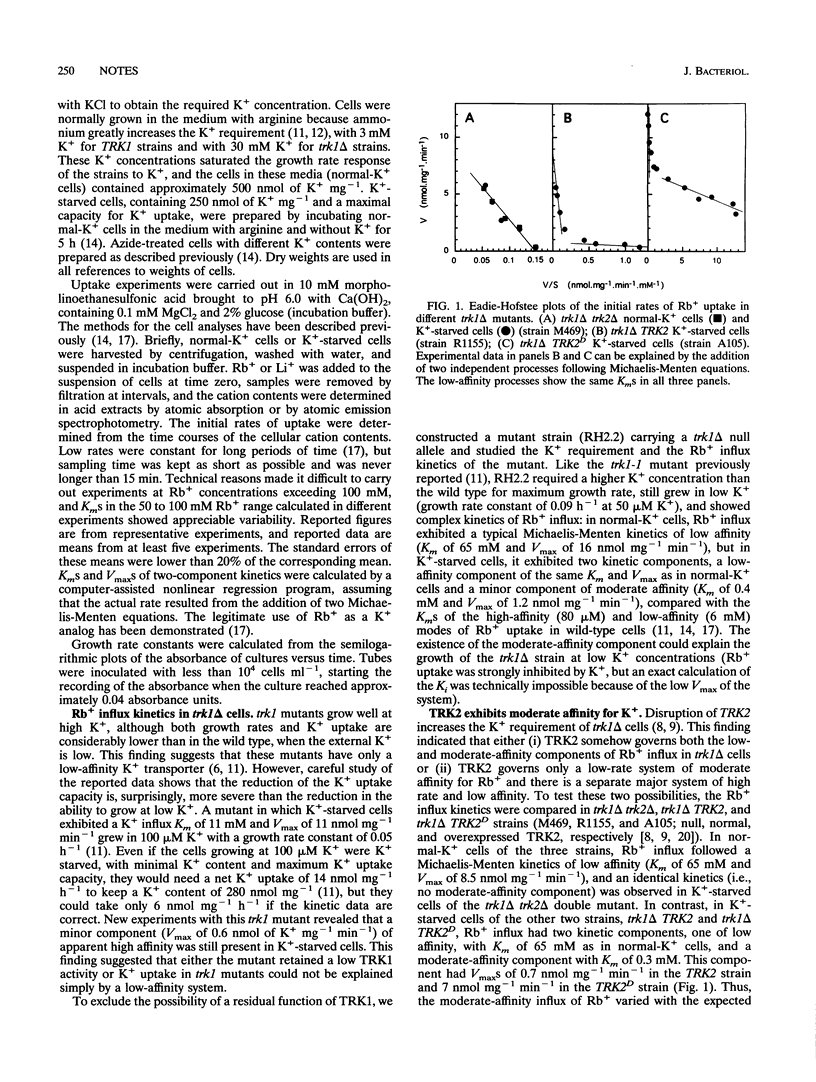
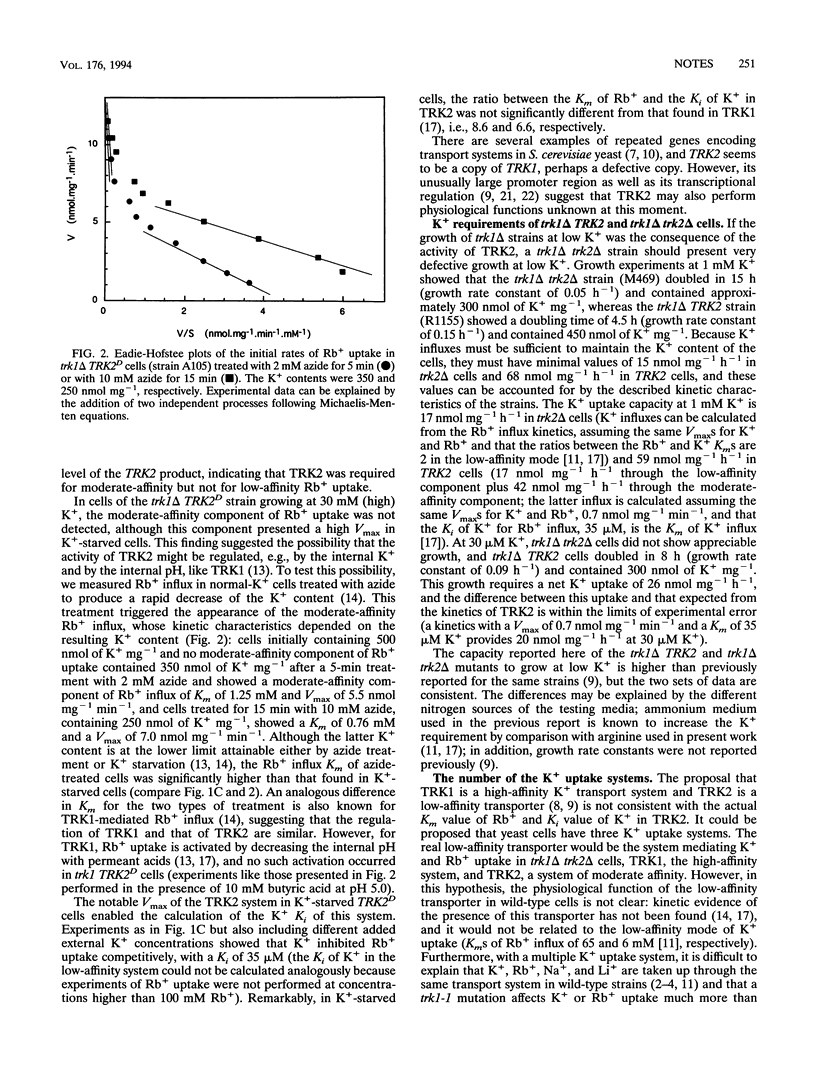
TRK1 and TRK2 encode proteins involved in K+ uptake in Saccharomyces cerevisiae. A kinetic study of Rb+ influx in trk1 TRK2, trk1 TRK2D, and trk1 trk2 mutants reveals that TRK2 shows moderate affinity for Rb+. K(+)-starved trk1 delta TRK2 cells show a low-affinity component accounting for almost the total Vmax of the influx and a moderate-affinity component exhibiting a very low Vmax. Overexpression of TRK2 in trk1 delta TRK2D cells increases the Vmax of the moderate-affinity component, and this component disappears in trk1 delta trk2 delta cells. In contrast, the low-affinity component of Rb+ influx in trk1 delta TRK2 cells is not affected by mutations in TRK2. Consistent with the different levels of activity of the moderate-affinity Rb+ influx, trk1 delta TRK2 cells grow slowly in micromolar K+, trk1 delta TRK2D cells grow rapidly, and trk1 delta trk2 delta cells fail to grow. The existence of a unique K+ uptake system composed of several proteins is also discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG W. M., ROTHSTEIN A. DISCRIMINATION BETWEEN ALKALI METAL CATIONS BY YEAST. I. EFFECT OF PH ON UPTAKE. J Gen Physiol. 1964 Sep;48:61–71. doi: 10.1085/jgp.48.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst-Pauwels G. W. Ion transport in yeast. Biochim Biophys Acta. 1981 Dec;650(2-3):88–127. doi: 10.1016/0304-4157(81)90002-2. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., DUGGAN F. A cation carrier in the yeast cell wall. Biochem J. 1958 Jun;69(2):265–274. doi: 10.1042/bj0690265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber R. F. Molecular genetics of yeast ion transport. Int Rev Cytol. 1992;137:299–353. doi: 10.1016/s0074-7696(08)62679-0. [DOI] [PubMed] [Google Scholar]
- Gaber R. F., Styles C. A., Fink G. R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblas B., Rubio F., Quintero F. J., Bañuelos M. A., Haro R., Rodríguez-Navarro A. Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol Gen Genet. 1993 Jan;236(2-3):363–368. doi: 10.1007/BF00277134. [DOI] [PubMed] [Google Scholar]
- Ko C. H., Buckley A. M., Gaber R. F. TRK2 is required for low affinity K+ transport in Saccharomyces cerevisiae. Genetics. 1990 Jun;125(2):305–312. doi: 10.1093/genetics/125.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. H., Gaber R. F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Aug;11(8):4266–4273. doi: 10.1128/mcb.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. H., Liang H., Gaber R. F. Roles of multiple glucose transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):638–648. doi: 10.1128/mcb.13.1.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J., Haro R., Alijo R., Rodríguez-Navarro A. Activation of the potassium uptake system during fermentation in Saccharomyces cerevisiae. J Bacteriol. 1992 Mar;174(6):2025–2027. doi: 10.1128/jb.174.6.2025-2027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J., Haro R., Rodríguez-Navarro A. Regulation of potassium fluxes in Saccharomyces cerevisiae. Biochim Biophys Acta. 1990 Nov 16;1029(2):211–217. doi: 10.1016/0005-2736(90)90156-i. [DOI] [PubMed] [Google Scholar]
- Ramos J., Rodríguez-Navarro A. Regulation and interconversion of the potassium transport systems of Saccharomyces cerevisiae as revealed by rubidium transport. Eur J Biochem. 1986 Jan 15;154(2):307–311. doi: 10.1111/j.1432-1033.1986.tb09398.x. [DOI] [PubMed] [Google Scholar]
- Rodréguez-Navarro A. Inhibition by sodium and lithium in osmophilic yeasts. Antonie Van Leeuwenhoek. 1971;37(2):225–231. doi: 10.1007/BF02218485. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Ramos J. Dual system for potassium transport in Saccharomyces cerevisiae. J Bacteriol. 1984 Sep;159(3):940–945. doi: 10.1128/jb.159.3.940-945.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Vidal M., Buckley A. M., Hilger F., Gaber R. F. Direct selection for mutants with increased K+ transport in Saccharomyces cerevisiae. Genetics. 1990 Jun;125(2):313–320. doi: 10.1093/genetics/125.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Gaber R. F. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Dec;11(12):6317–6327. doi: 10.1128/mcb.11.12.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Strich R., Esposito R. E., Gaber R. F. RPD1 (SIN3/UME4) is required for maximal activation and repression of diverse yeast genes. Mol Cell Biol. 1991 Dec;11(12):6306–6316. doi: 10.1128/mcb.11.12.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]


