Abstract
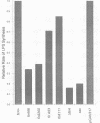
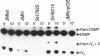
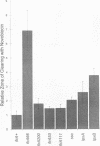
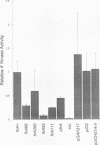
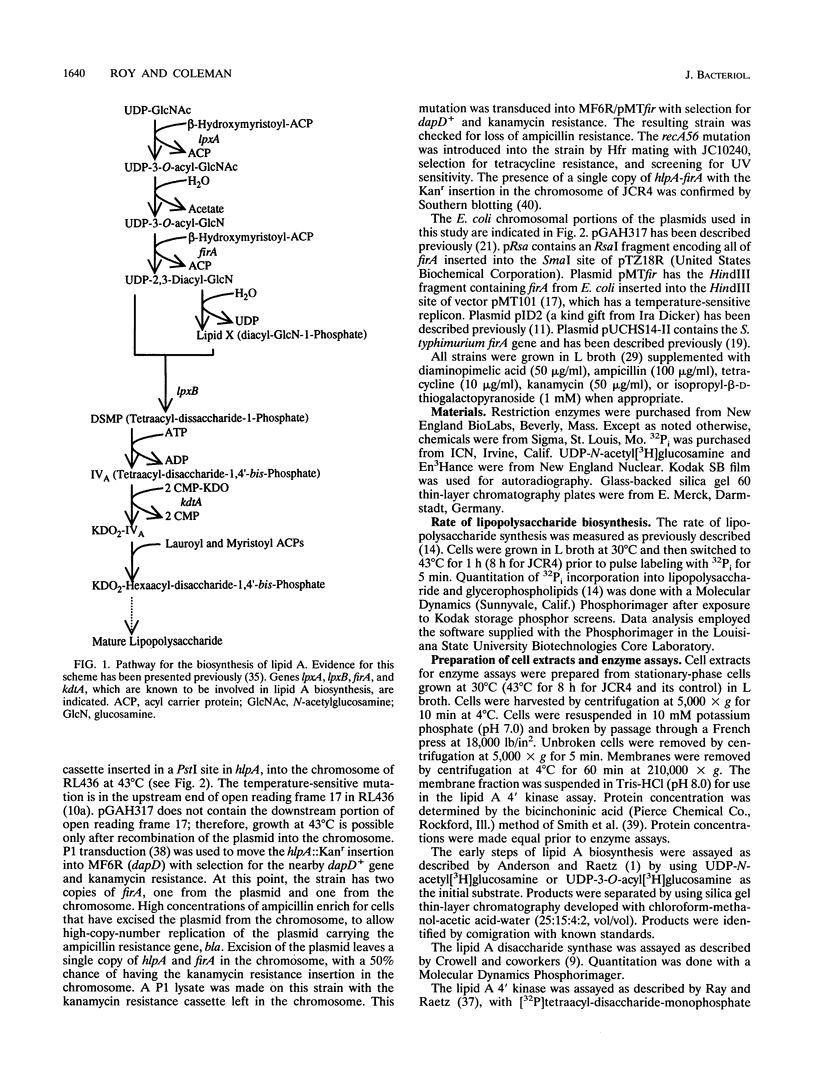
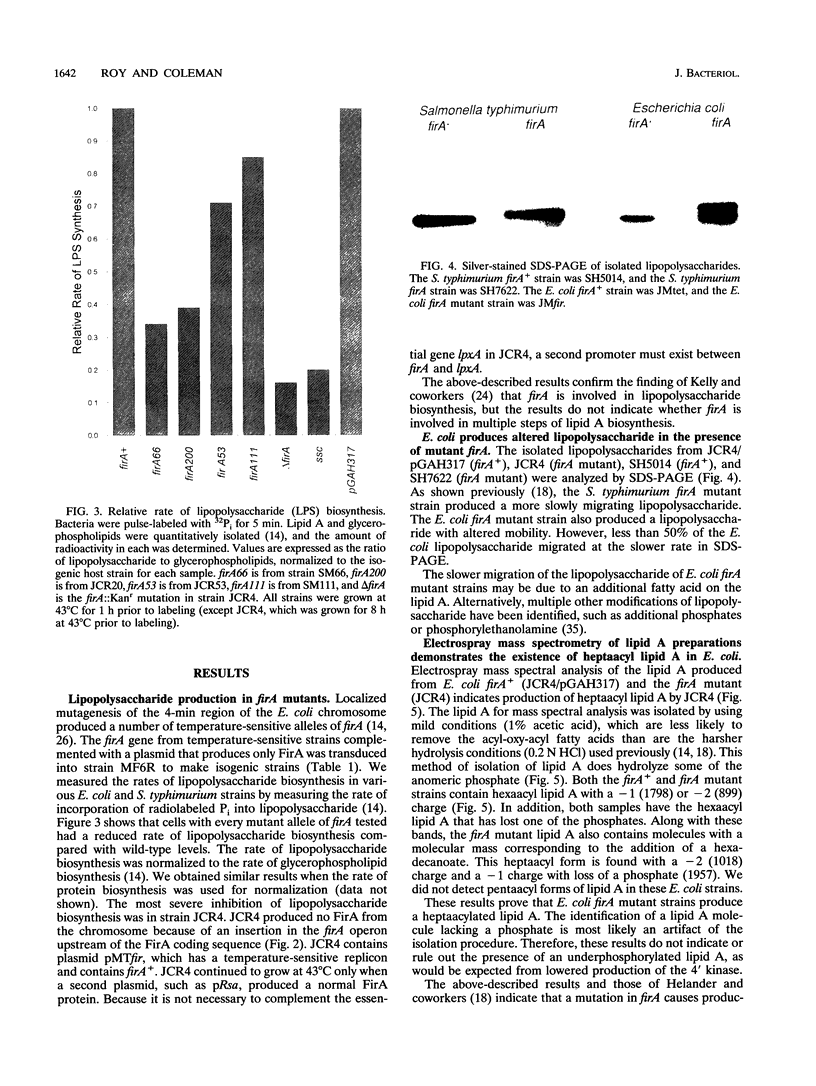
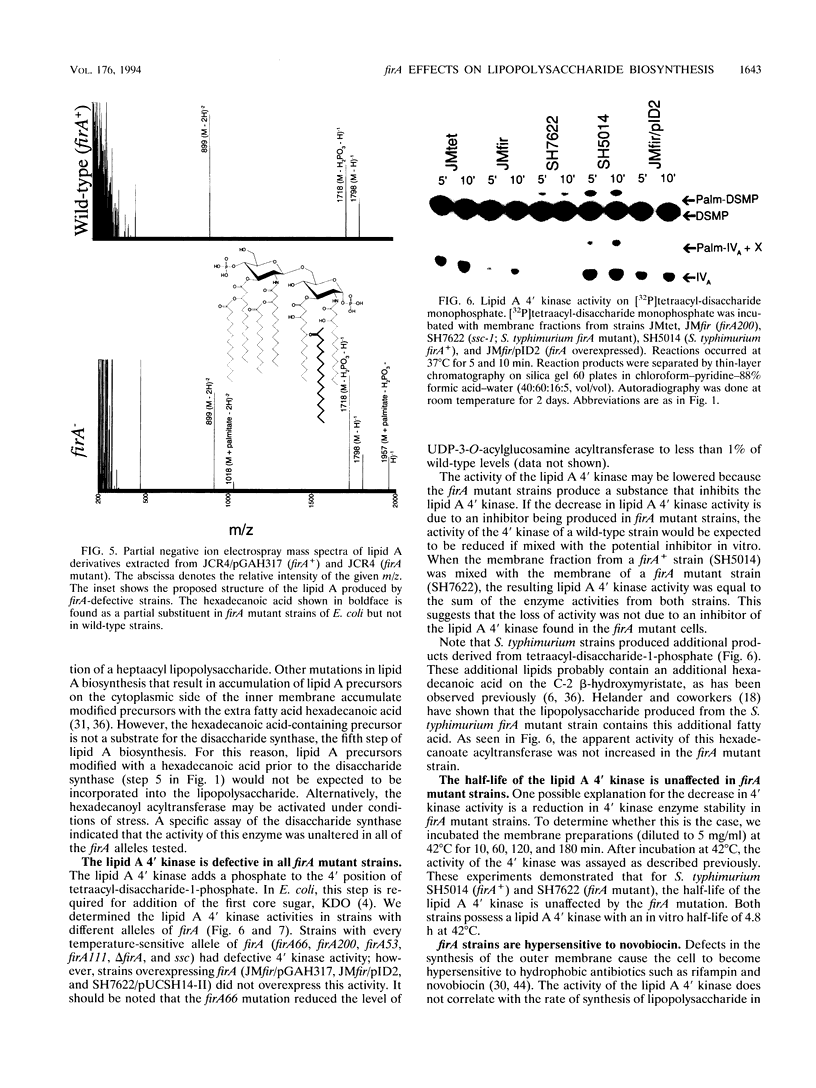
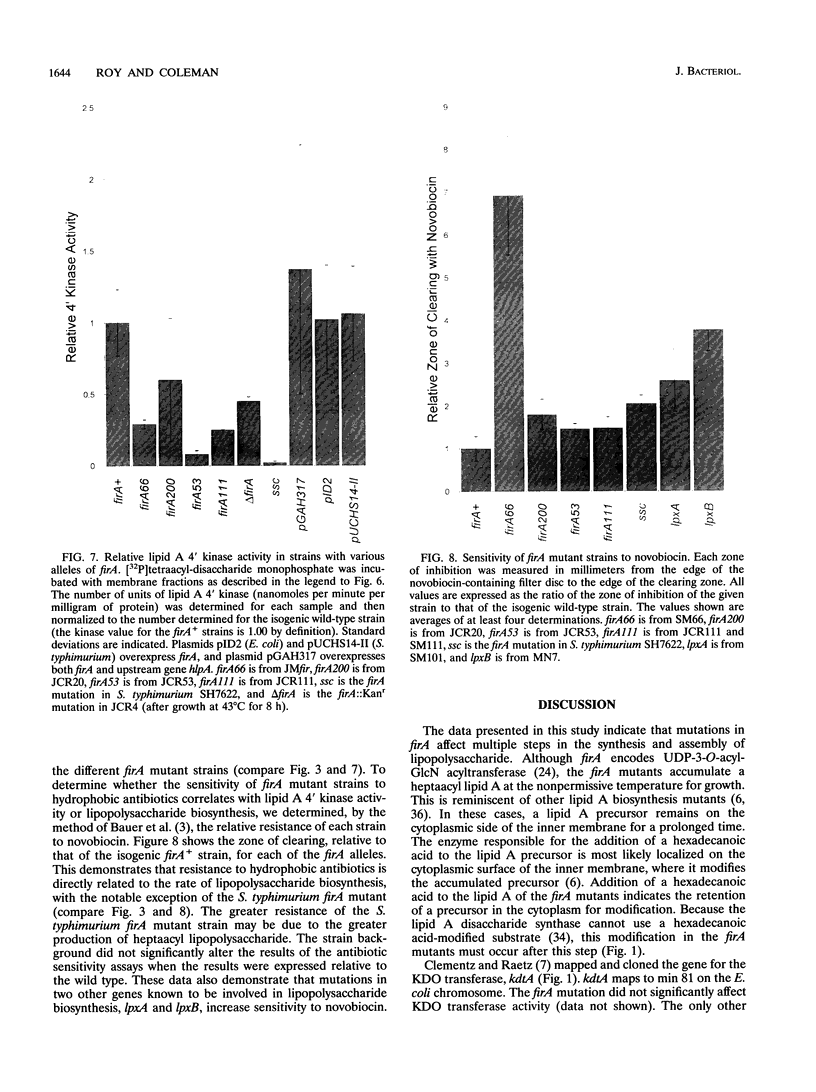
The product of the firA (ssc) gene is essential for growth and for the integrity of the outer membrane of Escherichia coli and Salmonella typhimurium. Recently, Kelly and coworkers (T. M. Kelly, S. A. Stachula, C. R. H. Raetz, and M. S. Anderson, J. Biol. Chem., 268:19866-19874, 1993) identified firA as the gene encoding UDP-3-O-(R-3-hydroxymyristoyl)-glucosamine N-acyltransferase, the third step in lipid A biosynthesis. We studied the effects of six different mutations in firA on lipopolysaccharide synthesis. All of the firA mutants of both E. coli and S. typhimurium examined had a decreased lipopolysaccharide synthesis rate. E. coli and S. typhimurium strains defective in firA produced a lipid A that contains a seventh fatty acid, a hexadecanoic acid, when grown at the nonpermissive temperature. Analysis of the enzymatic activity of other enzymes involved in lipid A biosynthesis revealed that the firA mutations pleiotropically affect lipopolysaccharide biosynthesis. In addition to that of UDP-3-O-(R-3-hydroxymyristoyl)-glucosamine N-acyltransferase, the enzymatic activity of the lipid A 4' kinase (the sixth step of lipid A biosynthesis) was decreased in strains with each of the firA mutations examined. However, overproduction of FirA was not accompanied by overexpression of the lipid A 4' kinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. S., Raetz C. R. Biosynthesis of lipid A precursors in Escherichia coli. A cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem. 1987 Apr 15;262(11):5159–5169. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Belunis C. J., Raetz C. R. Biosynthesis of endotoxins. Purification and catalytic properties of 3-deoxy-D-manno-octulosonic acid transferase from Escherichia coli. J Biol Chem. 1992 May 15;267(14):9988–9997. [PubMed] [Google Scholar]
- Brozek K. A., Bulawa C. E., Raetz C. R. Biosynthesis of lipid A precursors in Escherichia coli. A membrane-bound enzyme that transfers a palmitoyl residue from a glycerophospholipid to lipid X. J Biol Chem. 1987 Apr 15;262(11):5170–5179. [PubMed] [Google Scholar]
- Clementz T., Raetz C. R. A gene coding for 3-deoxy-D-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J Biol Chem. 1991 May 25;266(15):9687–9696. [PubMed] [Google Scholar]
- Coleman J., Raetz C. R. First committed step of lipid A biosynthesis in Escherichia coli: sequence of the lpxA gene. J Bacteriol. 1988 Mar;170(3):1268–1274. doi: 10.1128/jb.170.3.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell D. N., Anderson M. S., Raetz C. R. Molecular cloning of the genes for lipid A disaccharide synthase and UDP-N-acetylglucosamine acyltransferase in Escherichia coli. J Bacteriol. 1986 Oct;168(1):152–159. doi: 10.1128/jb.168.1.152-159.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):529–530. doi: 10.1128/jb.143.1.529-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker I. B., Seetharam S. Cloning and nucleotide sequence of the firA gene and the firA200(Ts) allele from Escherichia coli. J Bacteriol. 1991 Jan;173(1):334–344. doi: 10.1128/jb.173.1.334-344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker I. B., Seetharam S. What is known about the structure and function of the Escherichia coli protein FirA? Mol Microbiol. 1992 Apr;6(7):817–823. doi: 10.1111/j.1365-2958.1992.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Freudenberg M., Brade L., Schade U., Rietschel E. T., Kusumoto S., Shiba T. Biological activity of synthetic heptaacyl lipid A representing a component of Salmonella minnesota R595 lipid A. Eur J Biochem. 1986 Oct 1;160(1):55–59. doi: 10.1111/j.1432-1033.1986.tb09939.x. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Raetz C. R. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem. 1990 Apr 15;265(11):6394–6402. [PubMed] [Google Scholar]
- Ganong B. R., Raetz C. R. Massive accumulation of phosphatidic acid in conditionally lethal CDP-diglyceride synthetase mutants and cytidine auxotrophs of Escherichia coli. J Biol Chem. 1982 Jan 10;257(1):389–394. [PubMed] [Google Scholar]
- Goldman R. C., Doran C. C., Capobianco J. O. Analysis of lipopolysaccharide biosynthesis in Salmonella typhimurium and Escherichia coli by using agents which specifically block incorporation of 3-deoxy-D-manno-octulosonate. J Bacteriol. 1988 May;170(5):2185–2191. doi: 10.1128/jb.170.5.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock P. N., Dowhan W. Construction of a lethal mutation in the synthesis of the major acidic phospholipids of Escherichia coli. J Biol Chem. 1987 Sep 25;262(27):13044–13049. [PubMed] [Google Scholar]
- Helander I. M., Hirvas L., Tuominen J., Vaara M. Preferential synthesis of heptaacyl lipopolysaccharide by the ssc permeability mutant of Salmonella typhimurium. Eur J Biochem. 1992 Mar 15;204(3):1101–1106. doi: 10.1111/j.1432-1033.1992.tb16734.x. [DOI] [PubMed] [Google Scholar]
- Hirvas L., Koski P., Vaara M. Identification and sequence analysis of the gene mutated in the conditionally lethal outer membrane permeability mutant SS-C of Salmonella typhimurium. EMBO J. 1991 Apr;10(4):1017–1023. doi: 10.1002/j.1460-2075.1991.tb08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holck A., Kleppe K. Cloning and sequencing of the gene for the DNA-binding 17K protein of Escherichia coli. Gene. 1988 Jul 15;67(1):117–124. doi: 10.1016/0378-1119(88)90014-5. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Fujita N., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase: omega factor is responsible for the ppGpp sensitivity. Nucleic Acids Res. 1989 Nov 11;17(21):8755–8765. doi: 10.1093/nar/17.21.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M. Isolation and characterization of a second RNase H (RNase HII) of Escherichia coli K-12 encoded by the rnhB gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8587–8591. doi: 10.1073/pnas.87.21.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. M., Stachula S. A., Raetz C. R., Anderson M. S. The firA gene of Escherichia coli encodes UDP-3-O-(R-3-hydroxymyristoyl)-glucosamine N-acyltransferase. The third step of endotoxin biosynthesis. J Biol Chem. 1993 Sep 15;268(26):19866–19874. [PubMed] [Google Scholar]
- Labischinski H., Naumann D., Schultz C., Kusumoto S., Shiba T., Rietschel E. T., Giesbrecht P. Comparative X-ray and Fourier-transform-infrared investigations of conformational properties of bacterial and synthetic lipid A of Escherichia coli and Salmonella minnesota as well as partial structures and analogues thereof. Eur J Biochem. 1989 Feb 15;179(3):659–665. doi: 10.1111/j.1432-1033.1989.tb14598.x. [DOI] [PubMed] [Google Scholar]
- Lathe R., Buc H., Lecocq J. P., Bautz E. K. Prokaryotic histone-like protein interacting with RNA polymerase. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3548–3552. doi: 10.1073/pnas.77.6.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Lecocq J. P. The firA gene, a locus involved in the expression of rifampicin resistance in Escherichia coli. I. Characterisation of lambdafirA transducing phages constructed in vitro. Mol Gen Genet. 1977 Jul 7;154(1):43–51. doi: 10.1007/BF00265575. [DOI] [PubMed] [Google Scholar]
- Li S. J., Cronan J. E., Jr The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992 Aug 25;267(24):16841–16847. [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Bulawa C. E., Raetz C. R. Two interacting mutations causing temperature-sensitive phosphatidylglycerol synthesis in Escherichia coli membranes. J Bacteriol. 1981 Jan;145(1):113–121. doi: 10.1128/jb.145.1.113-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979 Aug 25;254(16):7837–7844. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Mascagni P., Honovich J., Wong R., Cotter R. J. Complete structural determination of lipopolysaccharide obtained from deep rough mutant of Escherichia coli. Purification by high performance liquid chromatography and direct analysis by plasma desorption mass spectrometry. J Biol Chem. 1988 Aug 25;263(24):11971–11976. [PubMed] [Google Scholar]
- Radika K., Raetz C. R. Purification and properties of lipid A disaccharide synthase of Escherichia coli. J Biol Chem. 1988 Oct 15;263(29):14859–14867. [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Meyer M. V., Qureshi N., Takayama K. Isolation and characterization of eight lipid A precursors from a 3-deoxy-D-manno-octylosonic acid-deficient mutant of Salmonella typhimurium. J Biol Chem. 1985 Dec 25;260(30):16080–16088. [PubMed] [Google Scholar]
- Ray B. L., Raetz C. R. The biosynthesis of gram-negative endotoxin. A novel kinase in Escherichia coli membranes that incorporates the 4'-phosphate of lipid A. J Biol Chem. 1987 Jan 25;262(3):1122–1128. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vuorio R., Vaara M. The lipid A biosynthesis mutation lpxA2 of Escherichia coli results in drastic antibiotic supersusceptibility. Antimicrob Agents Chemother. 1992 Apr;36(4):826–829. doi: 10.1128/aac.36.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]