Abstract
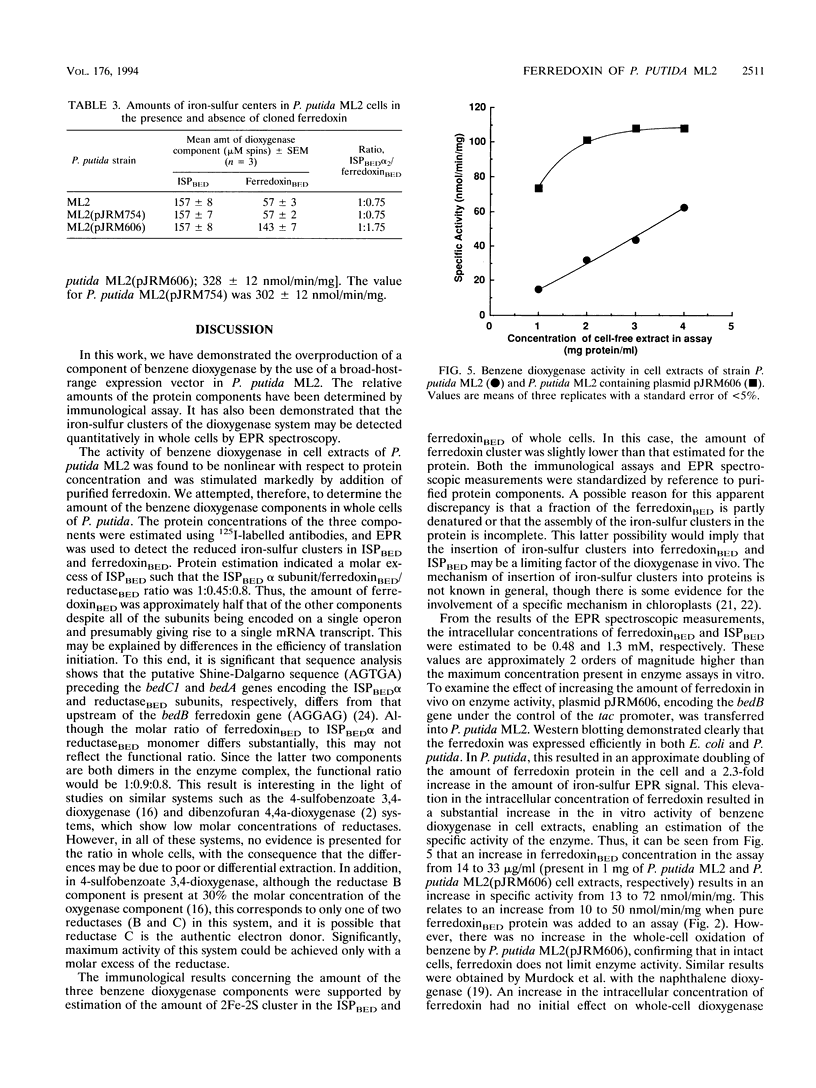
The benzene dioxygenase from Pseudomonas putida ML2 is a multicomponent complex comprising a flavoprotein reductase, a ferredoxin, and a terminal iron-sulfur protein (ISP). The catalytic activity of the isolated complex shows a nonlinear relationship with protein concentration in cell extracts, with the limiting factor for activity in vitro being ferredoxin(BED). The relative levels of the three components were analyzed by using 125I-labelled antibodies, and the functional molar ratio of ISP(BED), ferredoxin(BED), and reductase(BED) was shown to be 1:0.9:0.8, respectively. The concentration of ferredoxin(BED) was confirmed by quantitative electron paramagnetic resonance spectroscopy of the 2Fe-2S centers in ferredoxin(BED) and ISP(BED) of whole cells. These results demonstrate that the ferredoxin(BED) component is a limiting factor in dioxygenase activity in vitro. To determine if it is a limiting factor in vivo, a plasmid (pJRM606) overproducing ferredoxin(BED) was introduced into P. putida ML2. The benzene dioxygenase activity of this strain, measured in cell extracts, was fivefold greater than in the wild type, and the activity was linear with protein concentration in cell extracts above 2 mg/ml. Western blotting (immunoblotting) and electron paramagnetic resonance spectroscopic analysis confirmed an elevated level of ferredoxin(BED) protein and active redox centers in the recombinant strain. However, in these cells, the increased level of ferredoxin(BED) had no effect on the overall rate of benzene oxidation by whole cells. Thus, we conclude that ferredoxin(BED) is not limiting at the high intracellular concentration (0.48 mM) found in cells.
Full text
PDF
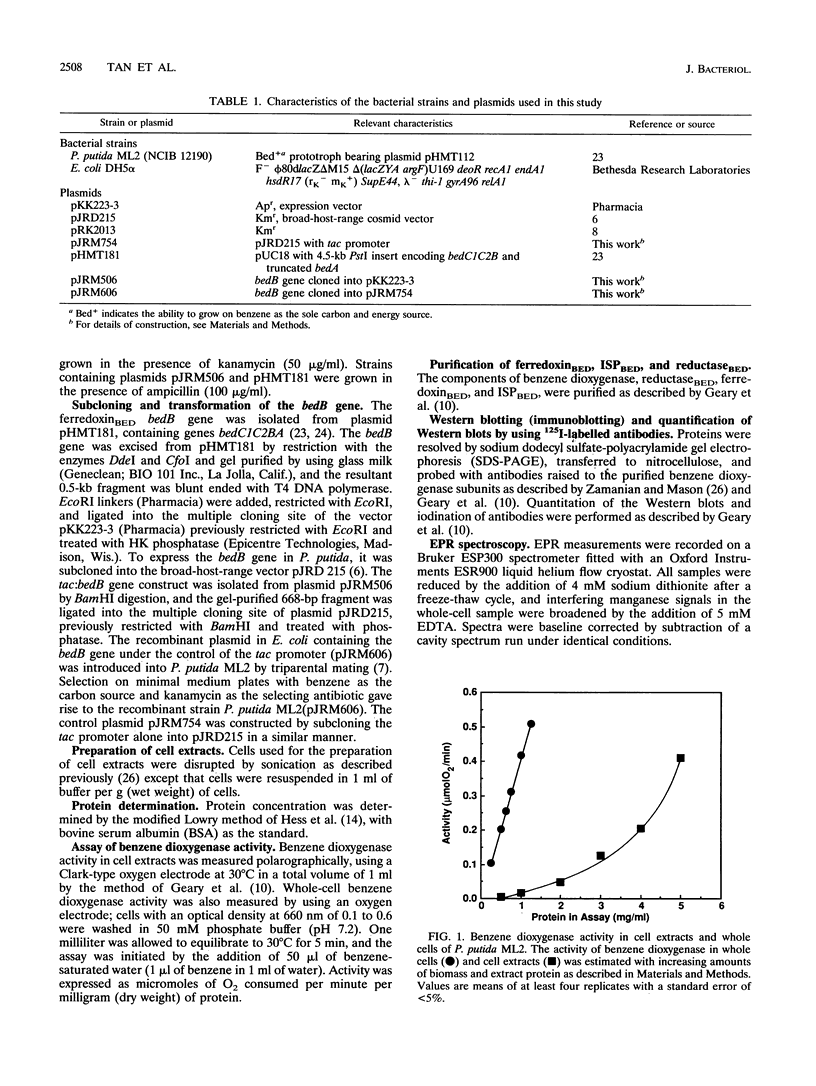
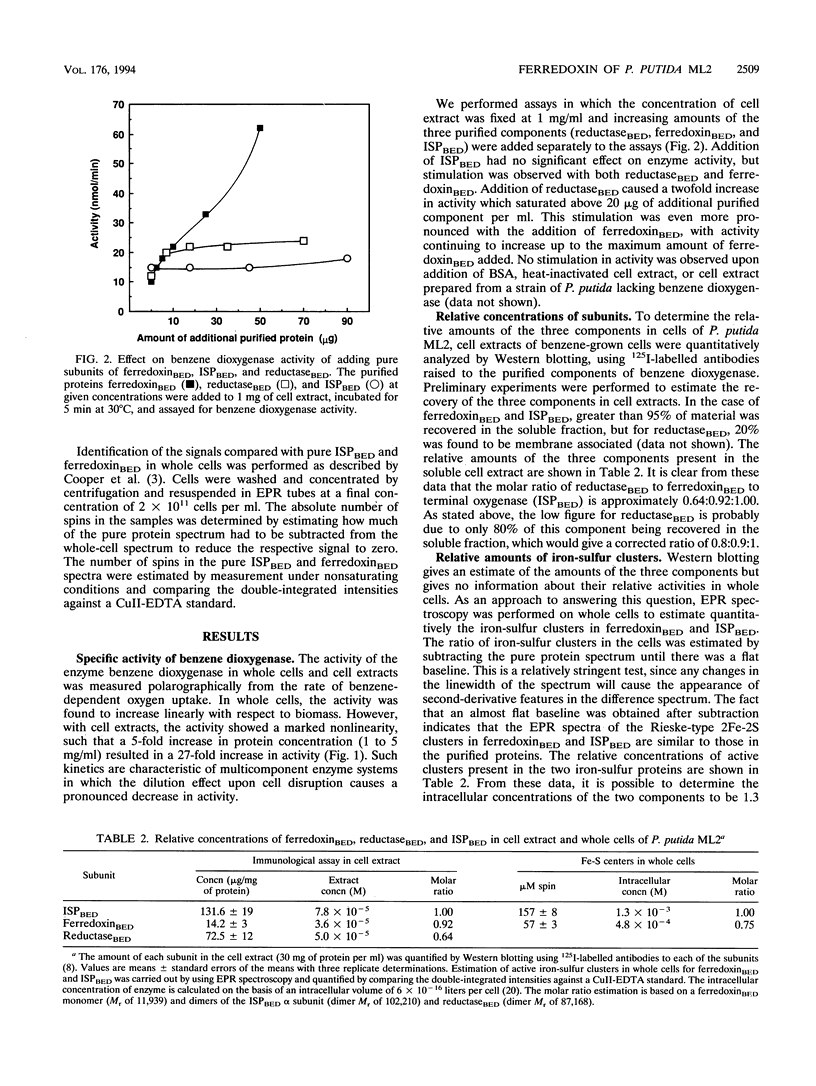
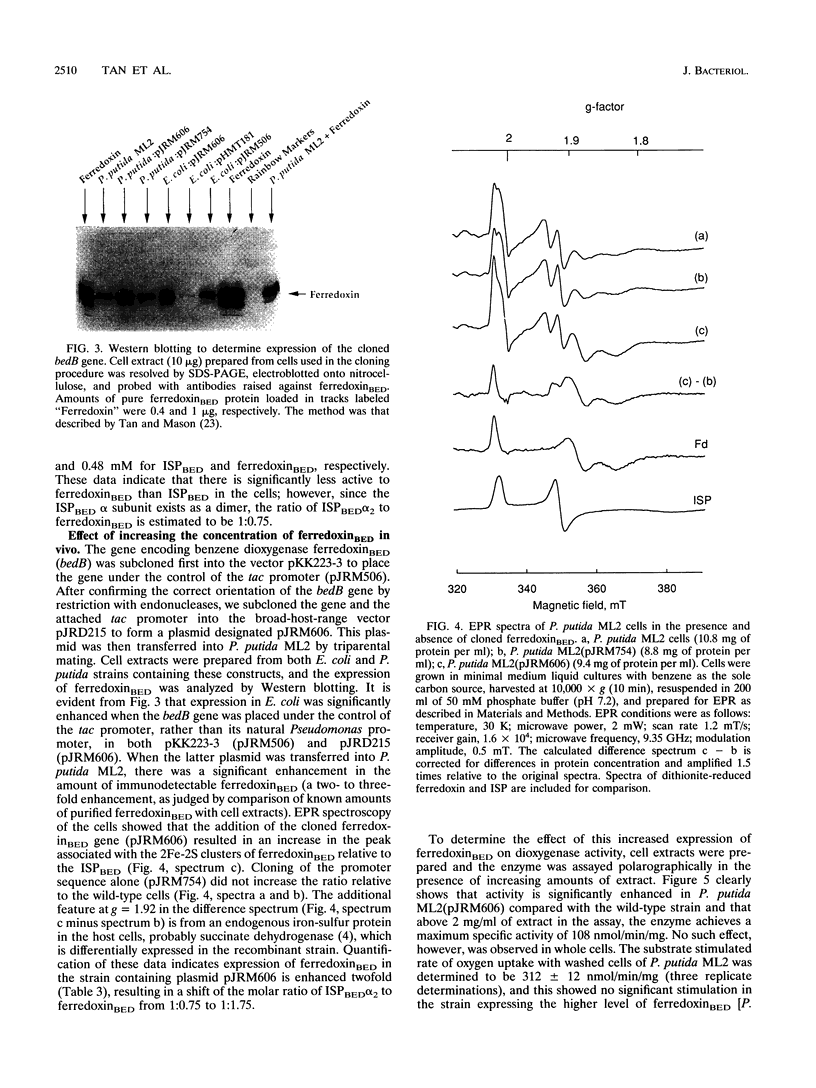

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünz P. V., Cook A. M. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: angular dioxygenation by a three-component enzyme system. J Bacteriol. 1993 Oct;175(20):6467–6475. doi: 10.1128/jb.175.20.6467-6475.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon C., Cammack R., Patil D. S., Owen P. The succinate dehydrogenase of Escherichia coli. Immunochemical resolution and biophysical characterization of a 4-subunit enzyme complex. J Biol Chem. 1985 Aug 5;260(16):9427–9434. [PubMed] [Google Scholar]
- Crutcher S. E., Geary P. J. Properties of the iron--sulphur proteins of the benzene dioxygenase system from Pseudomonas putida. Biochem J. 1979 Feb 1;177(2):393–400. doi: 10.1042/bj1770393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison J., Heusterspreute M., Chevalier N., Ha-Thi V., Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51(2-3):275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary P. J., Dickson D. P. Mössbauer spectroscopic studies of the terminal dioxygenase protein of benzene dioxygenase from Pseudomonas putida. Biochem J. 1981 Apr 1;195(1):199–203. doi: 10.1042/bj1950199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary P. J., Mason J. R., Joannou C. L. Benzene dioxygenase from Pseudomonas putida, ML2 (NCIB 12190). Methods Enzymol. 1990;188:52–60. doi: 10.1016/0076-6879(90)88012-y. [DOI] [PubMed] [Google Scholar]
- Geary P. J., Saboowalla F., Patil D., Cammack R. An investigation of the iron-sulphur proteins of benzene dioxygenase from Pseudomonas putida by electron-spin-resonance spectroscopy. Biochem J. 1984 Feb 1;217(3):667–673. doi: 10.1042/bj2170667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Jefcoate C. R. Mitochondrial cytochrome P-450scc. Mechanism of electron transport by adrenodoxin. J Biol Chem. 1980 Apr 10;255(7):3057–3061. doi: 10.1016/S0021-9258(19)85851-9. [DOI] [PubMed] [Google Scholar]
- Hess H. H., Lees M. B., Derr J. E. A linear Lowry--Folin assay for both water-soluble and sodium dodecyl sulfate-solubilized proteins. Anal Biochem. 1978 Mar;85(1):295–300. doi: 10.1016/0003-2697(78)90304-4. [DOI] [PubMed] [Google Scholar]
- Irie S., Doi S., Yorifuji T., Takagi M., Yano K. Nucleotide sequencing and characterization of the genes encoding benzene oxidation enzymes of Pseudomonas putida. J Bacteriol. 1987 Nov;169(11):5174–5179. doi: 10.1128/jb.169.11.5174-5179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher H. H., Leisinger T., Cook A. M. 4-Sulphobenzoate 3,4-dioxygenase. Purification and properties of a desulphonative two-component enzyme system from Comamonas testosteroni T-2. Biochem J. 1991 Mar 15;274(Pt 3):833–842. doi: 10.1042/bj2740833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. R., Cammack R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu Rev Microbiol. 1992;46:277–305. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- Morrice N., Geary P., Cammack R., Harris A., Beg F., Aitken A. Primary structure of protein B from Pseudomonas putida, member of a new class of 2Fe-2S ferredoxins. FEBS Lett. 1988 Apr 25;231(2):336–340. doi: 10.1016/0014-5793(88)80845-7. [DOI] [PubMed] [Google Scholar]
- Murdock D., Ensley B. D., Serdar C., Thalen M. Construction of metabolic operons catalyzing the de novo biosynthesis of indigo in Escherichia coli. Biotechnology (N Y) 1993 Mar;11(3):381–386. doi: 10.1038/nbt0393-381. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Takahashi Y., Mitsui A., Fujita Y., Matsubara H. Roles of ATP and NADPH in formation of the fe-s cluster of spinach ferredoxin. Plant Physiol. 1991 Jan;95(1):104–110. doi: 10.1104/pp.95.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Mitsui A., Hase T., Matsubara H. Formation of the iron-sulfur cluster of ferredoxin in isolated chloroplasts. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2434–2437. doi: 10.1073/pnas.83.8.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H. M., Mason J. R. Cloning and expression of the plasmid-encoded benzene dioxygenase genes from Pseudomonas putida ML2. FEMS Microbiol Lett. 1990 Nov;60(3):259–264. doi: 10.1016/0378-1097(90)90314-g. [DOI] [PubMed] [Google Scholar]
- Tan H. M., Tang H. Y., Joannou C. L., Abdel-Wahab N. H., Mason J. R. The Pseudomonas putida ML2 plasmid-encoded genes for benzene dioxygenase are unusual in codon usage and low in G+C content. Gene. 1993 Aug 16;130(1):33–39. doi: 10.1016/0378-1119(93)90343-2. [DOI] [PubMed] [Google Scholar]
- Tyson C. A., Lipscomb J. D., Gunsalus I. C. The role of putidaredoxin and P450 cam in methylene hydroxylation. J Biol Chem. 1972 Sep 25;247(18):5777–5784. [PubMed] [Google Scholar]
- Zamanian M., Mason J. R. Benzene dioxygenase in Pseudomonas putida. Subunit composition and immuno-cross-reactivity with other aromatic dioxygenases. Biochem J. 1987 Jun 15;244(3):611–616. doi: 10.1042/bj2440611. [DOI] [PMC free article] [PubMed] [Google Scholar]