Abstract
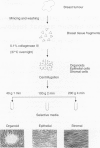
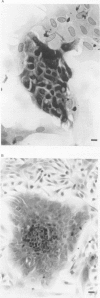
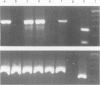
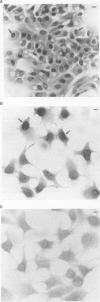
As experimental models for breast cancer, most studies rely on established human breast cancer cell lines. However, many of these lines were established over 20 years ago, many from pleural effusions rather than the primary tumour, so the validity of using them as representative models is questionable. This paper describes our experiences, over a 3-year period, in establishing short-term epithelial-cell-enriched preparations from primary breast tumours based on differential centrifugation followed by culture in selective media. Epithelial cells were successfully cultured from 55% of samples, but culture success did not appear to be correlated with tumour histology, stage, grade or node status. Epithelial cell-enriched cultures were immunopositive for broad-spectrum cytokeratin and epithelial membrane antigen (EMA). Positivity for keratin 19 confirmed that the cultures contained tumour-derived cells, which additionally showed significantly higher activity of the reductive pathway of the steroid-converting enzyme 17beta-hydroxysteroid dehydrogenase type I. That the cultures contained tumour and not normal epithelial cells was further substantiated by the complete absence of the calmodulin-like gene NB-1 in tumour-derived cultures; this is only associated with normal breast epithelia. Eighty-five per cent of cultures established from oestrogen receptor (ER)-positive tumours expressed ER in vitro; this was functional in 66% of cultures, although ER-positive phenotype was gradually lost over time. In conclusion, epithelial cells can be isolated and maintained as short-term cultures from primary breast tumours irrespective of histopathological or clinical details, providing a model system with a greater biological and clinical relevance than breast cancer cell lines.
Full text
PDF

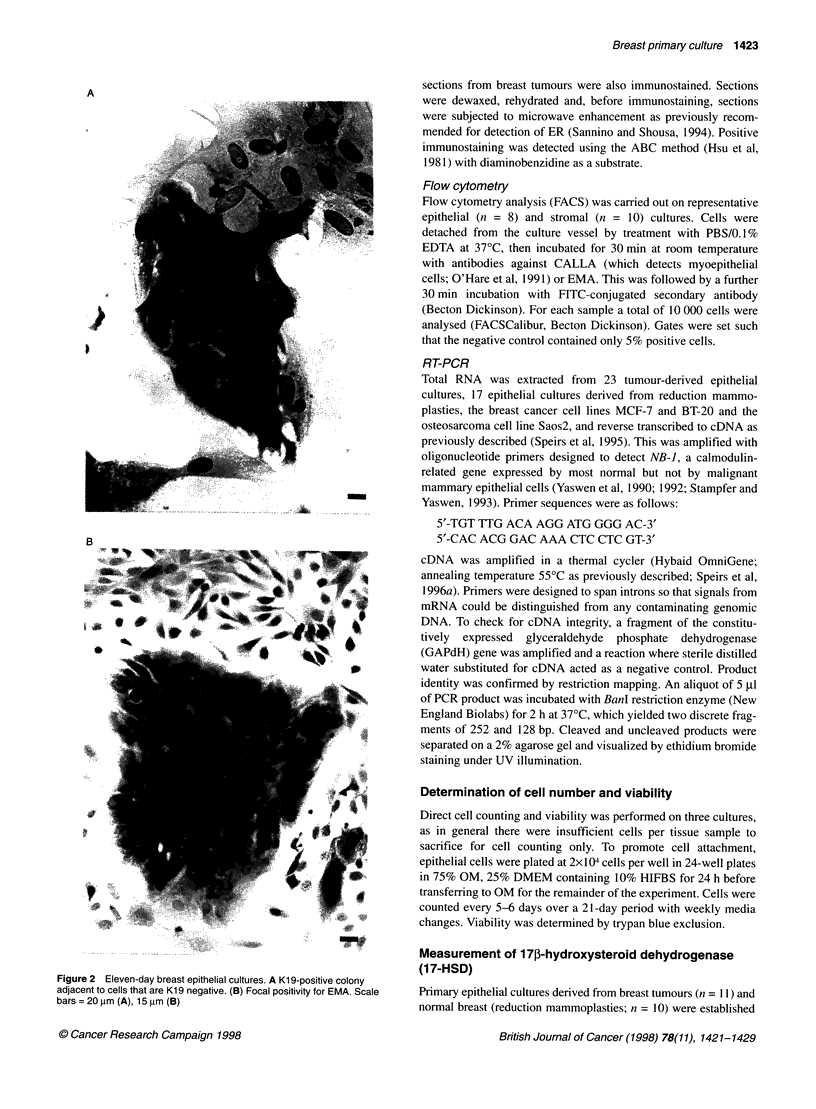
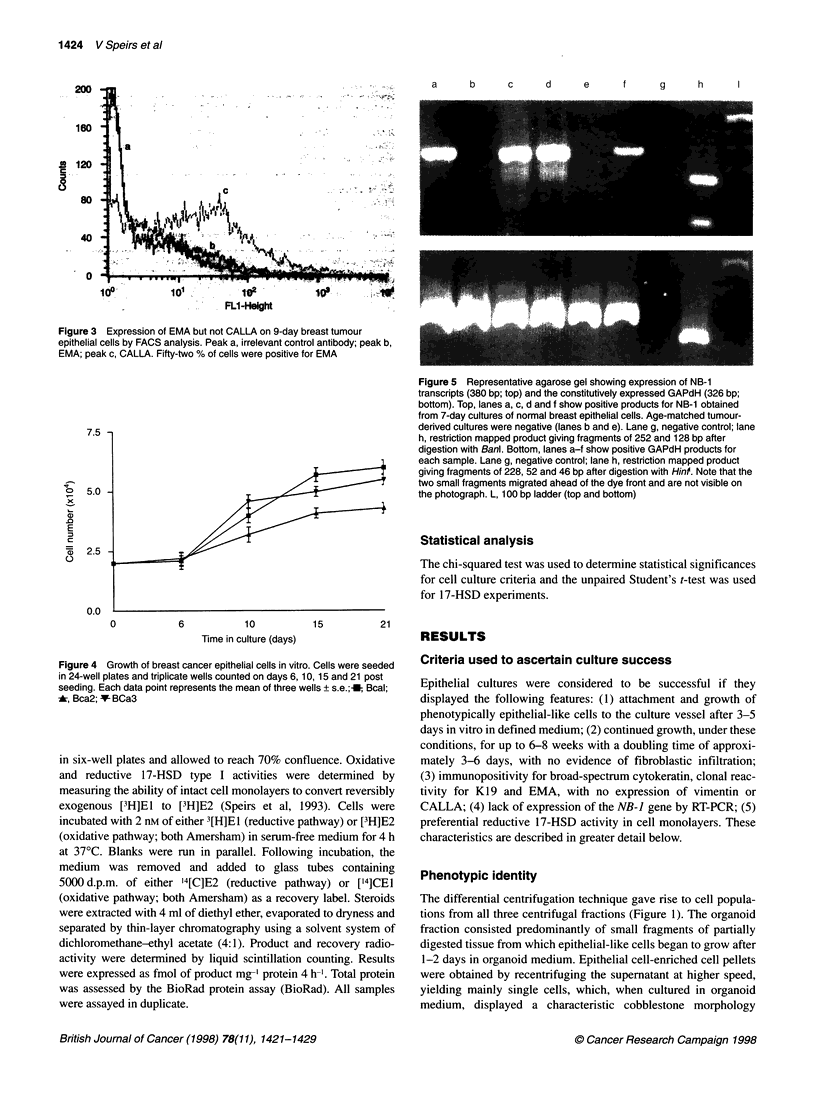
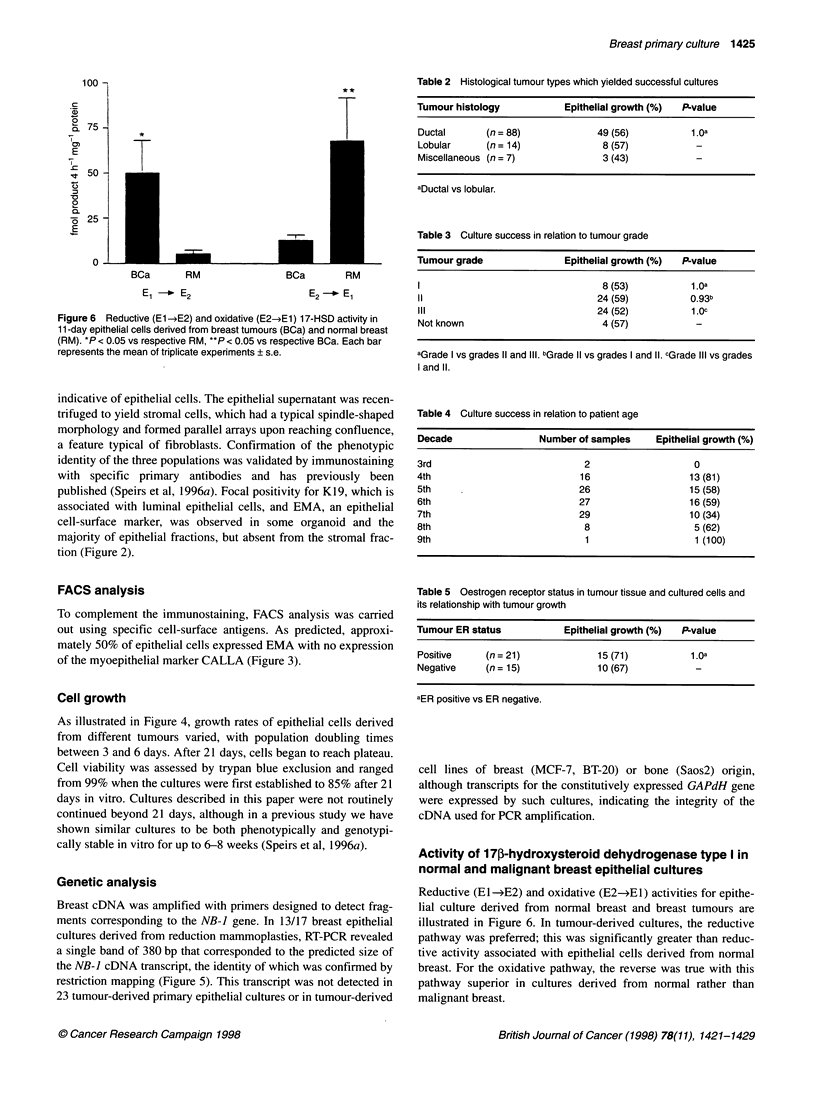
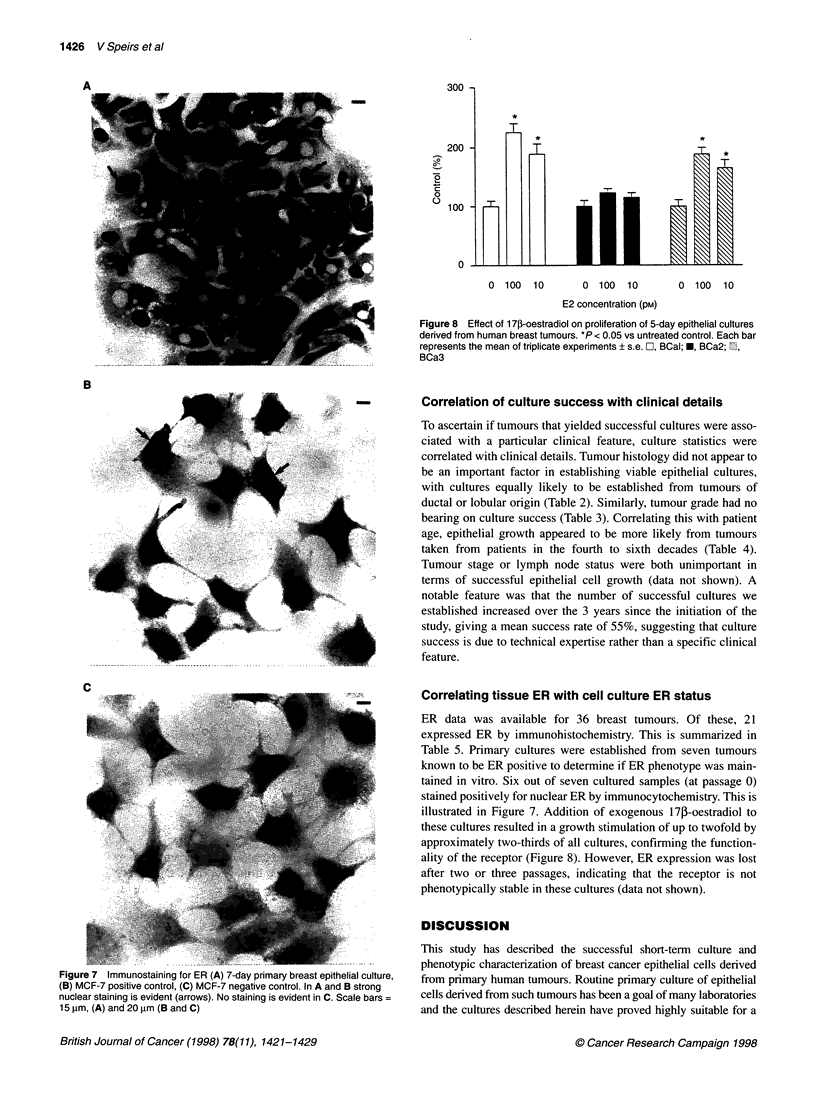



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. F., Coldham N. G., James V. H. Steroidal regulation of oestradiol-17 beta dehydrogenase activity of the human breast cancer cell line MCF-7. J Endocrinol. 1988 Jul;118(1):149–154. doi: 10.1677/joe.0.1180149. [DOI] [PubMed] [Google Scholar]
- Adams E. F., Newton C. J., Braunsberg H., Shaikh N., Ghilchik M., James V. H. Effects of human breast fibroblasts on growth and 17 beta-estradiol dehydrogenase activity of MCF-7 cells in culture. Breast Cancer Res Treat. 1988 May;11(2):165–172. doi: 10.1007/BF01805840. [DOI] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Swisshelm K., Trask D., Kulesa V., Cohen C., Connolly J., Sager R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990 Nov 15;50(22):7351–7357. [PubMed] [Google Scholar]
- Bergstraesser L. M., Weitzman S. A. Culture of normal and malignant primary human mammary epithelial cells in a physiological manner simulates in vivo growth patterns and allows discrimination of cell type. Cancer Res. 1993 Jun 1;53(11):2644–2654. [PubMed] [Google Scholar]
- Cailleau R., Young R., Olivé M., Reeves W. J., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974 Sep;53(3):661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnetta L. A., Granata O. M., Taibi G., Lo Casto M., Comito L., Oliveri G., Di Falco M., Carruba G. 17 beta-hydroxysteroid oxidoreductase activity in intact cells significantly differs from classical enzymology analysis. J Endocrinol. 1996 Sep;150 (Suppl):S73–S78. [PubMed] [Google Scholar]
- Clarke R. B., Howell A., Potten C. S., Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997 Nov 15;57(22):4987–4991. [PubMed] [Google Scholar]
- Dairkee S. H., Paulo E. C., Traquina P., Moore D. H., Ljung B. M., Smith H. S. Partial enzymatic degradation of stroma allows enrichment and expansion of primary breast tumor cells. Cancer Res. 1997 Apr 15;57(8):1590–1596. [PubMed] [Google Scholar]
- Dillner J., Kallin B., Ehlin-Henriksson B., Timar L., Klein G. Characterization of a second Epstein-Barr virus-determined nuclear antigen associated with the BamHI WYH region of EBV DNA. Int J Cancer. 1985 Mar 15;35(3):359–366. doi: 10.1002/ijc.2910350312. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Stingl J., Petersen A., Shpall E. J., Eaves C. J. Selective growth of freshly isolated human breast epithelial cells cultured at low concentrations in the presence or absence of bone marrow cells. Breast Cancer Res Treat. 1996;41(2):147–159. doi: 10.1007/BF01807160. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Wilkinson D. A. Routine culturing of normal, dysplastic and malignant human mammary epithelial cells from small tissue samples. In Vitro Cell Dev Biol. 1990 Dec;26(12):1186–1194. doi: 10.1007/BF02623697. [DOI] [PubMed] [Google Scholar]
- Ethier S. P., Mahacek M. L., Gullick W. J., Frank T. S., Weber B. L. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 1993 Feb 1;53(3):627–635. [PubMed] [Google Scholar]
- Green A. R., Green V. L., White M. C., Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. 1997 Sep 17;72(6):937–941. doi: 10.1002/(sici)1097-0215(19970917)72:6<937::aid-ijc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hofland L. J., van der Burg B., van Eijck C. H., Sprij D. M., van Koetsveld P. M., Lamberts S. W. Role of tumor-derived fibroblasts in the growth of primary cultures of human breast-cancer cells: effects of epidermal growth factor and the somatostatin analogue octreotide. Int J Cancer. 1995 Jan 3;60(1):93–99. doi: 10.1002/ijc.2910600114. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Kasid A., Gelmann E., Davidson N., McManaway M., Huff K., Bronzert D., Bates S., Swain S. Autocrine and paracrine growth regulation of human breast cancer. J Steroid Biochem. 1986 Jan;24(1):147–154. doi: 10.1016/0022-4731(86)90044-0. [DOI] [PubMed] [Google Scholar]
- McCallum H. M., Lowther G. W. Long-term culture of primary breast cancer in defined medium. Breast Cancer Res Treat. 1996;39(3):247–259. doi: 10.1007/BF01806153. [DOI] [PubMed] [Google Scholar]
- Meltzer P., Leibovitz A., Dalton W., Villar H., Kute T., Davis J., Nagle R., Trent J. Establishment of two new cell lines derived from human breast carcinomas with HER-2/neu amplification. Br J Cancer. 1991 May;63(5):727–735. doi: 10.1038/bjc.1991.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir J., Speirs V. Rapid and irreversible loss of estrogen receptor in human osteoblast-like cells following culture in phenol red-free medium. In Vitro Cell Dev Biol Anim. 1997 Apr;33(4):240–242. doi: 10.1007/s11626-997-0041-2. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Ormerod M. G., Monaghan P., Lane E. B., Gusterson B. A. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation. 1991 Apr;46(3):209–221. doi: 10.1111/j.1432-0436.1991.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Pétursdóttir I., Gudmundsdóttir I., Amundadóttir L., Rønnov-Jessen L., Petersen O. W. Effects of lymphocytes and fibroblasts on the growth of human mammary carcinoma cells studied in short-term primary cultures. In Vitro Cell Dev Biol Anim. 1993 Dec;29A(12):936–942. doi: 10.1007/BF02634232. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Arteaga C. L. Autocrine and paracrine growth regulation of breast cancer: clinical implications. Breast Cancer Res Treat. 1990 Jan;15(1):3–11. doi: 10.1007/BF01811884. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Hobbs K., Trent J. M. Biological differences among MCF-7 human breast cancer cell lines from different laboratories. Breast Cancer Res Treat. 1987;9(2):111–121. doi: 10.1007/BF01807363. [DOI] [PubMed] [Google Scholar]
- Peltoketo H., Isomaa V., Poutanen M., Vihko R. Expression and regulation of 17 beta-hydroxysteroid dehydrogenase type 1. J Endocrinol. 1996 Sep;150 (Suppl):S21–S30. [PubMed] [Google Scholar]
- Petersen O. W., van Deurs B., Nielsen K. V., Madsen M. W., Laursen I., Balslev I., Briand P. Differential tumorigenicity of two autologous human breast carcinoma cell lines, HMT-3909S1 and HMT-3909S8, established in serum-free medium. Cancer Res. 1990 Feb 15;50(4):1257–1270. [PubMed] [Google Scholar]
- Pink J. J., Bilimoria M. M., Assikis J., Jordan V. C. Irreversible loss of the oestrogen receptor in T47D breast cancer cells following prolonged oestrogen deprivation. Br J Cancer. 1996 Oct;74(8):1227–1236. doi: 10.1038/bjc.1996.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen M., Isomaa V., Peltoketo H., Vihko R. Role of 17 beta-hydroxysteroid dehydrogenase type 1 in endocrine and intracrine estradiol biosynthesis. J Steroid Biochem Mol Biol. 1995 Dec;55(5-6):525–532. doi: 10.1016/0960-0760(95)00201-4. [DOI] [PubMed] [Google Scholar]
- Reed M. J., Singh A., Ghilchik M. W., Coldham N. G., Purohit A. Regulation of oestradiol 17 beta hydroxysteroid dehydrogenase in breast tissues: the role of growth factors. J Steroid Biochem Mol Biol. 1991 Nov;39(5B):791–798. doi: 10.1016/0960-0760(91)90027-3. [DOI] [PubMed] [Google Scholar]
- Sannino P., Shousha S. Demonstration of oestrogen receptors in paraffin wax sections of breast carcinoma using the monoclonal antibody 1D5 and microwave oven processing. J Clin Pathol. 1994 Jan;47(1):90–92. doi: 10.1136/jcp.47.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld A., Luqmani Y., Sinnett H. D., Shousha S., Coombes R. C. Keratin 19 mRNA measurement to detect micrometastases in lymph nodes in breast cancer patients. Br J Cancer. 1996 Nov;74(10):1639–1642. doi: 10.1038/bjc.1996.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld A., Luqmani Y., Smith D., O'Reilly S., Shousha S., Sinnett H. D., Coombes R. C. Detection of breast cancer micrometastases in axillary lymph nodes by using polymerase chain reaction. Cancer Res. 1994 Jun 1;54(11):2986–2990. [PubMed] [Google Scholar]
- Shearer M., Bartkova J., Bartek J., Berdichevsky F., Barnes D., Millis R., Taylor-Papadimitriou J. Studies of clonal cell lines developed from primary breast cancers indicate that the ability to undergo morphogenesis in vitro is lost early in malignancy. Int J Cancer. 1992 Jun 19;51(4):602–612. doi: 10.1002/ijc.2910510417. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Speirs V., Adams E. F., Rafferty B., White M. C. Interactive effects of interleukin-6, 17 beta-estradiol and progesterone on growth and 17 beta-hydroxysteroid dehydrogenase activity in human breast carcinoma cells. J Steroid Biochem Mol Biol. 1993 Jul;46(1):11–15. doi: 10.1016/0960-0760(93)90203-9. [DOI] [PubMed] [Google Scholar]
- Speirs V., Birch M. A., Boyle-Walsh E., Green A. R., Gallagher J. A., White M. C. Interleukin-3: a putative protective factor against breast cancer which is secreted by male but not female breast fibroblasts. Int J Cancer. 1995 May 4;61(3):416–419. doi: 10.1002/ijc.2910610323. [DOI] [PubMed] [Google Scholar]
- Speirs V., White M. C., Green A. R. Collagenase III: a superior enzyme for complete disaggregation and improved viability of normal and malignant human breast tissue. In Vitro Cell Dev Biol Anim. 1996 Feb;32(2):72–74. doi: 10.1007/BF02723036. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R., Yaswen P. Culture systems for study of human mammary epithelial cell proliferation, differentiation and transformation. Cancer Surv. 1993;18:7–34. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Stampfer M., Bartek J., Lewis A., Boshell M., Lane E. B., Leigh I. M. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989 Nov;94(Pt 3):403–413. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- Vermeulen A., Deslypere J. P., Paridaens R., Leclercq G., Roy F., Heuson J. C. Aromatase, 17 beta-hydroxysteroid dehydrogenase and intratissular sex hormone concentrations in cancerous and normal glandular breast tissue in postmenopausal women. Eur J Cancer Clin Oncol. 1986 Apr;22(4):515–525. doi: 10.1016/0277-5379(86)90121-5. [DOI] [PubMed] [Google Scholar]
- Wolman S. R., Smith H. S., Stampfer M., Hackett A. J. Growth of diploid cells from breast cancers. Cancer Genet Cytogenet. 1985 Mar 1;16(1):49–64. doi: 10.1016/0165-4608(85)90077-9. [DOI] [PubMed] [Google Scholar]
- Yaswen P., Smoll A., Hosoda J., Parry G., Stampfer M. R. Protein product of a human intronless calmodulin-like gene shows tissue-specific expression and reduced abundance in transformed cells. Cell Growth Differ. 1992 Jun;3(6):335–345. [PubMed] [Google Scholar]
- Yaswen P., Smoll A., Peehl D. M., Trask D. K., Sager R., Stampfer M. R. Down-regulation of a calmodulin-related gene during transformation of human mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7360–7364. doi: 10.1073/pnas.87.19.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roozendaal C. E., van Ooijen B., Klijn J. G., Claassen C., Eggermont A. M., Henzen-Logmans S. C., Foekens J. A. Stromal influences on breast cancer cell growth. Br J Cancer. 1992 Jan;65(1):77–81. doi: 10.1038/bjc.1992.14. [DOI] [PMC free article] [PubMed] [Google Scholar]