Abstract
The formation of neuronal networks is governed by a limited number of guidance molecules, yet it is immensely complex. The complexity of guidance cues is augmented by posttranslational modification of guidance molecules and their receptors. We report here that cleavage of the floor plate guidance molecule F-spondin generates two functionally opposing fragments: a short-range repellent protein deposited in the membrane of floor plate cells and an adhesive protein that accumulates at the basement membrane. Their coordinated activity, acting respectively as a short-range repellant and a permissive short-range attractant, constricts commissural axons to the basement membrane beneath the floor plate cells. We further demonstrate that the repulsive activity of the inhibitory fragment of F-spondin requires its presentation by the lipoprotein receptor–related protein (LRP) receptors apolipoprotein E receptor 2, LRP2/megalin, and LRP4, which are expressed in the floor plate. Thus, proteolysis and membrane interaction coordinate combinatorial guidance signaling originating from a single guidance cue.
Introduction
Commissural axons cross the midline by elongating between the floor plate cells and the basement membrane. Dye tracing and ultrastructural studies have shown that commissural axons are restricted to the basal floor plate, growing between the floor plate cells and the underlying ECM/basement membrane (Bovolenta and Dodd, 1990). The avoidance of penetrating floor plate cells, the source of the chemoattractants netrin and sonic hedgehog (Kennedy et al., 1994; Serafini et al., 1994; Charron et al., 2003), is puzzling. Two possible mechanisms may account for this pattern of trajectory. The floor plate cells may produce a short-range repellent signal that prevents axonal growth into the floor plate cells or, alternatively, the basement membrane may positively attract axons.
The basement membrane of the floor plate is a site of deposition of various adhesion molecules (Yaginuma et al., 1991; Hunter et al., 1992), among them F-spondin (Burstyn-Cohen et al., 1999). F-spondin, a gene expressed at the ventral midline of the embryonic spinal cord (i.e., the floor plate) encodes a secreted, ECM-attached protein (Klar et al., 1992). It plays a dual role in patterning axonal trajectory in the embryonic spinal cord by promoting outgrowth of commissural axons and inhibiting outgrowth of motor axons and migration of neural crest cells (Burstyn-Cohen et al., 1999; Debby-Brafman et al., 1999; Tzarfaty-Majar et al., 2001a). The carboxyl half of F-spondin contains six thrombospondin type-one repeats (TSRs). The TSRs of F-spondin protein are typical of class-two TSRs (Tan et al., 2002). Vertebrate class-two TSR proteins are represented in the nervous system by F-spondin, mindin, subcommissural organ (SCO)–spondin and two remotely related proteins, heparin-binding growth-associated molecule and midkine (Feinstein and Klar, 2004).
The TSR domain of F-spondin is proteolytically processed. The cleaved products of F-spondin have different adhesive properties: the fifth and sixth TSRs (TSR5 and TSR6, respectively) bind to the ECM whereas the TSR1-4 fragment does not bind to the ECM (Tzarfaty-Majar et al., 2001b). In this paper we demonstrate that the two fragments of F-spondin, which are generated in vivo by a proteolytic cleavage, are spatially restricted to different extracellular compartments at the midline. An outgrowth-promoting fragment, TSR6, is deposited at the basement membrane that underlies the floor plate, whereas an outgrowth-inhibiting fragment, TSR1-4, is bound to the floor plate cell surface via binding to lipoprotein receptor–related protein (LRP) receptors apolipoprotein E receptor 2 (ApoER2), LRP2/megalin, and LRP4. The spatial localization of F-spondin fragments forces commissural axons to deflect from the repulsive TSR1-4 and extend on the permissive TSR6. Consequentially, commissural axons elongate between the basement membrane and the floor plate cells, rather than into the floor plate cells, which are the source of the chemoattractants netrin and sonic hedgehog. Hence, two novel posttranslational modifications elicit F-spondin activity: the coordinated generation of two functionally opposing polypeptides from a single protein by proteolysis and immobilization by membranal receptors.
Results
The dual activity of F-spondin in promoting and inhibiting neurite outgrowth resides from distinct TSR domains
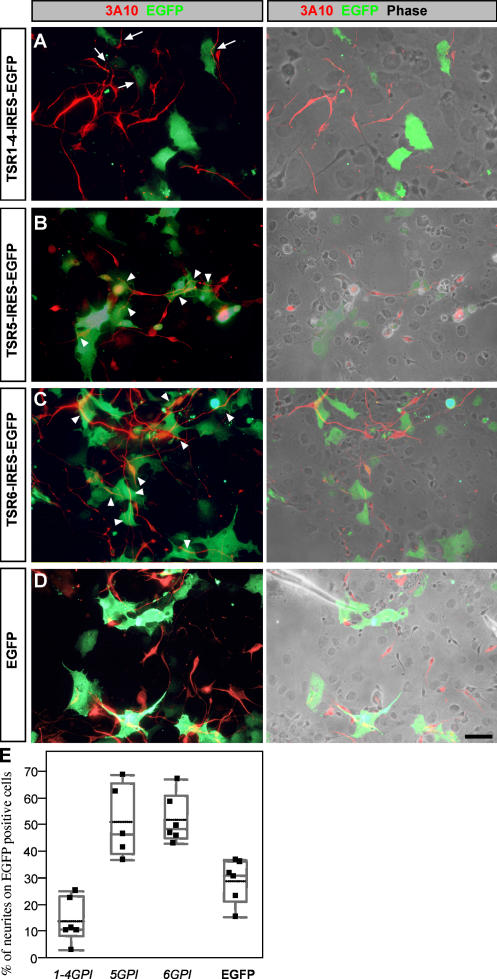
The outgrowth-promoting activity of F-spondin resides in its carboxyl half, the TSR domains. F-spondin is proteolytically processed in vivo between the amino-terminal reelin/spondin and the carboxyl-terminal TSR domain. The TSR domain of F-spondin is further cleaved into three fragments. Plasmin cleavage between repeats 1–4, 5, and 6 generates a nonadhesive TSR1-4 fragment and adhesive TSR5 and 6 fragments (Tzarfaty-Majar et al., 2001b). To test whether the diverse adhesive properties of the TSRs of F-spondin are also reflected in their outgrowth-promoting potential, we tested the outgrowth of E6 chick dorsal spinal cord neurons on cell surface–immobilized F-spondin substrates. Glycosylphosphatidylinositol (GPI)-anchored forms of TSR1-4, 5, and 6 were transiently expressed in COS cells. A confluent culture of mixed F-spondin–expressing cells marked by EGFP and nonexpressing cells served as a substrate for the dissociated neurons. Chick E6 dorsal spinal cord neurons preferably elongate on TSR5GPI and 6GPI (Fig. 1, B and C), and avoid growing on TSR1-4GPI (Fig. 1 A). The ratio between neurites growing on F-spondin fragments versus neurites growing on the nonexpressing cells was calculated. Cultured EGFP-expressing COS cells served as a control (Fig. 1 D). The outgrowth on TSR5GPI and 6GPI was significantly higher than the control whereas the outgrowth on TSR1-4GPI was significantly lower than the control (Fig. 1 E). Similar results were obtained in an outgrowth assay with rat E13 spinal cord neurons cultured on purified TSR1-4 and 6 proteins (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200702184/DC1). Thus, the dual activity of F-spondin in either promoting or inhibiting neurite outgrowth may result from the activity of different domains; the TSR1-4 fragment inhibits outgrowth whereas the TSR5 and 6 fragments support outgrowth.
Figure 1.
Differential outgrowth activity of TRSs of F-spondin. COS cells were transfected with pCAGG–TSR1-4GPI–IRES–EGFP (A), pCAGG–TSR5GPI–IRES–EGFP (B), pCAGG–TSR6GPI–IRES–EGFP (C), and pCAGG–EGFP (D). Dissociated E6 chick dorsal spinal cord was plated 24 h after transfection and cultured for an additional 48 h. Neurites are detected with 3A10 mAb. Fluorescence images are shown in the left, and combination of fluorescence and phase contrast images are shown in the right. Neurites are deflected from TSR1-4GPI–expressing cells (A, arrows) and grow preferentially on the adjacent nonexpressing cells. Neurites grow preferentially on TSR5GPI (B)- and TSR6GPI (C)-expressing cells (arrowheads) rather than on the neighboring nonexpressing cells. In the control experiment neurites grow uniformly on EGFP-expressing and nonexpressing cells (D). For quantification, images (n = 6 for each experiment) of the cultures were taken with a digital camera. The total neurite outgrowth was measured as a 3A10-positive area using ImageJ software. A total of 146 neurites were analyzed for TSR5GPI, 278 for TSR6GPI, 203 for TSR1-4GPI, and 455 for EGFP. The area occupied by neurites growing on EGFP-expressing cells was measured using the RG2B colocalization plugin of ImageJ software. The ratio between neurites extending on expressing cells versus total outgrowth is presented. Comparing each experimental group to the control using Dunnett's method (which takes into account multiple comparisons) shows a significant difference between the TSR5GPI (P = 0.004), TSR6GPI (P = 0.002), and TSR1-4GPI (P = 0.04) groups and the control EGFP group. Bar, 10 μm.
In vivo localization of F-spondin–cleaved fragments
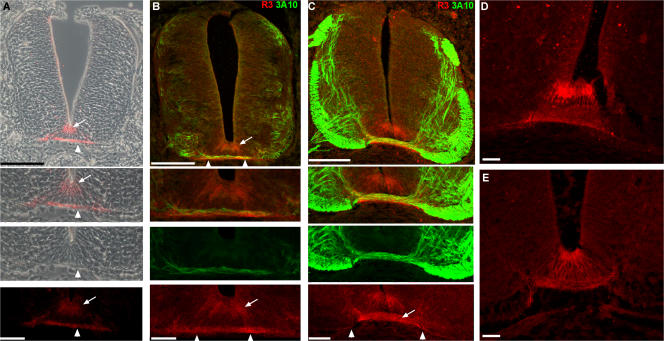
The pattern of expression of the TSR fragments of F-spondin protein in chick embryos was examined with R3 antibody raised against TSRs 3–6 of chick F-spondin (Burstyn-Cohen et al., 1999). Epitope mapping with the isolated TSRs indicates that R3 recognizes TSR3 and 5 (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200702184/DC1). Hence, R3 recognizes the repulsive (TSR3) and the outgrowth-promoting, ECM-attached domain (TSR5) of F-spondin that flank the plasmin cleavage site P2 (Tzarfaty-Majar et al., 2001b). F-spondin immunoreactivity is first detected at stage 21. As the pioneer commissural axons invade the midline, expression is detected on the apical floor plate cells and the pia that underlies the floor plate cells (Fig. 2 A). Costaining with axonal marker (antineurofilament 3A10 mAb) reveals that F-spondin accumulates beneath and along the pathway of the crossing axons (Fig. 2 B). After stage 24, expression was also detected on the crossing fibers of the commissural axons (stage 26; Fig. 2 C). The segregation between the apical floor plate cells and the axonal and pial staining persists through development (stage 27, the last stage analyzed was stage 29; Fig. 2 D). The pial staining is not restricted to the basement membrane underlying the floor plate. It also spreads lateral to the floor plate (Fig. 2 C, arrowheads). This suggests that F-spondin binding to the pia is governed by a ubiquitous, not midline-specific, basement membrane component.
Figure 2.
F-spondin protein is deposited in the apical floor plate and the basement membrane, and binds to commissural axons. F-spondin protein expression at stage 21 (A and B), 26 (C and E), and 27 (D) chick embryos as revealed by the R3 antibody. F-spondin accumulates at the apical floor plate (arrow) and the basement membrane (arrowhead). (B) The pioneer commissural axons (3A10 staining) extend on the F-spondin (R3 staining) that is deposited in the basement membrane as they cross the midline at stage 21. (C) At stage 26, the immunoreactivity of F-spondin is evident on the crossing fibers of the commissural axons (arrow) and basement membrane that flanks the midline ventral pia (arrowheads). Inhibition of serine proteases by in ovo aprotinin injection reduces the deposition of F-spondin at the basement membrane (E) and yields homogenous staining along the surface of floor plate cells. Fluorescence and phase-contrast images in A were taken with microscope using a digital camera. Fluorescence images in B–E were taken with a confocal microscope. Bar, 40 μm.
The differential ECM-binding capacities of the different F-spondin TSR fragments may be reflected in their extracellular localization at the midline and in their activities. That is, the adhesive TSR5 and 6 fragments may accumulate at the basement membrane, bind to commissural axons, and support commissural axon growth at the midline, whereas the nonadhesive TSR1-4 may bind to the apical floor plate cell membrane and deflect the axons away from floor plate cells. The anti–F-spondin antibody recognizes the adhesive TSR5 and the nonadhesive TSR3, which precludes its usage as a domain-specific antibody.
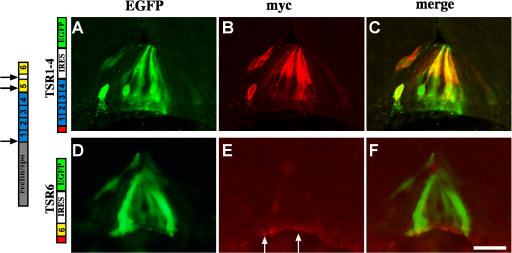
To test the subcellular deposition site of F-spondin fragments, the nonadhesive TSR1-4 and the adhesive TSR6 were expressed at the chick embryonic floor plate. Cell-specific expression was achieved by electroporation of DNA using the floor plate–specific enhancer III of the HoxA-1 gene (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200702184/DC1; Li and Lufkin, 2000). The TSR1-4 and 6 fragments were expressed at the floor plate at stages 12–14 along with cytoplasmic EGFP. F-spondin fragments were tagged with the myc epitope at the amino terminus. The localization of the ectopically expressed fragments was analyzed at stages 22–24. The TSR1-4 fragment labeled the floor plate cell membrane (Fig. 3, A–C, compare myc staining with the cytoplasmic EGFP staining). The staining obtained for the TSR1-4 fragment resembles the apical floor plate staining obtained with the R3 antibody (Fig. 2, A–D). The TSR1-4 fragment is likely to accumulate on the cell membranes of the expressing cells. In contrast, the TSR6 fragment labeled the basement membrane underlying the floor plate (Fig. 3, D–F), similar to the basal staining acquired with the R3 antibody (Fig. 2, A–D). Thus, the different TSR domains of F-spondin accumulate in different floor plate compartments. The nonadhesive TSR1-4 fragment binds to the surface of the floor plate cells, whereas the adhesive TSR6 fragment is deposited in the ECM that underlies the floor plate cells.
Figure 3.
Expression and protein localization of F-spondin domains at the floor plate. In all the experiments, enhancer III of HoxA-1 gene (Li and Lufkin, 2000) was used to drive expression of Cre recombinants (HoxA-1–Cre). A TSR1-4 conditional plasmid (loxP–STOP–loxP–TSR1-4–IRES–EGFP; A–C) and a TSR6 conditional plasmid (loxP–STOP–loxP–TSR6–IRES–EGFP; D–F) cloned in a pCAGG vector were electroporated into the spinal cord of stage 12–14 chick embryos. The F-spondin proteins were tagged with 4× myc epitope at their amino end. Cross-sections of stage 22–24 electroporated embryos were stained with antibodies to myc (B and E) and EGFP (A and D). TSR1-4 protein (B and C) labels the margins of cytoplasmic EGFP (A and C), reflecting its deposition along the membrane of the expressing cells. (E and F) TSR6 is deposited at the basement membrane that underlies the floor plate (arrows). (D) Note that the expressing cells, EGFP-positive cells, do not present the protein. The gray box represents the reelin/spondin domain of F-spondin. The blue boxes represent the nonadhesive TSR1-4 of F-spondin. The yellow boxes represent the adhesive TSR5 and 6 of F-spondin. The red box represents a cassette of 4× myc epitope. The black arrows point to the sites of cleavage of F-spondin. The white arrows point to the site of deposition of TSR6. Images were taken with a microscope using a digital camera. Bar, 25 μm.
F-spondin is processed in vivo
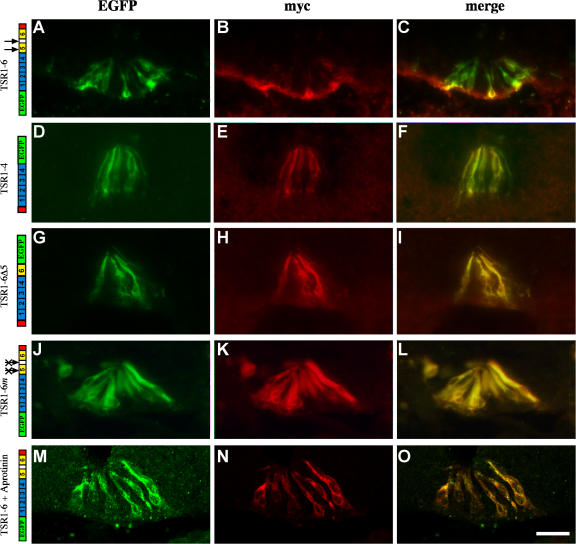
Two plasmin-sensitive sites were predicted based on amino acid sequence and were found, in vitro, to be plasmin sensitive: between the first and second antiparallel β strands of TSR5 and between TSR5 and 6 (Tzarfaty-Majar et al., 2001b). To test whether the predicted cleavage sites are indeed targets for floor plate–derived proteases, a double-tagged F-spondin protein was used. EGFP and myc epitope were fused to the amino and carboxyl ends of the TSR domain, respectively, and expressed in the floor plate at stages 12–14. At stages 22–24, the intact TSR domain EGFP–TSR1-6–myc gave rise to two distinct labeling patterns. TSR6 accumulated at the ECM that underlies the floor plate cells whereas the TSR1-4 domain bound to the floor plate cells (Fig. 4, A–C). A protein that lacks the adhesive fifth and sixth repeats, EGFP–TSR1-4–myc, was deposited on the floor plate cells, as revealed by identical staining with either myc or EGFP (Fig. 4, D–F). Thus, F-spondin is processed in vivo and gives rise to two proteolytic fragments that either bind to the membrane of floor plate cells or are deposited at the ECM.
Figure 4.
F-spondin is processed in vivo. In all the experiments the double-tagged F-spondin was cloned in a Cre-dependent plasmid and electroporated along with Hoxa-1–Cre. The myc epitope at the amino end contains four copies (E and H) and the myc epitope at the carboxyl end contains six copies (B, K, and N). (A–C) The TSR1-6 fragment of F-spondin is cleaved in vivo. The amino, EGFP-fused part of the protein stains the floor plate cells (A and C), whereas the carboxyl, myc-fused part of the protein stains the basement membrane (B and C). The TSR1-4 fragment of F-spondin (D–F), deletion of plasmin cleavage site (G–I), mutation of the plasmin cleavage sites (J–L), and inhibition of the endogenous serine proteases by aprotinin (M–O) resulted in the colocalization of the amino and carboxyl tags along the membrane of the floor plate cells, as revealed by the overlapping staining of the myc and EGFP epitopes. The black arrows point to the sites of cleavage of F-spondin. The crossed arrows indicate point mutations in the plasmin cleavage sites. Images in A–L were taken with a microscope using a digital camera. Images in M–O were taken with a confocal microscope. Bar, 50 μm.
Corroboration of the predicted cleavage sites was assessed with two mutant forms of F-spondin: deletion of the putative cleavage sites by deleting TSR5 and the region between TSR5 and 6 (TSR1-6Δ5) and point mutations in the plasmin cleavage sites (TSR1-6m; Tzarfaty-Majar et al., 2001b). The mutated proteins were flanked with EGFP and myc tags. On electroporation of the mutated proteins into the floor plate, the amino and carboxyl tags labeled the floor plate cells (Fig. 4, G–L). There was no accumulation of TSR6 at the basement membrane. The complete overlapping of the amino and carboxyl tags suggests that deleting or mutating the plasmin cleavage sites generates a plasmin-resistant F-spondin protein. The unprocessed TSR domain of F-spondin, generated by the various mutations, accumulates on the membrane of floor plate cells rather than at the ECM that underlies the floor plate.
To test whether plasmin is involved in the processing of F-spondin, the serine protease inhibitor aprotinin was used. Aprotinin was injected repeatedly into the lumen of the spinal cord in ovo. The pattern of the endogenous F-spondin and exogenous double-tagged F-spondin EGFP–TSR1-6–myc was studied. After aprotinin application, the endogenous protein, as revealed by the R3 antibody, binds uniformly to the floor plate cells (Fig. 2 E). The segregation in the deposition of F-spondin between the apical floor plate and the pia that is evident in the floor plate of untreated embryos (Fig. 2, A–D) was eradicated. In addition, the pial staining was reduced (Fig. 2 E), suggesting that the release of TSR5 and 6 from TSR1-4 is inhibited. To test directly whether aprotinin inhibits F-spondin processing, EGFP–TSR1-6–myc was expressed at the floor plate, followed by aprotinin treatment. The amino and carboxyl tags labeled the floor plate cells in an overlapping manner (Fig. 4, M–O), resembling the pattern of the mutated proteins (Fig. 4, G–L). The resistance of F-spondin to cleavage after aprotinin injection provides evidence that F-spondin processing occurs extracellularly. Thus, the cleavage of F-spondin by serine protease is required for the diffusion and accumulation of the adhesive TSRs at the basement membrane.
The membrane-bound TSR1-4 fragment of F-spondin is contact repellent for commissural axons
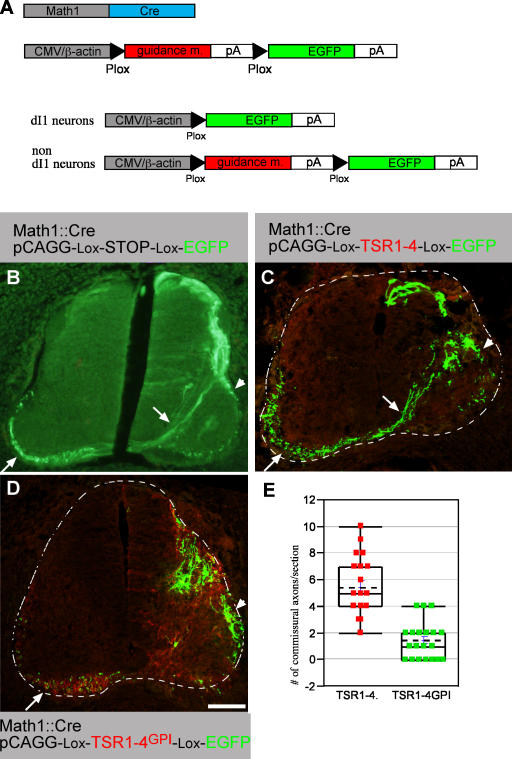
The localization of the TSR1-4 fragment, together with the finding that it does not support neurite outgrowth in vitro, implies that it may prevent the growth of commissural axons into the floor plate cells. To test the potential inhibitory effect of the TSR1-4 fragment on commissural axons, the fragment was ectopically expressed in the lateral chick neural tube. Expression of a guidance molecule in the presumed responding neurons can hinder the ability of the neurons to respond to the exogenous, target-derived guidance molecule. Thus, an alternate reporter/guidance molecule approach was used. This method generates two different expression patterns: EGFP in the dI1 neuronal subpopulation using the Math1 enhancer (Helms and Johnson, 1998) and a guidance molecule in nondI1 cells using a cytomegalovirus (CMV) enhancer (Fig. 5 A). dI1 interneurons give rise to commissural and ipsilaterally projecting axons (Fig. 5 B and Fig. S3, B–D). Embryos were electroporated at stages 17 and 18 and analyzed at stage 26. The expression of TSR1-4 in nondI1 cells was barely detectable (Fig. 5 C), suggesting that the ectopically expressed TSR1-4 does not bind avidly to the cell surface of the lateral spinal cord neurons. The presence of TSR1-4 along the axonal trajectory pathway of dI1 axons did not alter their projection. dI1 cells projected axons in commissural and ipsilateral patterns (Fig. 5 C). The projection pattern is similar to the axonal pattern of embryos electroporated with Math1-Cre and EGFP-Cre–dependent plasmids (Fig. 5 A).
Figure 5.
Membrane-tethered TSR1-4 inhibits commissural outgrowth in vivo. (A, top) Two plasmids are coelectroporated into the chick neural tube. Math1 enhancer, which drives expression in the dI1 dorsal interneurons (Helms and Johnson, 1998), was used to drive expression of Cre recombinase and an alternate guidance molecule/EGFP plasmid. This plasmid contains a CMV enhancer followed by a guidance molecule (F-spondin or ApoER2) flanked with two Plox sites, followed by EGFP. (A, bottom) On electroporation into the chick spinal cord, the guidance molecule will be excised in dI1 cells that express Cre, leading to EGFP expression, whereas nondI1 cells present along the dI1 axonal pathway will express the guidance molecule. EGFP (B), TSR1-4 (C), and TSR1-4GPI (D) were electroporated using an EGFP alternating cassette along with a Math1-Cre plasmid. An alternating cassette contains a Plox-TSR-Plox-EGFP cloned in pCAGG vector. Electroporation was performed at stages 17–18 and embryos were analyzed with anti-myc (Cy3) and anti-EGFP (Cy2) antibodies at stage 26. (B) Expression of EGFP in dI1 cells. dI1 neurons project their axon toward and across the floor plate (arrow) and ipsilaterally (arrowhead). Commissural axons projecting diagonally toward the floor plate and across it to the contralateral side are observed (arrow). (C) dI1 EGFP-expressing cells are present at the dorsal, dorsal-lateral, and medial-lateral spinal cord. Commissural axons projecting diagonally toward the floor plate and across it to the contralateral side are observed (arrow). In addition, the medial-lateral dI1 subpopulation projects ipsilaterally (arrowhead). (D) Very few axons are extending diagonally toward the floor plate at the TSR1-4GPI domain (myc epitope in red). EGFP-expressing axons and TSR1-4GPI–expressing axons projecting longitudinally at the contralateral side are evident (arrow). (E) Quantification of the extent of commissural projection in B and C. Sections from four different embryos (for each group) with matching EGFP intensity were selected. 25 sections of the TSR1-4GPI and 20 sections of the TSR1-4 were analyzed. The number of diagonally crossing axons toward the floor plate was scored. Using a t test shows a significant difference between the TSR1-4 and TSR1-4GPI groups, under a significant level of α = 0.05. The dashed lines demarcate the spinal cord. The image in A was taken with a microscope using a digital camera. Images in C and D were taken with a confocal microscope. Bar, 100 μm.
The inability of TSR1-4 to inhibit axonal outgrowth in vivo contradicts the inhibitory activity obtained in vitro with a substrate-bound protein (Fig. 1 and Fig. S1; Tzarfaty-Majar et al., 2001a), thus suggesting that the repulsive activity of TSR1-4 is context dependent and may require immobilization of the protein. It is conceivable that the TSR1-4 protein is anchored to the floor plate cells by binding to a floor plate–specific receptor, whereas the ectopically expressed protein is soluble. We hypothesized that a membrane-tethered form of F-spondin may mimic the immobilized form of the protein, yielding its inhibitory effect. Therefore, a GPI signal was added to the carboxyl end of the TSR1-4 fragment.
A TSR1-4GPI/EGFP alternate plasmid with a Math1-Cre plasmid were expressed in the chick neural tube. In contrast to TSR1-4, the staining of ectopic TSR1-4GPI is intense, and cell bodies as well as the axons express it, as revealed by the myc- positive axons at the contralateral side of the spinal cord (Fig. 5 D). The expression of TSR1-4GPI along the trajectory pathway of dI1 axons resulted in substantial elimination of the diagonally crossing commissural axons (Fig. 5 D). The number of diagonally crossing axons toward the floor plate was evaluated. A mean of 5.45 ± 2.2 axons per section was scored in the TSR1-4–treated embryos (n = 20) whereas 1.48 ± 1.35 axons were scored in the TSR1-4GPI–treated embryos (n = 25; Fig. 5 E). The repulsion of dI1 axons for TSR1-4GPI, demonstrated in this protocol, is specific for commissural axons because motor neurons expressing either TSR1-4GPI or EGFP projected laterally in a normal manner (unpublished data). These results support the hypothesis that the endogenous TSR1-4 fragment of F-spondin should be associated with the floor plate cell surface to exert its repulsive effect. TSR1-4 and TSR1-4GPI have no effect on cell fate, as determined by cell fate markers (Fig. S4, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200702184/DC1).
Immobilization of the TSR1-4 fragment of F-spondin by LRP receptors
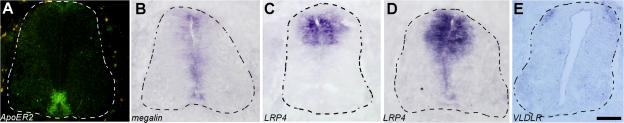
The TSR1-4 fragment of F-spondin binds to the ApoER2 (Hoe et al., 2005). Fluorescent in situ hybridization with the chick ApoER2 probe demonstrates that ApoER2 is expressed in the lateral floor plate and in the ventral ventricular zone. A low level of expression is also evident at the midline (Fig. 6 A). Thus, ApoER2 is expressed at the floor plate, mostly at the lateral floor plate cells. Therefore, ApoER2 may bind to F-spondin, immobilize it, and present it to the commissural axons.
Figure 6.
Expression pattern of LRP receptors in the chick embryonic spinal cord. In situ hybridization of ApoER2 (A), LRP2/megalin (B), and LRP4 receptors at E5 chick spinal cord (D), and LRP4 (C) VLDLR (E) at E4 chick spinal cord. (A) ApoER2 expression is confined to the midline, the lateral floor plate cells, and the ventral ventricular zone. Lower levels are detected at the medial floor plate. (B) Megalin is expressed at the ventricular zone, including the floor plate. (C) LRP4 is expressed at E4 in the dorsal third neural tube, at the ventricular zone, and laterally to the ventricular zone. (D) At E5, LRP4 expression spreads to the ventricular zone and the floor plate. (E) VLDLR is expressed in subpopulation of dorsal interneurons. Colabeling with Lhx2 reveals that VLDLR is expressed in dI1 neurons (not depicted). The dashed lines demarcate the spinal cord. Bars: (A, B, and D) 75 μm; (C and E) 100 μm.
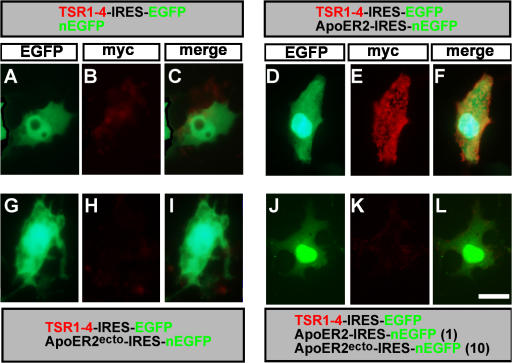
The possible immobilization of TSR1-4 by ApoER2 was tested in vitro. COS cells were transfected with a myc-tagged TSR1-4 or cotransfected with TSR1-4 and ApoER2. Surface staining (unfixed cells) with 9E10 mAb was used to reveal immobilization of TSR1-4 to the cell membranes. Cells expressing (Fig. 7, A–C) or coexpressing TSR1-4 and a secreted form of ApoER2, ApoER2ecto (Fig. 7, G–I), did not present TSR1-4 on their cell surface. However, coexpression of ApoER2 and TSR1-4 resulted in the immobilization and presentation of TSR1-4 on the cell surface of the expressing cells (Fig. 7, D–F). TSR1-4 did not bind to DCC when coexpressed in COS cells (Fig. S5, A–C, available at http://www.jcb.org/cgi/content/full/jcb.200702184/DC1). Thus, ApoER2 is required for the cell surface immobilization of the TSR1-4 fragment of F-spondin. Consistent with the in vitro experiment, when expressed in the chick hemitube, the combination of ApoER2 and TSR1-4 yielded intense cell surface staining in vivo (Fig. 8, A and B). The staining, however, is confined to cell bodies and is not detected on axons.
Figure 7.
F-spondin is immobilized to the cell surface by the ApoER2 receptors. ApoER2 is required for the binding of myc-tagged TSR1-4 to the cell surface of COS cells. COS cells were transfected with TSR1-4–IRES–EGFP and nEGFP (A–C), TSR1-4–IRES–EGFP and ApoER2-IRES-nEGFP (D–F), a secreted form of ApoER2 (ApoER2ecto-IRES-nEGFP and TSR1-4–IRES–EGFP [G–I]), ApoER2ecto-IRES-nEGFP, ApoER2-IRES-EGFP, and TSR1-4–IRES–EGFP (J–L). At 48 h after the transfection, surface staining (without fixation) of the cells was preformed. TSR1-4 is bound to the cell surface, where it is coexpressed with ApoER2 (D–F). Bar, 5 μm.
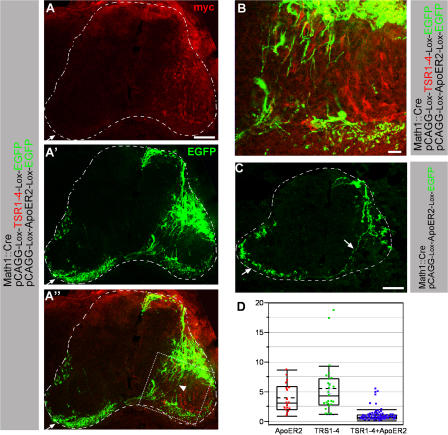
Figure 8.
ApoER2 is required for eliciting TSR1-4 repulsive activity. ApoER2 (A–C) was coelectroporated with TSR1-4 (A and B) using EGFP-alternating cassettes along with a Math1-Cre plasmid. An alternating cassette containing Plox-ApoER2-Plox-EGFP cloned in a pCAGG vector was used for ApoER2 expression. (A and B) The dotted square in A′ is presented as an enlargement in B. ApoER2 coexpressed with TSR1-4 in dI1-negative cells and EGFP in dI1 cells. dI1 axons are circumventing ApoER2 + TSR1-4–expressing domains (A and B). Many axons are deflected laterally (arrowhead). The number of diagonally crossing axons is reduced. (C) ApoER2 was expressed in dI1- negative cells and EGFP in dI1 cells. The dI1 cell projects axons toward the floor plate (arrow). The dashed lines demarcate the spinal cord. (D) Quantification of erroneous projection of dI1 axons. Sections with matching EGFP intensity were selected: ApoER2 18 (from two embryos), TSR1-4 25 (from two embryos), and ApoER2 + TSR1-4 73 (from five embryos). In each section, the number of axons projecting diagonally toward the floor plate versus the axons that are projecting laterally in the motor neurons domain was determined by quantifying EGFP brightness intensity using National Institutes of Health image software. The ordinate is the ratio between the normal diagonally crossing axons and the erroneous axon projecting at the ventral lateral spinal cord. The projection pattern of dI1 axons growing in a TSR1-4 milieu was compared with ApoER2 and ApoER2 + TRS1-4 milieus. Comparisonsfor all pairs using the Tukey-Kramer honestly significant difference test shows a significant difference between the ApoER2 + TRS1-4 group and the TSR1-4 and ApoER2 groups, and no difference between the TSR1-4 and ApoER2 groups, with a significance level of α = 0.05. Images in A and B were taken with a confocal microscope. The image in C was taken with a microscope using a digital camera. The ends of the box in D are the 25th and 75th percentiles. The line and the dashed line across the box identify the median and the mean, respectively. The horizontal line above the box represents the outermost data point that falls within the 75th percentile plus 1.5 the interquartile range (75th minus 25th percentile). The same applies for the line below the box. Bars, (A and C) 150 μm; (B) 20 μm.
The possible binding of F-spondin to other LRP receptors was tested using the aforementioned immobilization assay. The TSR1-4 fragment of F-spondin binds to very-low-density lipoprotein receptor (VLDLR), LRP4, and megalin (Fig. S5, D–L). The expression pattern of LRP genes was analyzed by mRNA in situ hybridization. Megalin is expressed in the floor plate and in the ventricular zone (Fig. 6 B). LRP4 is expressed at embryonic day (E) 4 in the dorsal third neural tube (Fig. 6 C). Its expression overlaps the region of commissural neurons. At E5 the expression of LRP4 spreads to the ventricular zone and the floor plate (Fig. 6 D). VLDLR is expressed in the dI1 subpopulation of commissural neurons (Fig. 6 E). Thus, LRP receptors that bind F-spondin are expressed at the floor plate and in commissural axons.
Immobilization of the TSR1-4 fragment of F-spondin by LRP receptors results in exertion of its repulsive activity
To test whether ApoER2 may collaborate with the TSR1-4 fragment of F-spondin in inhibiting commissural axon outgrowth, TSR1-4 and ApoER2 were jointly expressed unilaterally at the chick neural tube using TSR1-4/EGFP and ApoER2/EGFP alternating plasmids with a Math1-Cre plasmid. Ectopic expression of ApoER2 at stage 18 had no effect on cell fate as determined by cell fate markers (Fig. S4 C). Coexpression of TSR1-4 and ApoER2 along the dI1 axonal pathway resulted in an axonal failure to turn diagonally toward the floor plate (Fig. 8, A and B). Axons seem to circumvent the ApoER2/TRS1-4 domains. The ratio between the accurate, diagonally crossing axons and the erroneous axon growing in the ventral lateral neural tube is 1.09 ± 1.07 (n = 76; Fig. 8 D). Electroporation of ApoER2 did not cause any dI1 axonal errors and axons projected diagonally toward the floor plate (Fig. 8 C). The ratio between normal and erroneous axonal projection in ApoER2 + TSR1-4 ectopic expression is significantly different from ectopic ApoER2 (4 ± 2.41, n = 18) and ectopic TSR1-4 (5.58 ± 4.39, n = 25; Fig. 8 D).
In ectopic TSR1-4/ApoER2 expression, EGFP axonal labeling is evident in the white matter on the ipsi- and contralateral sides of the floor plate, indicating that dI1 axons elongated in the white matter rather than in the neuroepithelium expressing ApoER2/TSR1-4. Thus, the attraction to the floor plate is retained. The extent of commissural axonal lateral deflection and the failure to cross the midline to the contralateral side in ApoER2/TSR1-4 is smaller than the erroneous phenotype obtained with TSR1-4GPI. This may result from the larger spinal cord domain that is occupied by TSR1-4GPI neuronal cell bodies and axonal processes as opposed to ApoER2-immobilized TSR1-4 that is restricted to cell bodies.
Impeding F-spondin/LRP binding facilitates the growth of commissural axons into the floor plate cells
The redundancy in numerous F-spondin binding receptors precludes the use of loss-of-function approach. As an alternative, we chose to impede the endogenous interaction of F-spondin and F-spondin family proteins with LRP receptors.
ApoER2 binds to the nonadhesive TSR of F-spondin TSR1-4. The adhesive TSR5 and 6 do not bind to ApoER2 (Hoe et al., 2005). This provides the means to specifically block the immobilization of the endogenous TSR1-4 fragment of F-spondin using a secreted (ecto) form of ApoER2. To test whether ApoER2ecto can block the binding of TSR1-4 to the membranal ApoER2, COS cells were transfected with myc-tagged TSR1-4, ApoER2, and various concentrations of ApoER2ecto. At an ApoER2/ApoER2ecto ratio of 1:10, no binding of TSR1-4 to the cell surface was detected (Fig. 7, J–L). Coexpression experiments with ApoER2ecto and other LRP demonstrated that it blocks the binding of F-spondin to LRP4, megalin, and VLDLR (unpublished data).
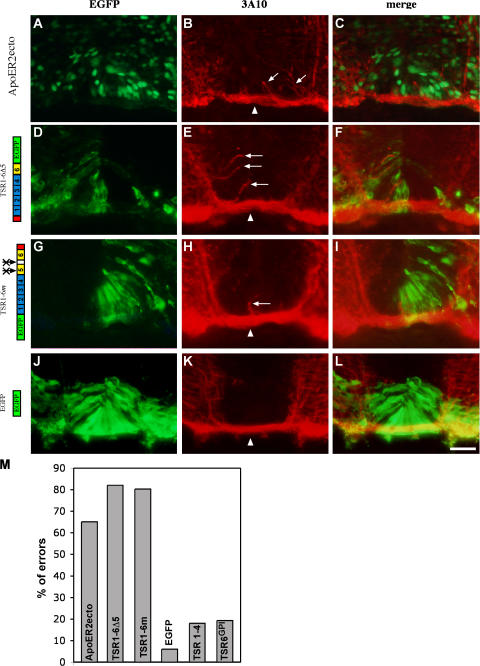
Thus, ApoER2ecto can serve as a dominant-negative form of ApoER2 that blocks the binding and immobilization of the endogenous TSR1-4 to the endogenous floor plate–derived LRPs ApoER2, megalin, and LRP4. ApoER2ecto was expressed ectopically at the ventral spinal cord. The ventral ectopic expression of ApoER2ecto yielded erroneous axonal projection at the midline. Axons are detached from the ventral bundle that underlies the floor plate and turn dorsally within the floor plate cells (64%, n = 115; Fig. 9, A–C and M). ApoER2ecto has no patterning effect when expressed in the lateral and ventral spinal cord and no axonal guidance effects when expressed in the lateral spinal cord (unpublished data). However, it cannot be excluded that ApoER2ecto may directly affect axon guidance specifically at the floor plate.
Figure 9.
Inhibiting LRPs/F-spondin binding enables growth of commissural axons into the floor plate cells. ApoER2ecto (A–C) or F-spondin EGFP-tagged isoforms (D–L) were electroporated into the ventral spinal cord at stages 12–14. Cross-sections of stage 22–24 embryos were stained with anti-EGFP (A, D, G, and J) or antineurofilament mAb 3A10 (B, E, H, and K). In the control experiment, expression of EGFP (J–L), commissural axons cross the midline as a tight bundle (arrowhead) under the floor plate cells whereas expression of ApoER2ecto (A–C) and the mutated F-spondin's isoforms (D–L) resulted in a dorsal erroneous neurites projection into the floor plate cells (B, E, and H, arrows). (M) Quantification of the extent of pathfinding errors at the midline. For each protein ∼100 sections were inspected. A cross-section with axons extending dorsally at the floor plate was scored as an error. The crossed arrows indicate point mutations in the plasmin cleavage sites. Bar, 50 μm.
F-spondin activity at the midline might be shared by other class-two TSR proteins that are expressed at the central nervous system. Mindin and SCO-spondin (SCO-spondin contains 13 class-one and 12 class-two TSR repeats) are expressed at the floor plate (Higashijima et al., 1997; Lichtenfeld et al., 1999; Guinazu et al., 2002). The TSR of mindin and 19 out of the 25 SCO-spondin TSRs are not adhesive (as predicted by the absence of basic amino acids at their third antiparallel β strand) and thus resemble TSR1-4 of F-spondin. The TSRs of SCO-spondin bind with ApoER2 (Fig. S5, M–R) and thus may also serve as a repellent cue for commissural axons. The binding of the endogenous LRPs and class-two TSR proteins can be blocked by secreted LRP and also by the dominant-negative form of TSR1-4. In vitro TSR1-4 does not promote outgrowth of spinal cord neurons. However, the inclusion of an adhesive TSR together with TSR1-4 (as in the TSR1-5 protein) converts in vitro the outgrowth activity of F-spondin to a promoting one (Burstyn-Cohen et al., 1999). The mutated noncleavable forms of F-spondin bind to the surface of floor plate cells (Fig. 4, G–L) via binding to the floor plate LRPs and probably compete with the endogenous TSR1-4. The recruitment of the adhesive TSR5 and 6 (TSR1-6m) or the TSR6 (TSR1-6Δ5) to the cell surface of floor plate cells may either serve as a gain of function (positioning of the outgrowth promoting modules on the surface of floor plate cells) or a loss of function (competing away the repulsive TSR1-4 with a nonrepulsive TSR mutant proteins).
The mutant forms of the TSR domain were electroporated into the ventral spinal cord. Commissural axons encountering the TSR1-6Δ5 (82%, n = 100; Fig. 9, D–F and M) and TSR1-6m (81%, n = 147; Fig. 9, G–I and M) turned within the floor plate dorsally into the floor plate cells. Electroporation of EGFP (6%, n = 100) or the nonadhesive fragment TSR1-4 (18%, n = 100) did not alter axonal trajectory at the floor plate (Fig. 9, J–M). The erroneous projection of the commissural axons was always confined to the ventral midline, though the mutated proteins were expressed nonspecifically throughout the entire ventral or lateral/ventral neural tube. This suggests that the adhesive TSR5 and 6 are not sufficient to alter commissural outgrowth but rather function as dominant-negative TSR1-4 that inhibits the binding of the endogenous TSR1-4 to the endogenous floor plate LRPs. In support of this, expression of a membrane-tethered form of TSR6 (TSR6GPI) at the ventral neural tube did not cause pathfinding errors at the floor plate (19%, n = 100; Fig. 9 M). These results support the hypothesis that the TSR1-4 fragment of F-spondin serves as a repellent that prevents the invasion of commissural axons into the floor plate.
Discussion
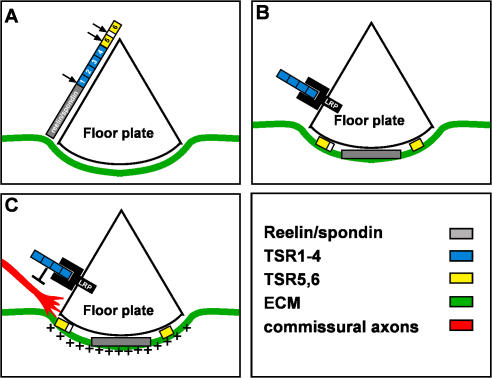
The floor plate boundary at the entering ipsilateral side of the neural tube is a checkpoint for commissural axons. Commissural axons, which extend in the neuroepithelium, advancing in a netrin gradient (Serafini et al., 1994; Kennedy et al., 2006), are faced with new environmental conditions. The growth cone turns from the neuroepithelium to the region between the floor plate cells and the pia, extending in the basement membrane that underlies the floor plate. The turning at the midline is accompanied by exposure to a new set of short-range guidance molecules expressed at the floor plate and a change in the pattern of receptors presented on the growth cone. We have provided evidence that F-spondin protein expressed at the floor plate participates in shaping the precise turning into the ventral midline. F-spondin processing in vivo generates two functionally opposing fragments, an inhibitory TSR1-4 and an adhesive TSR6, that are deposited in different extracellular milieus that flank or associate with, respectively, the crossing fibers of commissural axon (Fig. 10, A and B). In vivo gain- and loss-of-function experiments demonstrate that the repulsive TSR1-4 fragment of F-spondin contributes to the constriction of commissural axons to the basement membrane under the floor plate cells (Fig. 10 C). The coordinated posttranslational generation of two functionally antagonistic polypeptides from a single protein is a novel mechanism for increasing the complexity of a given guidance molecule. Revealing the inhibitory activity of TSR1-4 requires immobilization and presentation by LRP receptors. Thus, membrane proteins, like proteoglycans, contribute to the regulation and activation of guidance molecules.
Figure 10.
The role of F-spondin in midline crossing. (A) F-spondin is expressed and secreted from the floor plate cells. F-spondin is subjected to proteolysis by serine proteases within the amino end of TSR5 and between TSR5 and 6 (arrows). A yet unidentified protease cleaves F-spondin between the spondin domain and TSR1 (arrow). (B) The reelin/spondin domain (Burstyn-Cohen et al., 1999) and the adhesive TSR5 and 6 bind to the ECM that underlies the floor plate. The TSR1-4 fragment binds to the apical floor plate cells via interaction with the LRP receptors ApoER2, megalin, and LRP4. (C) The cell surface–tethered TSR1-4 repels commissural axons and prevents their penetration into the floor plate cells. The adhesive TSRs provide an outgrowth supportive substrate for the commissural axons.
Short-range repulsion from the floor plate cells and short-range attraction to midline basement membrane
The phenotype obtained after perturbation of ApoER2/TSR1-4 binding resembles the phenotype of the Slits and Robo1 null mice. Interaction between the Slit-repellent proteins, expressed at the floor plate, and the Robo receptors, expressed in commissural neurons, is required for midline crossing (Long et al., 2004; Sabatier et al., 2004). In the triple Slits and Robo1 null mice, axons fail to leave the floor plate and commissural axons collapse at the midline. In addition, many axons project dorsally at the floor plate toward the ventricular zone (Long et al., 2004). Thus, Slit proteins are likely to play a repulsive role in deflecting commissural axons away from their site of expression—the floor plate cells. In both mutations, the triple Slits and Robo1, most of the axons still elongate under the floor plate cells (Long et al., 2004), suggesting that a repulsive floor plate–derived activity might be shared with other proteins. F-spondin may cooperate with Slit proteins in squeezing commissural axons between the floor plate cells and the basement membrane. This cooperation is likely to be mediated by parallel signaling pathways because F-spondin does not bind to Robo receptors (unpublished data).
Ectopic expression of a membrane-tethered form of TSR6 did not alter the projection pattern of commissural axons before and while crossing the floor plate. The inability to affect axonal projection as the axons elongate toward the floor plate may result from spatially restricted expression of putative TSR6 receptors, expressed only as the axons transverse the floor plate. In support of this, the TSR1-6 fragment of F-spondin fused to alkaline phosphatase–labeled floor plate cells and axons under the floor plate (unpublished data). However, even at the midline, axons are not attracted to the ectopic cell surface–associated TSR6. The repositioning of TSR6 along the cell surface may not shift the ratio between repulsive and attractive cues in the floor plate cells. The repulsive activity of TSR1-4 and Slits may dominate the attractive activity of the exogenous TSR6. Furthermore, the short-range attraction of the midline basement membrane, mediated by the endogenous TSR5 and 6 of F-spondin, together with other adhesive molecules, is potentially more robust than the adhesiveness of the ectopic TSR6. Inhibition of both the repulsive activity of endogenous TSR1-4 and the deposition of TSR5 and 6 at the basement membrane, together with ectopic expression of TSR6, is required to elucidate the presumed short-range attractive role of TSR6.
Immobilization of TSR1-4 by LRPs
Guidance molecules are presented to the growth cone as either membrane-bound or ECM-attached molecules. Semaphorins 4, 5, and 6; eprinBs; and the Eph receptors that reverse signal through interaction with ephrinAs are membranal proteins. EphrinAs are GPI encored. Netrin, Slits, and semaphorin 3 bind to the ECM via interaction with proteoglycans. Global blocking of glycosaminoglycan synthesis results in many axonal guidance defects, many of them at the midline (for review see Van Vactor et al., 2006). The immobilized TSR1-4 fragment of F-spondin was shown in vitro to inhibit neurite outgrowth of spinal cord neurons (Fig. 1 A; Tzarfaty-Majar et al., 2001a). However, TSR1-4 does not bind to proteoglycans or the ECM (Tzarfaty-Majar et al., 2001b), and subsequently, when applied ectopically in vivo, does not perturb axonal wiring. The inhibitory activity of TSR1-4 is exerted by a novel mechanism, i.e., binding to a cell surface receptor expressed in cis: the ApoER2, megalin, and the LRP4 receptor. A knockdown of ApoER2, megalin, and LRP4 is required to substantiate the requirement of LRPs for TSR1-4 immobilization to the floor plate cells.
Two LRPs are expressed in commissural axons. Expression of LRP4 in the dorsal neural tube is in the ventricular zone and also spreads to cells lateral to the ventricular zone. It is formally possible that LRP4 protein is stable and expressed in postmitotic commissural neurons. However, VLDLR is expressed in the postmitotic dI1 neurons. LRP4 and VLDLR may serve as a signaling receptor for F-spondin.
Other TSR proteins at the floor plate
Several class-two TSR proteins are expressed at the floor plate. F-spondin and SCO-spondin are expressed at the floor plate of zebrafish, Xenopus laevis, chicks, and rodents (Lichtenfeld et al., 1999) whereas mindin is expressed at the floor plate of zebrafish (Higashijima et al., 1997). The structural homology between F-spondin, mindin, and SCO-spondin, and their extensive overlapping expression domains, is likely to represent redundancy in their biological activities. This notion is reinforced by the expression pattern of SCO-spondin protein at the floor plate. Immunolabeling with anti–SCO-spondin in various vertebrates reveals that SCO-spondin protein is expressed on the floor plate cells above the midline bundle of the commissural axons (Lichtenfeld et al., 1999; del Brio et al., 2000; Richter et al., 2001), similar to the site of deposition of TSR1-4.
The amino acid composition at the third antiparallel strand of SCO-spondin suggests that some TSRs of SCO-spondin are adhesive (TSR2, 3, 8, 14, 18, 19, and 24), and may play a redundant role to TSR5 and 6 of F-spondin. However, it is not known whether SCO-spondin is subjected to proteolysis that is required for release of the adhesive domains to the ECM.
The foremost factors directing the projection of commissural axons toward the floor plate are the long-range guidance molecules emanating from the midlines of the neural tube: the roof plate and the floor plate, BMP7, and netrin (Serafini et al., 1994; Augsburger et al., 1999). At choice points along the pathway (entering and exiting to the floor plate), additional short-range guidance molecules (neuronglia cell adhesion molecule–related cell adhesion molecule, Slits, and F-spondin) contribute to the accurate projection of commissural axons (Klar et al., 1992; Stoeckli and Landmesser, 1995; Brose et al., 1999). An additional layer of complexity is attained by the proteolytic processing of F-spondin. Similar posttranslational modifications may augment the versatility of other guidance molecules and thus intensify the diversity of guidance cues.
Materials and methods
In ovo electroporations
Fertilized white leghorn chicken eggs were incubated at 38.5–39°C. A DNA solution of 5 mg/ml was injected into the lumen of the neural tube at either Hamburger and Hamilton stages 12–14 (CMV enhancer in pCAGG plasmid) or stages 17 and 18 (Math1 and HoxA1 enhancers, provided by T. Lufkin, Mount Sinai School of Medicine, New York, NY). Electroporation was performed using 3 × 50 ms pulses at 25 V applied across the embryo using a 0.5-mm tungsten wire and an electroporator (ECM 830; BTX). Embryos were incubated for 2–3 d before analysis.
In ovo aprotinin treatment
Aprotinin (Protosol [500,000 kallikrein inactivator units/ml]; Kamada) was injected into the lumen of the spinal cord at 10-h intervals from stages 16–23 (five injections). Embryos were analyzed at stage 25.
Immunohistochemistry
Embryos were fixed overnight at 4°C in 4% paraformaldehyde/0.1 M phosphate buffer, washed twice with PBS, incubated in 30% sucrose/PBS for 24 h, and embedded in optimal cutting temperature compound. 14-mm cryostat sections were collected on Superfrost Plus slides (Fisher Scientific) and kept at −70°C. The following antibodies were used: 3A10 (dilution 1:10), 4D7, HNF3β (dilution 1:10), MNR2 (dilution 1:100), Pax7 (dilution 1:5), Nkx2.2 (dilution 1:10; all provided by T. Jessell, Columbia University, New York, NY), and 9E10 (dilution 1:200). The rabbit polyclonal GFP antibody (dilution 1:500) was obtained from Invitrogen.
For the R3 antibody, embryos were fixed in 4% paraformaldehyde/0.1 M phosphate buffer and processed for embedding in paraffin. 8-μm sections were cut and collected on Superfrost Plus slides. Paraffin was removed by immersion in xylene and the sections were rehydrated using a graded ethanol/H2O series. For antigen retrieval, slides were submerged completely in 10 mM sodium citrate, pH 6.0, in a glass histology box. The buffer was then brought to boiling in a pressure cooker (PickCell Laboratories), and the slides were boiled in buffer for 1 h. Slides immersed in buffer were allowed to cool for 15 min and washed twice for 5 min in PBS at room temperature.
Images were taken on a microscope (Axioscope 2; Zeiss) with a digital camera (DP70; Olympus) or confocal microscope (Eclipse C1; Nikon). Cy2 and 3 were used as fluorochromes.
Outgrowth assay
E13 rat and E6 chick dorsal spinal cord neurons were obtained and plated on F-spondin fragments as described previously (Burstyn-Cohen et al., 1999). F-spondin proteins TSR1-4_HIS, and TSR6_HIS were affinity purified on an affinity column (Talon; CLONTECH Laboratories, Inc.).
DNA
The floor plate–specific enhancer Hoxa-1 enhancer III was provided by T. Lufkin. The human ApoER2 was obtained from the I.M.A.G.E. Consortium (6143442). The chick LRP4 (ChEST392o8) was obtained from the Chick EST Project. To generate the ApoER2ecto the sequences downstream to amino acid 472 were deleted using a BglII site. To generate TSR1-4GPI, TSR5 GPI, and TSR6GPI, a synthetic DNA sequence encoding the prion GPI signal (TCTAGATCCAGCGCGGTGCTGTTCTCCTCCCCTCCTGTGATCCTCCTCATTTCCTTTCTCATCTTCCTGATGGTGGGATGA) was cloned in frame downstream from the TSR fragments of F-spondin.
A megalin minigene was constructed from two partially overlapping EST clones (ChEST548d5 and ChEST437i20) that encompass the third complement-type repeats of chick megalin gene (amino acids 2704–3102), obtained from the Chick Est sequencing project. The ESTs were annealed and “filled in” by PCR using a 5′ (5′-CGAAGCTTTGGAAATGTGACAACGACAATG-3′) and 3′ primer (5′-TAGGATCCCACTGCATTCATTTATACCAC-3′). The PCR product was subcloned into a pSecTagB vector (Invitrogene). A membranal form of megalin was generated by fusing the secreted chick minigene of megalin to the carboxyl end of ApoER2 (transmembrane and cytoplasmatic domains).
TSR12 and 26 of the mouse SCO-spondin were obtained by PCR from genomic mouse DNA. PCR products were cloned in pSecTagB.
Fluorescence in situ hybridization
Fluorescence in situ hybridization of chick spinal cords was performed as described in the TSA plus protocol (PerkinElmer).
Online supplemental material
Fig. S1 depicts the outgrowth of rat E13 dorsal spinal cord neurons on a substrate of purified TSR1-4 and 6. Neurons extend neurites only when cultured on TSR6. Fig. S2 depicts epitope mapping of the R3 antibody. R3 recognizes TSR3 and 5. Fig. S3 describes the expression pattern of the floor plate–specific enhancer HoxA-1 and the commissural-specific enhancer Math-1. Fig. S4 depicts the effect of TSR1-4, TSR1-4GPI, and ApoER2 on the patterning of the neural tube. None of these proteins changes the patterning of neural tube when expressed ectopically in the spinal cord. Fig. S5 illustrates the binding of F-spondin's and SCO-spondin's TSR to LRP receptors. The TSR1-4 fragment of F-spondin binds to LRP2, LRP4, and VLDLR. TSR12 and 26 of SCO-spondin bind to ApoER2. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200702184/DC1).
Supplementary Material
Acknowledgments
The authors thank Thomas Jessell for the 3A10, HNF3β, Nkx2.2, MNR2, 4D7, and Pax7 antibodies; Artur Kania for the chick ApoER2 gene; and Dr. Thomas Lufkin for the HoxA-1 enhancer. We are also grateful to Esther Stoeckli, Artur Kania, Mike Fainzibler, and Sam Pffaf for comments on the manuscript.
This work was supported by grants to A. Klar from the Israel-USA Binational Science Foundation (2001/182), the Israel Science Foundation (928/04), and the German-Israel Foundation (G-739.07.1/2002).
V. Tzarfaty-Majar's present address is Department of Physiology, Hebrew University, Jerusalem 91120, Israel.
Abbreviations used in this paper: ApoER2, apolipoprotein E receptor 2; CMV, cytomegalovirus; E, embryonic day; GPI, glycosylphosphatidylinositol; LRP, lipoprotein receptor–related protein; SCO, subcommissural organ; TSR, thrombospondin type-one repeat; VLDLR, very-low-density lipoprotein receptor.
References
- Augsburger, A., A. Schuchardt, S. Hoskins, J. Dodd, and S. Butler. 1999. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 24:127–141. [DOI] [PubMed] [Google Scholar]
- Bovolenta, P., and J. Dodd. 1990. Guidance of commissural growth cones at the floor plate in the embryonic rat spinal cord. Development. 109:435–447. [DOI] [PubMed] [Google Scholar]
- Brose, K., K.S. Bland, K.H. Wang, D. Arnott, W. Henzel, C.S. Goodman, M. Tessier-Lavigne, and T. Kidd. 1999. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 96:795–806. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen, T., V. Tzarfaty, A. Frumkin, Y. Feinstein, E. Stoeckli, and A. Klar. 1999. F-spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron. 23:233–246. [DOI] [PubMed] [Google Scholar]
- Charron, F., E. Stein, J. Jeong, A.P. McMahon, and M. Tessier-Lavigne. 2003. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 113:11–23. [DOI] [PubMed] [Google Scholar]
- Debby-Brafman, A., T. Burstyn-Cohen, A. Klar, and C. Kalcheim. 1999. F-spondin, expressed in somitic regions avoided by neural crest cells, mediates inhibition of distinct somite domain to neural crest migration. Neuron. 22:475–488. [DOI] [PubMed] [Google Scholar]
- del Brio, M.A., P. Riera, R.I. Munoz, H. Montecinos, and E.M. Rodriguez. 2000. The metencephalic floor plate of chick embryos expresses two secretory glycoproteins homologous with the two glycoproteins secreted by the subcommissural organ. Histochem. Cell Biol. 113:415–426. [DOI] [PubMed] [Google Scholar]
- Feinstein, Y., and A. Klar. 2004. The neuronal class 2 TSR proteins F-spondin and Mindin: a small family with divergent biological activities. Int. J. Biochem. Cell Biol. 36:975–980. [DOI] [PubMed] [Google Scholar]
- Guinazu, M.F., H.G. Richter, and E.M. Rodriguez. 2002. Bovine floor plate explants secrete SCO-spondin. Cell Tissue Res. 308:177–191. [DOI] [PubMed] [Google Scholar]
- Helms, A.W., and J.E. Johnson. 1998. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 125:919–928. [DOI] [PubMed] [Google Scholar]
- Higashijima, S., A. Nose, G. Eguchi, Y. Hotta, and H. Okamoto. 1997. Mindin/F-spondin family: novel ECM proteins expressed in the zebrafish embryonic axis. Dev. Biol. 192:211–227. [DOI] [PubMed] [Google Scholar]
- Hoe, H.S., D. Wessner, U. Beffert, A.G. Becker, Y. Matsuoka, and G.W. Rebeck. 2005. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell. Biol. 25:9259–9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, D.D., R. Llinas, M. Ard, J.P. Merlie, and J.R. Sanes. 1992. Expression of s-laminin and laminin in the developing rat central nervous system. J. Comp. Neurol. 323:238–251. [DOI] [PubMed] [Google Scholar]
- Kennedy, T.E., T. Serafini, J.R. de la Torre, and M. Tessier-Lavigne. 1994. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 78:425–435. [DOI] [PubMed] [Google Scholar]
- Kennedy, T.E., H. Wang, W. Marshall, and M. Tessier-Lavigne. 2006. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J. Neurosci. 26:8866–8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar, A., M. Baldassare, and T.M. Jessell. 1992. F-spondin: a gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell. 69:95–110. [DOI] [PubMed] [Google Scholar]
- Li, X., and T. Lufkin. 2000. Cre recombinase expression in the floorplate, notochord and gut epithelium in transgenic embryos driven by the Hoxa-1 enhancer III. Genesis. 26:121–122. [PubMed] [Google Scholar]
- Lichtenfeld, J., J. Viehweg, J. Schutzenmeister, and W.W. Naumann. 1999. Reissner's substance expressed as a transient pattern in vertebrate floor plate. Anat. Embryol. (Berl.). 200:161–174. [DOI] [PubMed] [Google Scholar]
- Long, H., C. Sabatier, L. Ma, A. Plump, W. Yuan, D.M. Ornitz, A. Tamada, F. Murakami, C.S. Goodman, and M. Tessier-Lavigne. 2004. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 42:213–223. [DOI] [PubMed] [Google Scholar]
- Richter, H.G., R.I. Munoz, C.S. Millan, M.F. Guinazu, C.R. Yulis, and E.M. Rodriguez. 2001. The floor plate cells from bovines express the mRNA encoding for SCO-spondin and its translation products. Brain Res. Mol. Brain Res. 93:137–147. [DOI] [PubMed] [Google Scholar]
- Sabatier, C., A.S. Plump, M. Le, K. Brose, A. Tamada, F. Murakami, E.Y. Lee, and M. Tessier-Lavigne. 2004. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 117:157–169. [DOI] [PubMed] [Google Scholar]
- Serafini, T., T.E. Kennedy, M. Galko, C. Mirzayan, T.M. Jessell, and M. Tessier-Lavigne. 1994. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 78:409–424. [DOI] [PubMed] [Google Scholar]
- Stoeckli, E.T., and L.T. Landmesser. 1995. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 14:1165–1179. [DOI] [PubMed] [Google Scholar]
- Tan, K., M. Duquette, J.H. Liu, Y. Dong, R. Zhang, A. Joachimiak, J. Lawler, and J.H. Wang. 2002. Crystal structure of the TSP-1 type 1 repeats: a novel layered fold and its biological implication. J. Cell Biol. 159:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzarfaty-Majar, V., T. Burstyn-Cohen, and A. Klar. 2001. a. F-spondin is a contact-repellent molecule for embryonic motor neurons. Proc. Natl. Acad. Sci. USA. 98:4722–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzarfaty-Majar, V., R. López-Alemany, Y. Feinstein, L. Gombau, O. Goldshmidt, E. Soriano, P. Muñoz-Cánoves, and A. Klar. 2001. b. Plasmin-mediated release of the guidance molecule F-spondin from the extracellular matrix. J. Biol. Chem. 276:28233–28241. [DOI] [PubMed] [Google Scholar]
- Van Vactor, D., D.P. Wall, and K.G. Johnson. 2006. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr. Opin. Neurobiol. 16:40–51. [DOI] [PubMed] [Google Scholar]
- Yaginuma, H., S. Homma, R. Kunzi, and R.W. Oppenheim. 1991. Pathfinding by growth cones of commissural interneurons in the chick embryo spinal cord: a light and electron microscopic study. J. Comp. Neurol. 304:78–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.