Abstract
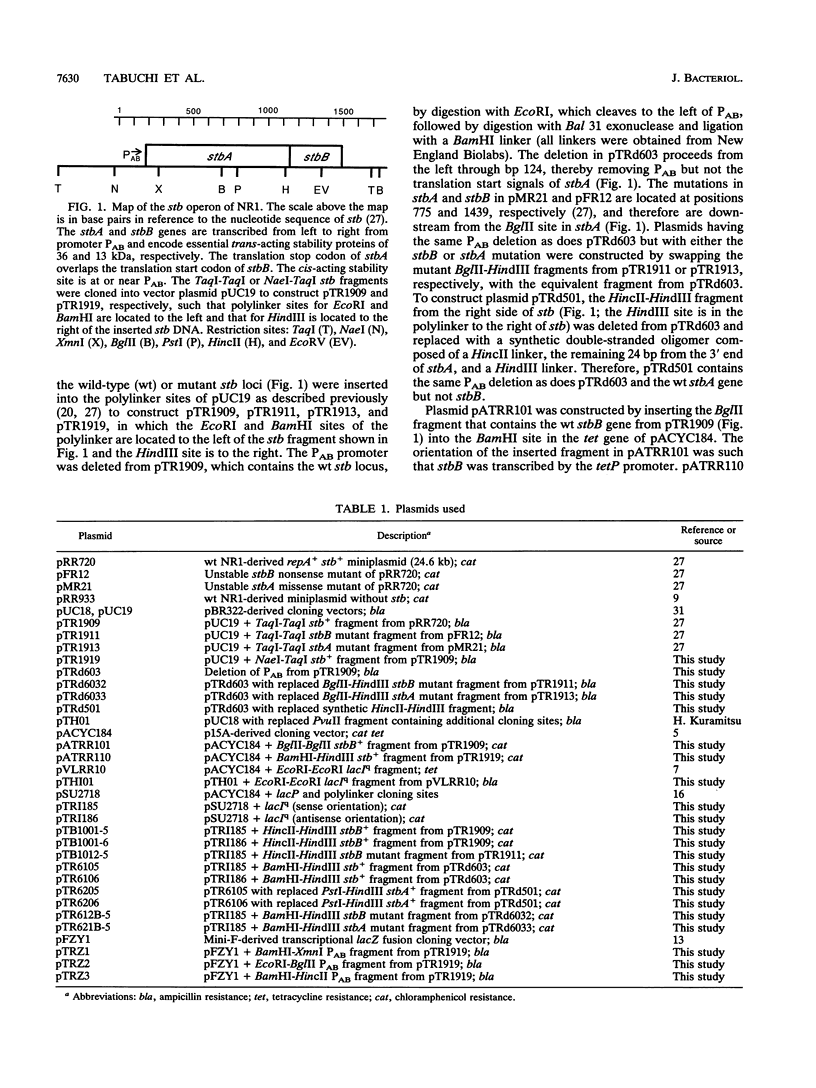
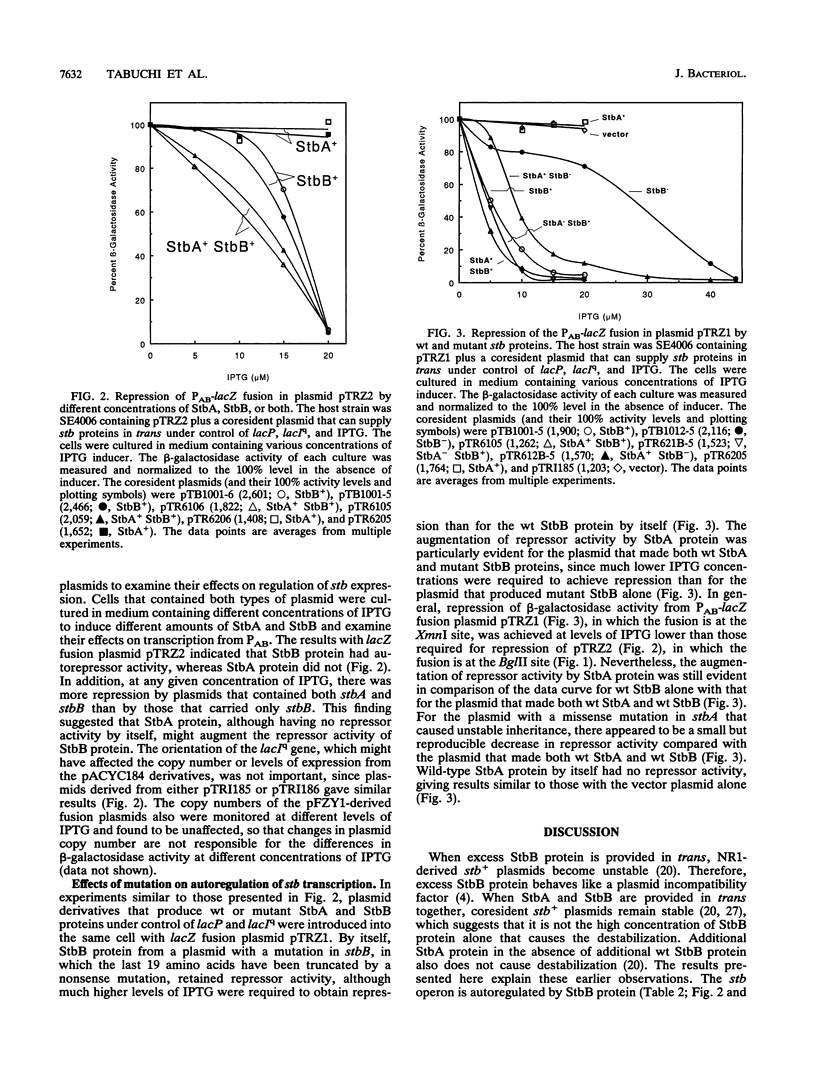
The stb locus of IncFII plasmid NR1, which mediates stable inheritance of the plasmid, is composed of an essential cis-acting DNA site located upstream from two tandem genes that encode essential stability proteins. The two tandem genes, stbA and stbB, are transcribed as an operon from promoter PAB. Using PAB-lacZ gene fusions, it was found that the stb operon is autoregulated. A low-copy-number stb+ plasmid introduced into the same cell with the PAB-lacZ fusion plasmid repressed beta-galactosidase activity about 5-fold, whereas a high-copy-number stb+ plasmid repressed beta-galactosidase about 15-fold. The details of autoregulation were analyzed by varying the concentrations of StbA and StbB to examine their effects on expression from the PAB-lacZ fusion plasmid. StbB protein by itself had autorepressor activity. Although StbA protein by itself had no detectable repressor activity, plasmids that encoded both stbA and stbB repressed more effectively than did those that encoded stbB alone. Plasmids with a mutation in stbA had reduced repressor activity. One mutation in stbB that inactivated the stability function also reduced, but did not eliminate, repressor activity. Repressor activity of the mutant StbB protein was effectively enhanced by stbA. These results indicate that StbB serves two functions, one for stable inheritance and one for autoregulation of the stb operon, both of which may be influenced by StbA protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin S. J. Plasmid partition. Plasmid. 1988 Jul;20(1):1–9. doi: 10.1016/0147-619x(88)90001-7. [DOI] [PubMed] [Google Scholar]
- Austin S., Abeles A. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J Mol Biol. 1983 Sep 15;169(2):373–387. doi: 10.1016/s0022-2836(83)80056-4. [DOI] [PubMed] [Google Scholar]
- Austin S., Abeles A. The partition functions of P1, P7, and F miniplasmids. Basic Life Sci. 1985;30:215–226. doi: 10.1007/978-1-4613-2447-8_18. [DOI] [PubMed] [Google Scholar]
- Austin S., Nordström K. Partition-mediated incompatibility of bacterial plasmids. Cell. 1990 Feb 9;60(3):351–354. doi: 10.1016/0092-8674(90)90584-2. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. A., Austin S. J. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 1988 Jun;7(6):1881–1888. doi: 10.1002/j.1460-2075.1988.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Womble D. D., Luckow V. A., Rownd R. H. Regulation of transcription of the repA1 gene in the replication control region of IncFII plasmid NR1 by gene dosage of the repA2 transcription repressor protein. J Bacteriol. 1985 Feb;161(2):544–551. doi: 10.1128/jb.161.2.544-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Easton A. M., Rownd R. H. The incompatibility product of IncFII R plasmid NR1 controls gene expression in the plasmid replication region. J Bacteriol. 1982 Nov;152(2):829–839. doi: 10.1128/jb.152.2.829-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. A., Austin S. J. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid. 1988 Mar;19(2):103–112. doi: 10.1016/0147-619x(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Molin S. Partitioning of plasmid R1. Structural and functional analysis of the parA locus. J Mol Biol. 1986 Aug 5;190(3):269–279. doi: 10.1016/0022-2836(86)90001-x. [DOI] [PubMed] [Google Scholar]
- Helsberg M., Eichenlaub R. Twelve 43-base-pair repeats map in a cis-acting region essential for partition of plasmid mini-F. J Bacteriol. 1986 Mar;165(3):1043–1045. doi: 10.1128/jb.165.3.1043-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Ruiz A. A., Godson G. N. Promotion, termination, and anti-termination in the rpsU-dnaG-rpoD macromolecular synthesis operon of E. coli K-12. Mol Gen Genet. 1984;195(3):391–401. doi: 10.1007/BF00341439. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Friedman S. A., Austin S. J. Partition site of the P1 plasmid. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8544–8547. doi: 10.1073/pnas.84.23.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., Bartolomé B., de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZ alpha reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988 Aug 15;68(1):159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Cloning of replication, incompatibility, and stability functions of R plasmid NR1. J Bacteriol. 1980 Jan;141(1):87–99. doi: 10.1128/jb.141.1.87-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y. N., Tabuchi A., Fan Y. L., Womble D. D., Rownd R. H. Complementation of mutants of the stability locus of IncFII plasmid NR1. Essential functions of the trans-acting stbA and stbB gene products. J Mol Biol. 1988 Nov 20;204(2):345–356. doi: 10.1016/0022-2836(88)90581-5. [DOI] [PubMed] [Google Scholar]
- Min Y. N., Tabuchi A., Womble D. D., Rownd R. H. Transcription of the stability operon of IncFII plasmid NR1. J Bacteriol. 1991 Apr;173(7):2378–2384. doi: 10.1128/jb.173.7.2378-2384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo A., Ohshima A., Ogura T., Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986 Nov 5;192(1):1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- Mori H., Mori Y., Ichinose C., Niki H., Ogura T., Kato A., Hiraga S. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J Biol Chem. 1989 Sep 15;264(26):15535–15541. [PubMed] [Google Scholar]
- Nordström K., Austin S. J. Mechanisms that contribute to the stable segregation of plasmids. Annu Rev Genet. 1989;23:37–69. doi: 10.1146/annurev.ge.23.120189.000345. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Tabuchi A., Min Y. N., Kim C. K., Fan Y. L., Womble D. D., Rownd R. H. Genetic organization and nucleotide sequence of the stability locus of IncFII plasmid NR1. J Mol Biol. 1988 Aug 5;202(3):511–525. doi: 10.1016/0022-2836(88)90282-3. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Womble D. D., Rownd R. H. Genetic and physical map of plasmid NR1: comparison with other IncFII antibiotic resistance plasmids. Microbiol Rev. 1988 Dec;52(4):433–451. doi: 10.1128/mr.52.4.433-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


