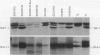
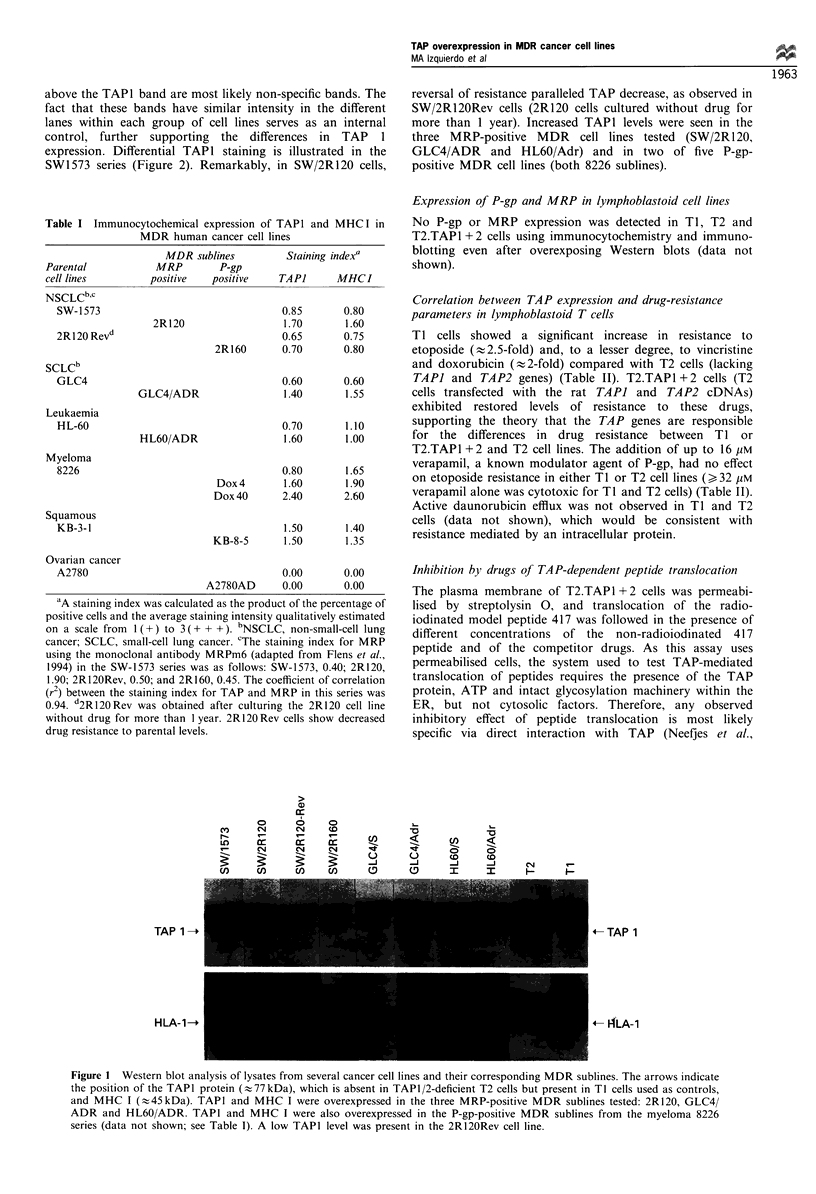
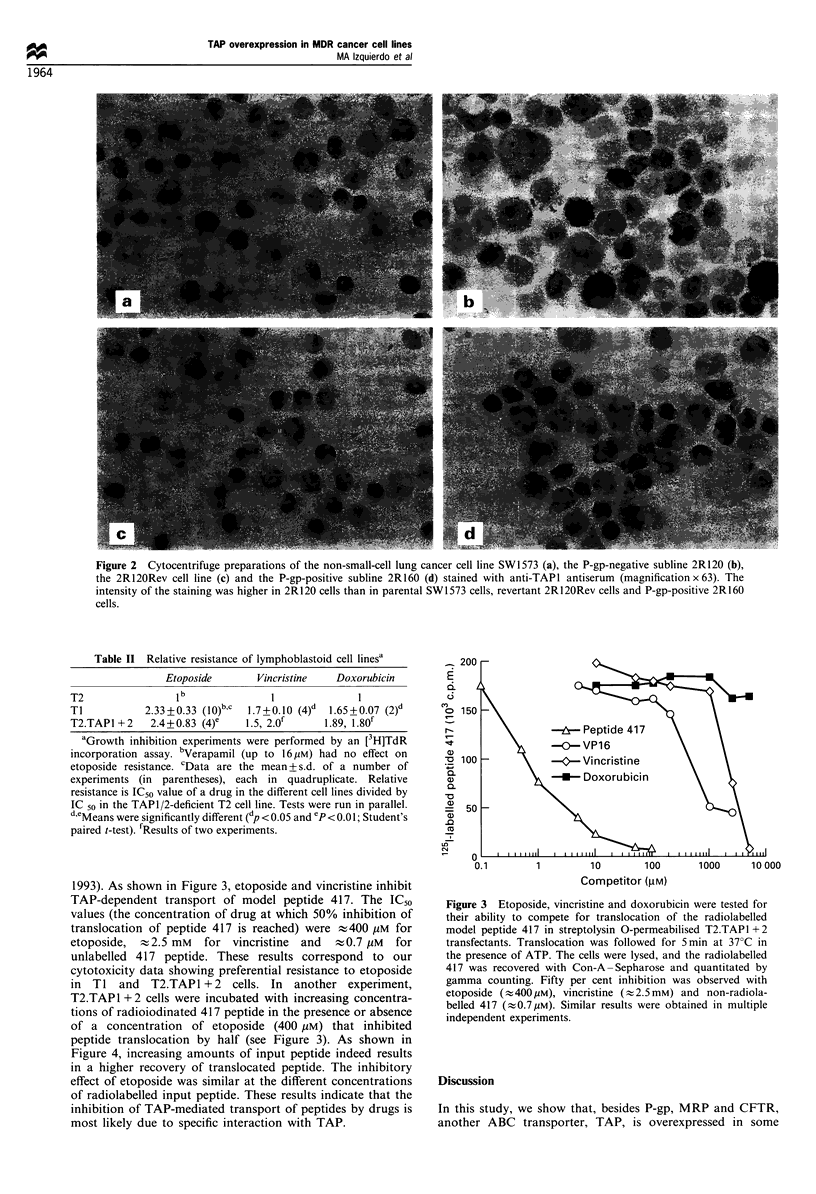
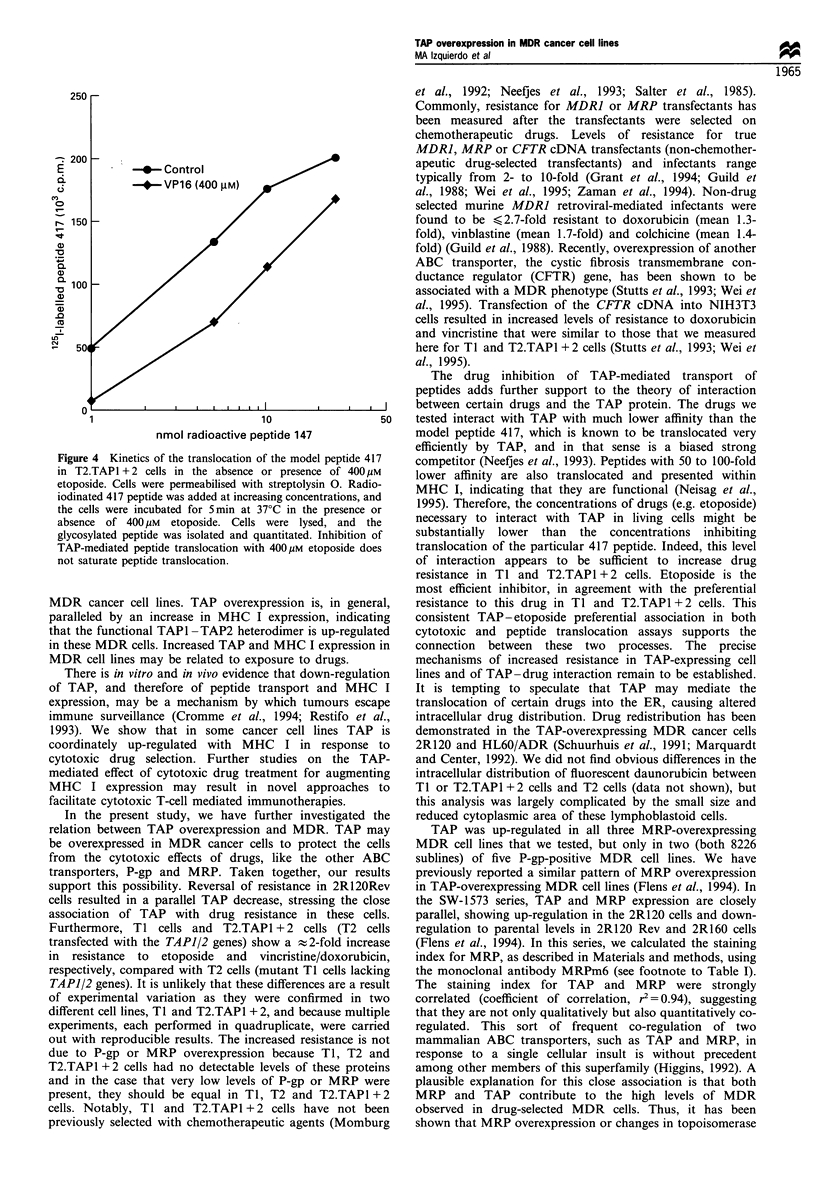
Abstract
Multidrug resistance (MDR) to anti-cancer drugs has been associated with the overexpression of P-glycoprotein (P-gp) and the multidrug resistance-associated protein (MRP), both being members of the ATP-binding cassette (ABC) superfamily of transporters. We investigated whether in addition to P-gp and MRP, another ABC transporter, the transporter associated with antigen processing (TAP), is associated with MDR. TAP plays a major role in MHC class I-restricted antigen presentation by mediating peptide translocation over the endoplasmic reticulum membrane. TAP1 and P-gp share a significant degree of homology among their transmembrane domains, which are thought to be the primary determinants of substrate specificity, and both can apparently mediate the translocation of peptides. Using immunocytochemistry and Western blot, TAP was overexpressed in parallel with MHC class I in several MDR human cancer cell lines. TAP was overexpressed more frequently in MRP-positive MDR cell lines (three out of three) than in P-gp positive MDR cells (two out of five). Reversal of resistance resulted in a decrease in TAP levels. Transfection of the TAP genes into TAP-deficient lymphoblastoid T2 cells conferred mild resistance to etoposide, vincristine and doxorubicin (2- to 2.5-fold). Furthermore, etoposide and vincristine inhibited TAP-dependent peptide translocation to the endoplasmic reticulum. Collectively, our results suggest that TAP may modestly contribute to the MDR phenotype, in particular in MRP- overexpressing MDR cells. Further insight into the role of TAP in MDR will require the study of other transfectants, as well as the investigation of TAP expression in P-gp and MRP-negative MDR cancer cell lines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Childs S., Ling V. The MDR superfamily of genes and its biological implications. Important Adv Oncol. 1994:21–36. [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Sparks K. E., Fraser K., Loe D. W., Grant C. E., Wilson G. M., Deeley R. G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994 Nov 15;54(22):5902–5910. [PubMed] [Google Scholar]
- Cromme F. V., Airey J., Heemels M. T., Ploegh H. L., Keating P. J., Stern P. L., Meijer C. J., Walboomers J. M. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med. 1994 Jan 1;179(1):335–340. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan G. D., Borgnia M. J., Regev R., Assaraf Y. G. Transport of polypeptide ionophores into proteoliposomes reconstituted with rat liver P-glycoprotein. J Biol Chem. 1994 Oct 21;269(42):26058–26065. [PubMed] [Google Scholar]
- Flens M. J., Izquierdo M. A., Scheffer G. L., Fritz J. M., Meijer C. J., Scheper R. J., Zaman G. J. Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res. 1994 Sep 1;54(17):4557–4563. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Grant C. E., Valdimarsson G., Hipfner D. R., Almquist K. C., Cole S. P., Deeley R. G. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994 Jan 15;54(2):357–361. [PubMed] [Google Scholar]
- Guild B. C., Mulligan R. C., Gros P., Housman D. E. Retroviral transfer of a murine cDNA for multidrug resistance confers pleiotropic drug resistance to cells without prior drug selection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1595–1599. doi: 10.1073/pnas.85.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7785–7789. doi: 10.1073/pnas.83.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Manavalan P., Smith A. E., McPherson J. M. Sequence and structural homology among membrane-associated domains of CFTR and certain transporter proteins. J Protein Chem. 1993 Jun;12(3):279–290. doi: 10.1007/BF01028190. [DOI] [PubMed] [Google Scholar]
- Marquardt D., Center M. S. Drug transport mechanisms in HL60 cells isolated for resistance to adriamycin: evidence for nuclear drug accumulation and redistribution in resistant cells. Cancer Res. 1992 Jun 1;52(11):3157–3163. [PubMed] [Google Scholar]
- Momburg F., Ortiz-Navarrete V., Neefjes J., Goulmy E., van de Wal Y., Spits H., Powis S. J., Butcher G. W., Howard J. C., Walden P. Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature. 1992 Nov 12;360(6400):174–177. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Momburg F., Hämmerling G. J. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993 Aug 6;261(5122):769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Ploegh H. L. Allele and locus-specific differences in cell surface expression and the association of HLA class I heavy chain with beta 2-microglobulin: differential effects of inhibition of glycosylation on class I subunit association. Eur J Immunol. 1988 May;18(5):801–810. doi: 10.1002/eji.1830180522. [DOI] [PubMed] [Google Scholar]
- Neisig A., Roelse J., Sijts A. J., Ossendorp F., Feltkamp M. C., Kast W. M., Melief C. J., Neefjes J. J. Major differences in transporter associated with antigen presentation (TAP)-dependent translocation of MHC class I-presentable peptides and the effect of flanking sequences. J Immunol. 1995 Feb 1;154(3):1273–1279. [PubMed] [Google Scholar]
- Restifo N. P., Esquivel F., Kawakami Y., Yewdell J. W., Mulé J. J., Rosenberg S. A., Bennink J. R. Identification of human cancers deficient in antigen processing. J Exp Med. 1993 Feb 1;177(2):265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Howell D. N., Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Müller M., Homolya L., Holló Z., Seprödi J., Germann U. A., Gottesman M. M., Price E. M., Boucher R. C. Interaction of bioactive hydrophobic peptides with the human multidrug transporter. FASEB J. 1994 Jul;8(10):766–770. doi: 10.1096/fasebj.8.10.7914178. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., de Lange J. H., Pinedo H. M., van Heijningen T. H., Kuiper C. M., Scheffer G. L., Scheper R. J., van Kalken C. K., Baak J. P. Early multidrug resistance, defined by changes in intracellular doxorubicin distribution, independent of P-glycoprotein. Br J Cancer. 1991 Nov;64(5):857–861. doi: 10.1038/bjc.1991.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. C., Inoue S., Roitelman J., Schimke R. T., Simoni R. D. Peptide transport by the multidrug resistance pump. J Biol Chem. 1992 Mar 25;267(9):5731–5734. [PubMed] [Google Scholar]
- Slapak C. A., Fracasso P. M., Martell R. L., Toppmeyer D. L., Lecerf J. M., Levy S. B. Overexpression of the multidrug resistance-associated protein (MRP) gene in vincristine but not doxorubicin-selected multidrug-resistant murine erythroleukemia cells. Cancer Res. 1994 Nov 1;54(21):5607–5613. [PubMed] [Google Scholar]
- Stutts M. J., Gabriel S. E., Olsen J. C., Gatzy J. T., O'Connell T. L., Price E. M., Boucher R. C. Functional consequences of heterologous expression of the cystic fibrosis transmembrane conductance regulator in fibroblasts. J Biol Chem. 1993 Sep 25;268(27):20653–20658. [PubMed] [Google Scholar]
- Takeda Y., Nishio K., Niitani H., Saijo N. Reversal of multidrug resistance by tyrosine-kinase inhibitors in a non-P-glycoprotein-mediated multidrug-resistant cell line. Int J Cancer. 1994 Apr 15;57(2):229–239. doi: 10.1002/ijc.2910570217. [DOI] [PubMed] [Google Scholar]
- Wei L. Y., Stutts M. J., Hoffman M. M., Roepe P. D. Overexpression of the cystic fibrosis transmembrane conductance regulator in NIH 3T3 cells lowers membrane potential and intracellular pH and confers a multidrug resistance phenotype. Biophys J. 1995 Sep;69(3):883–895. doi: 10.1016/S0006-3495(95)79962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G. J., Flens M. J., van Leusden M. R., de Haas M., Mülder H. S., Lankelma J., Pinedo H. M., Scheper R. J., Baas F., Broxterman H. J. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra J. G., de Vries E. G., Mulder N. H. Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1987 Apr 1;47(7):1780–1784. [PubMed] [Google Scholar]