Abstract
In this article, we report on the efficient synthesis of well defined, homogeneous [n]rotaxanes (n up to 11) by a template-directed thermodynamic clipping approach. By employing dynamic covalent chemistry in the form of reversible imine bond formation, [n]rotaxanes with dialkylammonium ion (–CH2NH2+CH2–) recognition sites, encircled by [24]crown-8 rings, were prepared by a thermodynamically controlled, template-directed clipping procedure, that is, by mixing together a dumbbell compound containing a discrete number of  CH2NH2+CH2– ion centers with appropriate amounts of a dialdehyde and a diamine to facilitate the [n]rotaxane formation. A 21-component self-assembly process is operative during the formation of the [11]rotaxane. The oligomeric dumbbells containing
CH2NH2+CH2– ion centers with appropriate amounts of a dialdehyde and a diamine to facilitate the [n]rotaxane formation. A 21-component self-assembly process is operative during the formation of the [11]rotaxane. The oligomeric dumbbells containing  CH2NH2+CH2– ion recognition sites were prepared by a stepwise protocol. Several of the dynamic [n]rotaxanes were converted into their kinetically stable counterparts, first by reduction (“fixing”) of imine bonds with the BH3·THF complex, then by protonation of the complex by addition of acid.
CH2NH2+CH2– ion recognition sites were prepared by a stepwise protocol. Several of the dynamic [n]rotaxanes were converted into their kinetically stable counterparts, first by reduction (“fixing”) of imine bonds with the BH3·THF complex, then by protonation of the complex by addition of acid.
Keywords: dynamic covalent chemistry, molecular recognition, polyrotaxanes, self-assembly, template-directed synthesis
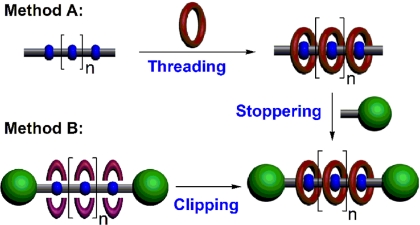
Mechanically interlocked and knotted compounds, such as rotaxanes (1–5), catenanes (6–10), suitanes (11, 12), trefoil knots (13–17), Borromean rings (18–20), and Solomon knots (21), represent challenging synthetic goals that have nevertheless been realized. These molecular compounds are usually synthesized by a template-directed approach (22) that depends on molecular recognition and self-assembly processes. Recently, their potential applications as molecular switches for nanoelectronics (23, 24) and molecular actuators for constructing artificial muscles (25), for fabricating smart surface materials (26), and for controlling the nanoscale release of molecules trapped in mesoporous silica (27–29) were demonstrated. Polyrotaxanes and well defined, homogeneous oligorotaxanes, in which the recognition sites on a dumbbell (an axle terminated by bulky stoppers) are encircled by large rings or macrocycles (wheels) by dint of molecular recognition, have become (30–36) one of the most intensively investigated subjects in mechanical chemistry. A general synthetic method for making rotaxanes, namely, the “threading-followed-by-stoppering” approach (Fig. 1, method A), involves (30–32) several macrocycles. First, the macrocycles are threaded onto oligomeric or polymeric axles carrying recognition sites at prescribed intervals along the axles to form pseudorotaxanes, then both ends of the axles are stoppered with bulky groups. Although this approach is relatively simple, it does not provide complete control over the number of threaded macrocycles, that is, the rings or beads are often not threaded onto all of the available recognition sites on the axles. Alternatively, a template-directed “clipping” approach (Fig. 1, method B), in which the macrocycles are formed from acyclic precursors in the presence of templating recognition sites on the dumbbells, has provided (33–36) a versatile means for the construction of some lower-order rotaxanes. Nonetheless, the efficient synthesis of well defined, homogeneous, higher-order polyrotaxanes continues to be a challenge to synthetic chemists.
Fig. 1.
Conceptual approaches to the template-directed syntheses of polyrotaxanes by using different protocols. (Method A) The “threading-followed-by-stoppering” approach. (Method B) The thermodynamically controlled clipping approach.
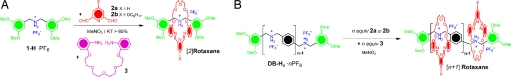
Recently, dynamic covalent chemistry (37–40), exemplified by reversible imine formation (41, 42), metal–ligand exchange (43), and olefin metathesis (44, 45), has been demonstrated to be an effective tool for the preparation of various exotic mechanically interlocked molecular compounds. It has been found that, in the presence of an appropriate template, one of the possible compounds in the dynamic library, after mixing the different components, can be amplified to give the thermodynamically most stable product. We have reported (see refs. 46–49) an example of such a template-directed synthesis of linear and branched [n]rotaxanes (n = 2–4) by employing dynamic covalent chemistry in the form of reversible imine formation (Fig. 2A). In the presence of the dumbbell-shaped compound 1-H·PF6 containing a  CH2NH2+CH2– ion recognition site, the condensation of 2,6-pyridinedicarboxaldehyde (2a) and tetraethyleneglycol bis(2-aminophenyl)ether (3) forms selectively and near quantitatively a [24]crown-8 ring that becomes clipped onto the dumbbell. Such thermodynamically controlled, template-directed amplification is driven by a series of noncovalent bonding interactions that include [N+–H···X] (X = O or N) and [N+C–H···O] hydrogen bonds and aromatic π–π interactions between the dumbbell and the ring. The thermodynamic product, a [2]rotaxane, was converted into a stable [2]rotaxane by reduction (“fixing”) of the two imine bonds. Moreover, such a template-directed, thermodynamic clipping approach has proven (48, 49) to be effective and efficient in the synthesis of sterically bulky, mechanically interlocked dendrimers. Inspired by the success of this thermodynamically controlled approach, we became interested in synthesizing well defined, homogeneous, oligo- and polyrotaxanes under template control. In particular, we questioned whether mixing well defined homogeneous, dumbbell compounds DB-Hn·nPF6 that already contain a known number of n
CH2NH2+CH2– ion recognition site, the condensation of 2,6-pyridinedicarboxaldehyde (2a) and tetraethyleneglycol bis(2-aminophenyl)ether (3) forms selectively and near quantitatively a [24]crown-8 ring that becomes clipped onto the dumbbell. Such thermodynamically controlled, template-directed amplification is driven by a series of noncovalent bonding interactions that include [N+–H···X] (X = O or N) and [N+C–H···O] hydrogen bonds and aromatic π–π interactions between the dumbbell and the ring. The thermodynamic product, a [2]rotaxane, was converted into a stable [2]rotaxane by reduction (“fixing”) of the two imine bonds. Moreover, such a template-directed, thermodynamic clipping approach has proven (48, 49) to be effective and efficient in the synthesis of sterically bulky, mechanically interlocked dendrimers. Inspired by the success of this thermodynamically controlled approach, we became interested in synthesizing well defined, homogeneous, oligo- and polyrotaxanes under template control. In particular, we questioned whether mixing well defined homogeneous, dumbbell compounds DB-Hn·nPF6 that already contain a known number of n  CH2NH2+CH2– ion recognition sites, together with n equivalents of the dialdehyde 2a (or its alkoxy derivative 2b) and n equivalents of the diamine 3, would afford (Fig. 2B) an [n + 1]rotaxane in a one-pot, multicomponent self-assembly process. This process is much more challenging than the synthesis of randomly threaded polyrotaxanes for the following reasons: (i) it requires the synthesis of dumbbell-shaped templates with a well defined number of ion centers; (ii) subsequently, the formation of the [n]rotaxanes relies on successful and efficient template-directed condensations of (2n − 1) components [one dumbbell plus (n − 1) 2a or 2b plus (n − 1) 3 in one pot]; and (iii) the fixing of the dynamic [n]rotaxanes to give kinetically stable [n]rotaxanes after reduction of (2n − 2) imine bonds in a one-step, one-pot reaction. Herein, we report a detailed investigation of the efficient syntheses of well defined, homogeneous, higher-order oligo- and polyrotaxanes, employing the template-directed, thermodynamically controlled clipping approach (Fig. 1, method B).
CH2NH2+CH2– ion recognition sites, together with n equivalents of the dialdehyde 2a (or its alkoxy derivative 2b) and n equivalents of the diamine 3, would afford (Fig. 2B) an [n + 1]rotaxane in a one-pot, multicomponent self-assembly process. This process is much more challenging than the synthesis of randomly threaded polyrotaxanes for the following reasons: (i) it requires the synthesis of dumbbell-shaped templates with a well defined number of ion centers; (ii) subsequently, the formation of the [n]rotaxanes relies on successful and efficient template-directed condensations of (2n − 1) components [one dumbbell plus (n − 1) 2a or 2b plus (n − 1) 3 in one pot]; and (iii) the fixing of the dynamic [n]rotaxanes to give kinetically stable [n]rotaxanes after reduction of (2n − 2) imine bonds in a one-step, one-pot reaction. Herein, we report a detailed investigation of the efficient syntheses of well defined, homogeneous, higher-order oligo- and polyrotaxanes, employing the template-directed, thermodynamically controlled clipping approach (Fig. 1, method B).
Fig. 2.
Extrapolating from the past to the future in the synthesis of rotaxanes. (A) An example of the template-directed synthesis of a [2]rotaxane by using a clipping reaction. (B) The proposed template-directed synthesis of [n + 1]rotaxanes by employing clipping reactions on the dumbbells DB-Hn·nPF6 as templates.
Results and Discussion
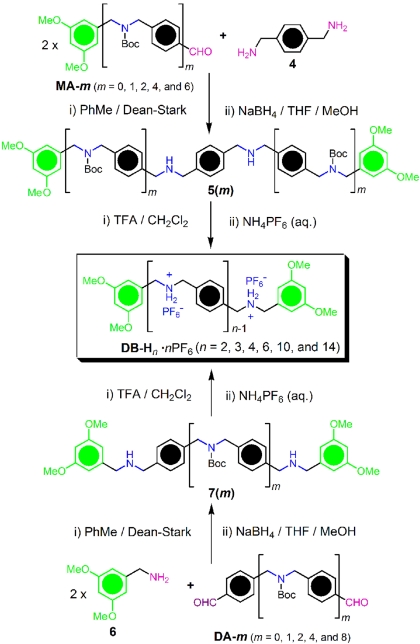
The stepwise synthesis of the oligomeric dumbbell templates DB-Hn·nPF6 is summarized in Fig. 3. The  CH2NH2+CH2– ion recognition centers were generated first by reductive amination with derivatives of benzylamines and benzaldehydes, then by protonation of the secondary amines and counterion exchange. In the synthesis of DB-Hn·nPF6, 1 eq of p-xylenediamine (4) and 2 eq of the monoformyl-terminated half dumbbells MA-m (m = 0, 1, 2, 4, and 6), which contain a well defined number of tert-butoxycarbonyl (Boc)-protected dialkylamine functions, were condensed, affording the corresponding imines. In particular, the 3,5-dimethoxybenzyl groups serve as bulky stoppers to prevent the dethreading of rings from the axles of dumbbell components of the rotaxanes. Subsequently, the imine functions obtained after condensation were converted quantitatively into dialkylamino groups in 5(m) by reduction with NaBH4. Treatment of 5(m) with trifluoroacetic acid (TFA) resulted in the quantitative removal of all of the Boc protecting groups to afford the DB-Hn·nTFA derivatives. After counterion exchange with saturated aqueous NH4PF6 solution, the corresponding dumbbell compounds DB-Hn·nPF6 containing
CH2NH2+CH2– ion recognition centers were generated first by reductive amination with derivatives of benzylamines and benzaldehydes, then by protonation of the secondary amines and counterion exchange. In the synthesis of DB-Hn·nPF6, 1 eq of p-xylenediamine (4) and 2 eq of the monoformyl-terminated half dumbbells MA-m (m = 0, 1, 2, 4, and 6), which contain a well defined number of tert-butoxycarbonyl (Boc)-protected dialkylamine functions, were condensed, affording the corresponding imines. In particular, the 3,5-dimethoxybenzyl groups serve as bulky stoppers to prevent the dethreading of rings from the axles of dumbbell components of the rotaxanes. Subsequently, the imine functions obtained after condensation were converted quantitatively into dialkylamino groups in 5(m) by reduction with NaBH4. Treatment of 5(m) with trifluoroacetic acid (TFA) resulted in the quantitative removal of all of the Boc protecting groups to afford the DB-Hn·nTFA derivatives. After counterion exchange with saturated aqueous NH4PF6 solution, the corresponding dumbbell compounds DB-Hn·nPF6 containing  CH2NH2+CH2– ion recognition sites were obtained in high yield. Alternatively, the synthesis of DB-Hn·nPF6 could also be performed (Fig. 3) by treating 3,5-dimethoxybenzylamine (6) and the diformyl-terminated oligomers DA-m (m = 0, 1, 2, 4, and 8) and employing synthetic protocols similar to those described earlier in this paragraph.
CH2NH2+CH2– ion recognition sites were obtained in high yield. Alternatively, the synthesis of DB-Hn·nPF6 could also be performed (Fig. 3) by treating 3,5-dimethoxybenzylamine (6) and the diformyl-terminated oligomers DA-m (m = 0, 1, 2, 4, and 8) and employing synthetic protocols similar to those described earlier in this paragraph.
Fig. 3.
Synthetic route to the dumbbell templates DB-Hn·nPF6.
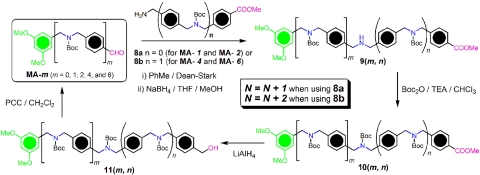
The mono- and diformyl-terminated oligomers, MA-m and DA-m, respectively, are key intermediates in the synthesis of the dumbbell templates. Both compounds were prepared by efficient repetitive protocols. The syntheses of MA-m started with condensation between the commercially available 3,5-dimethoxybenzaldehyde (MA-0) and methyl 4-(aminomethyl)benzoate (8a), affording the expected imine, which was then converted (Fig. 4) into the free amine 9(0,0) on treatment with NaBH4. The amino group was protected with Boc groups by reacting the product with Boc2O and triethylamine in CHCl3 to yield the fully protected compound 10(0,0). The ester group in 10(0,0) was then converted into a hydroxymethyl group in 11(0,0) by reduction with lithium aluminum hydride in THF. Finally, the hydroxymethyl group was converted into a formyl function (compound MA-1) by oxidation of 11(0,0) with pyridinium chlorochromate (PCC) in CH2Cl2. The aldehyde MA-1 has a molecular structure similar to that of the aldehyde MA-0, except that it possesses an additional  CH2N(Boc)CH2– unit. Thus, by repeating this iterative synthetic cycle, the monoformyl-terminated compound MA-2 was obtained after a four-step procedure.
CH2N(Boc)CH2– unit. Thus, by repeating this iterative synthetic cycle, the monoformyl-terminated compound MA-2 was obtained after a four-step procedure.
Fig. 4.
Synthetic route to the monoformyl-terminated oligomers MA-m.
Although this repetitive synthetic approach is straightforward and can lead conceptually to higher oligomers with m > 3, the growth of the repeating units is too tedious, requiring, as it does, multiple-step synthesis. To expedite the synthesis of the higher oligomers, that is, MA-m (m > 2), a compound analogous to 8a, namely 8b, which carries an additional  CH2N(Boc)CH2– unit, was synthesized [see supporting information (SI) Scheme 3] and then used as another key building block in the subsequent synthetic work. By using the already established synthetic protocol, the formyl oligomers MA-4 and MA-6 were prepared in four and eight steps, respectively, from the aldehyde MA-2 by involving the iterative synthesis cycles with 8b as the building block in place of 8a. Similarly, the bisformyl derivatives DA-m (m = 2, 4, and 8) were prepared (Fig. 5) by the same iterative procedure. The syntheses started with terephthaldehyde (DA-0). The dialdehyde DA-2 was prepared by using 8a as a building block. After each synthetic cycle, the number of N(Boc) units increases by 2 and 4, respectively. With all these intermediates to hand, dumbbells DB-Hn·nPF6 (with n = 2, 3, 4, 6, 10, and 14) were produced in amounts in excess of 100 mg. All of these intermediates, as well as the final target compounds, were characterized by standard spectroscopic techniques (see SI Text). For example, the high-resolution electrospray ionization mass spectra (HR-ESI-MS) of the dumbbells DB-Hn·nPF6, after neutralization with base, showed (SI Fig. 9) well defined isotopic distribution patterns for the [M + H]+ molecular mass peaks. At the same time, they exhibit symmetrical-looking 1H NMR spectra in agreement with the assigned molecular structures.
CH2N(Boc)CH2– unit, was synthesized [see supporting information (SI) Scheme 3] and then used as another key building block in the subsequent synthetic work. By using the already established synthetic protocol, the formyl oligomers MA-4 and MA-6 were prepared in four and eight steps, respectively, from the aldehyde MA-2 by involving the iterative synthesis cycles with 8b as the building block in place of 8a. Similarly, the bisformyl derivatives DA-m (m = 2, 4, and 8) were prepared (Fig. 5) by the same iterative procedure. The syntheses started with terephthaldehyde (DA-0). The dialdehyde DA-2 was prepared by using 8a as a building block. After each synthetic cycle, the number of N(Boc) units increases by 2 and 4, respectively. With all these intermediates to hand, dumbbells DB-Hn·nPF6 (with n = 2, 3, 4, 6, 10, and 14) were produced in amounts in excess of 100 mg. All of these intermediates, as well as the final target compounds, were characterized by standard spectroscopic techniques (see SI Text). For example, the high-resolution electrospray ionization mass spectra (HR-ESI-MS) of the dumbbells DB-Hn·nPF6, after neutralization with base, showed (SI Fig. 9) well defined isotopic distribution patterns for the [M + H]+ molecular mass peaks. At the same time, they exhibit symmetrical-looking 1H NMR spectra in agreement with the assigned molecular structures.
Fig. 5.
Synthetic route to the bisformyl-terminated oligomers DA-m.
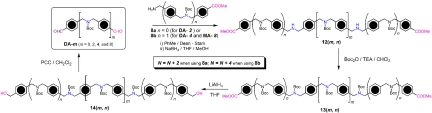
The clipping reactions to form the dynamic [n]rotaxanes were conducted in nitromethane (MeNO2) by mixing together the dumbbell template DB-Hn·nPF6 with n eq each of compounds 2a and 3. The condensations were followed by 1H NMR spectroscopy and HR-ESI-MS analyses. The dumbbell templates DB-Hn·nPF6 (especially in the higher oligomers) exhibited poor solubilities in MeNO2, forming suspensions. Upon addition of 2a and 3, the mixtures turned into a clear, golden-yellow solution in a few minutes when the dumbbell templates DB-Hn·nPF6 (where n = 2, 3, 4, and 6) were used, affording [3]-, [4]-, [5]-, and [7]rotaxanes, respectively. The 1H NMR spectra demonstrated (Fig. 6) the complete formation of the corresponding [n + 1]rotaxanes. The distinct, sharp peaks that correlate with the [24]crown-8 macrocycle are observed; for example, the peaks for imine protons (H C
C N), the peaks for pyridine rings (a and b) and aryl rings (c–f), and ethylene glycol chains (not shown), are all in agreement with the peaks of the dynamic [2]rotaxane investigated in ref. 46. Interestingly, two sets of resonances are observed for the aromatic protons of the macrocycles present in the [4]-, [5]-, and [7]rotaxanes, the ratios between the two sets of signals calculated by integration of the spectra, being 2:1, 1:1, and 1:2, respectively. These observations can be explained by the constitutionally heterotopic environments of the macrocycles surrounding the dumbbells, that is, in the [4]rotaxane, the two homotopic macrocycles adjacent to the stopper (RA, signals a–f) are heterotopic with respect to the central macrocycle (RB, signals a′–f′). In the [5]rotaxane, rings RA are different from rings RB, and in [7]rotaxane, rings RB and RC share very similar chemical environments that differ from rings RA. The slight difference between rings RB and RC is even expressed in the separation of the peaks for the imine protons (H′
N), the peaks for pyridine rings (a and b) and aryl rings (c–f), and ethylene glycol chains (not shown), are all in agreement with the peaks of the dynamic [2]rotaxane investigated in ref. 46. Interestingly, two sets of resonances are observed for the aromatic protons of the macrocycles present in the [4]-, [5]-, and [7]rotaxanes, the ratios between the two sets of signals calculated by integration of the spectra, being 2:1, 1:1, and 1:2, respectively. These observations can be explained by the constitutionally heterotopic environments of the macrocycles surrounding the dumbbells, that is, in the [4]rotaxane, the two homotopic macrocycles adjacent to the stopper (RA, signals a–f) are heterotopic with respect to the central macrocycle (RB, signals a′–f′). In the [5]rotaxane, rings RA are different from rings RB, and in [7]rotaxane, rings RB and RC share very similar chemical environments that differ from rings RA. The slight difference between rings RB and RC is even expressed in the separation of the peaks for the imine protons (H′ C
C N; Fig. 6D). In addition, the secondary dialkylammonium sites (–NH2+–) on the dumbbells also show two sets of signals for the higher [n]rotaxanes. The formation of these [n]rotaxanes is further supported (see SI Fig. 10) by their HR-ESI-MS. Intense peaks associated with the corresponding ions after the loss of a certain numbers of PF6− counterions are clearly observed in the mass spectra. All of these MS and 1H NMR spectroscopic data prove that the template-directed, thermodynamic clipping approach already used in the preparation (46, 47) of the [2]rotaxane is also applicable for the higher-order [n]rotaxanes, at least as far as n = 7.
N; Fig. 6D). In addition, the secondary dialkylammonium sites (–NH2+–) on the dumbbells also show two sets of signals for the higher [n]rotaxanes. The formation of these [n]rotaxanes is further supported (see SI Fig. 10) by their HR-ESI-MS. Intense peaks associated with the corresponding ions after the loss of a certain numbers of PF6− counterions are clearly observed in the mass spectra. All of these MS and 1H NMR spectroscopic data prove that the template-directed, thermodynamic clipping approach already used in the preparation (46, 47) of the [2]rotaxane is also applicable for the higher-order [n]rotaxanes, at least as far as n = 7.
Fig. 6.
Partial 1H NMR spectra (400 MHz) of the dynamic [n]rotaxanes (n = 3, 4, 5, and 7) after mixing the corresponding dumbbells DB-Hn·nPF6, 2a, and 3 in CD3NO2 (δ = 5.8–10.2 ppm). Signals labeled with a–f are correlated to the resonances of the ring close to the stoppers (RA), and the signals labeled with a′–f′ are assigned to the resonances of other rings (RB and RC). The peaks for protons i and j locate at about δ = 4.68 ppm (not shown).
The formation of the [11]rotaxane (a 21-component self-assembly) and the [15]rotaxane (a 29-component self-assembly) using similar clipping protocols, however, encountered practical problems associated, most likely, with the extremely poor solubilities of the dumbbell templates in MeNO2, conferring low solubilities on the rotaxanes as well. Specifically, the clipping reaction for the formation of the [11]rotaxane was performed under moderately dilute conditions, for example, 2.5 mg of the dumbbell in 10 ml of CD3NO2, wherein the mixture became nearly clear within 2 h. 1H NMR spectra and HR-ESI-MS (not shown) revealed partial formation of the desired [11]rotaxane with other products dominating the reaction mixture. Changing the solvent to CD3CN did not enhance the formation of the desired [11]rotaxane. The situation surrounding the formation of the [15]rotaxane was even more discouraging insofar as the suspension in the reaction did not become clear even under highly dilute conditions and with stirring at room temperature for several days. Heating of the reaction mixture led to decomposition of the starting materials.
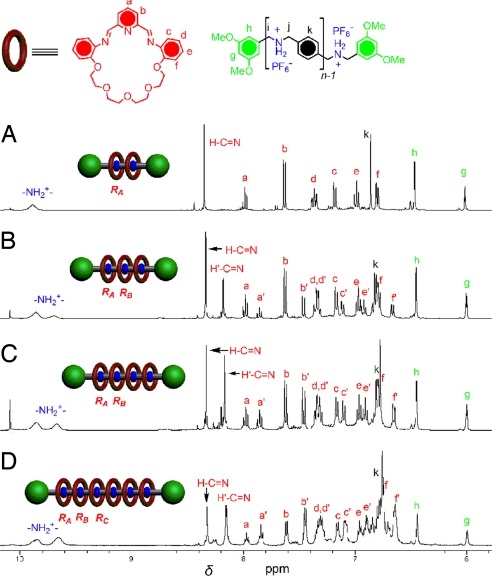
To address these issues, we decided to use alkyloxyl pyridinedicarboxaldehyde 2b (see SI Scheme 4 for its preparation) in which the additional octyloxyl unit is expected to improve significantly the solubilities of the polyrotaxanes formed. The clipping reactions of the dumbbells DB-Hn·nPF6 with 2b and 3 were conducted under conditions similar to those used with 2a clipping reactions and work well in the formation of [n]rotaxanes (where n = 3, 4, 5, and 7), as indicated by the 1H NMR spectroscopy and the HR-ESI-MS (see SI Figs. 11 and 12). Benefiting from the solubilizing groups present in 2b, the clipping reaction of the dumbbell DB-H10·10PF6 with 2b and 3 proceeds as rapidly as for the smaller [n]rotaxanes (where n = 3, 4, 5, and 7). A golden-yellow solution was obtained within a few minutes after mixing the components and the 1H NMR spectrum shows (Fig. 7) clearly that the major species present in the CD3NO2 solution is the desired [11]rotaxane. It is similar to that of the [7]rotaxane prepared under similar conditions. Two sets of imine signals are observed for all the [n]rotaxanes when n > 3. The ratios calculated (Fig. 7B) from the peaks for the imine protons, H C
C Nto H′
Nto H′ C
C N is 1:4, confirm the efficient formation of the [11]rotaxane in which heterotopic rings RB, RC, RD, and RE have similar chemical environments and are markedly different from those of the rings RA. The HR-ESI-MS of the mixture reveals intense peaks associated with the molecular ions [M − 8PF6]8+, [M − 7PF6]7+, [M − 6PF6]6+, [M − 5PF6]5+, and [M − 4PF6]4+ in the reliable mass/charge range (500–2,500) of the instrument (see SI Fig. 12e), once again supporting the formation of the [11]rotaxane.
N is 1:4, confirm the efficient formation of the [11]rotaxane in which heterotopic rings RB, RC, RD, and RE have similar chemical environments and are markedly different from those of the rings RA. The HR-ESI-MS of the mixture reveals intense peaks associated with the molecular ions [M − 8PF6]8+, [M − 7PF6]7+, [M − 6PF6]6+, [M − 5PF6]5+, and [M − 4PF6]4+ in the reliable mass/charge range (500–2,500) of the instrument (see SI Fig. 12e), once again supporting the formation of the [11]rotaxane.
Fig. 7.
Partial 1H NMR spectra (400 MHz) of the dynamic [n]rotaxanes (n = 7 and 11) after mixing the corresponding dumbbells DB-Hn, 2b, and 3 in CD3NO2 (δ = 5.8–10.4 ppm). Signals labeled with a–e are correlated with the resonances of the rings close to the stoppers (RA), and the signals labeled with a′–e′ are assigned to the resonances of other rings (RB, RC, RD, and RE). The peaks for protons i and j locate at about δ = 4.69 ppm (not shown).
Alas, however, mixing of the dumbbell DB-H14·14PF6 with 2b and 3 in MeNO2 failed to give a clear solution; even under highly dilute conditions and after prolonged stirring time, no convincing experimental data were obtained that pointed to the formation of the desired [15]rotaxane. The very low solubility of the dumbbell template finally put a limit on the template-directed, thermodynamic synthesis of the linear polyrotaxanes in one-pot reactions, at least with hexafluorophosphate anions as the counterions.
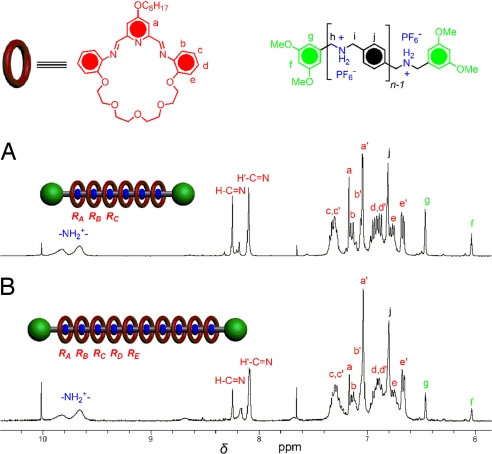
The dynamic [2]rotaxanes (see refs. 46 and 47) and some of the branched [4]rotaxane dendrimers (see refs. 48 and 49) containing imine bonds were “fixed” in their kinetically stable forms by reduction of the imine bonds with the BH3·THF complex without any need for chromatographic separations. The reduction of the [n]rotaxanes (n = 3, 4, 5, 7, and 10) reported here is not such an easy task because all of the (2n − 2) imine bonds in the macrocycles arranged along the dumbbell template have to be reduced at the same time. The dynamic [n]rotaxanes in MeNO2 were reduced by addition of 1 M BH3·THF complex (2 eq per imine bond). This solution was stirred at room temperature for 16 h. After removal of the solvent, the residue was treated with 2 M NaOH (aq) and extracted with CHCl3 to give the neutral [n]rotaxanes. After purification by preparative TLC, the neutral [n]rotaxanes were acidified with TFA, and counterion exchange with saturated NH4PF6 (aq) afforded the fixed [n]rotaxanes. However, the efficiency of the fixing process has its limitations with the increasing numbers of macrocycles. The pure fixed [3]-, [4]-, and [5]rotaxanes were isolated in 77%, 74%, and 40% yields, respectively. All of these [n]rotaxanes were purified by preparative TLC to remove impurities remaining after reduction. The reduction of dynamic [7]rotaxane and [11]rotaxane yielded large amounts of by-products from which the fully fixed rotaxanes could not be separated. This limitation is ascribed to the partial cleavage and dissociation of the macrocycles from the dumbbell templates during the reduction. This observation is comparable to the fixing process in the higher-order branched [4]rotaxane dynamic dendrimers, where steric hindrance is also operative (49). The diffusion of the BH3·THF to the imine bonds and the subsequent reduction has to compete with the imine dissociation process, and this balance is more difficult to control with the higher-order [n]rotaxanes. The 1H NMR spectra of the pure [3]-, [4]-, and [5]rotaxanes are shown in Fig. 8. The [4]- and [5]rotaxanes display two sets of resonance signals for the fixed macrocycles, with the integration ratios of 2:1 and 1:1, respectively. This observation, again, can be explained by the environments of rings RA compared with rings RB. Similarly, two sets of resonance signals for the  NH2+– protons can also be observed. The structural assignments of the [3]-, [4]-, and [5]rotaxanes are further supported (SI Fig. 13) by the HR-ESI-MS, wherein intense peaks correspond to positive ions after the loss of a certain number of PF6− ions. All the peaks give isotopic distributions in agreement with the calculated values.
NH2+– protons can also be observed. The structural assignments of the [3]-, [4]-, and [5]rotaxanes are further supported (SI Fig. 13) by the HR-ESI-MS, wherein intense peaks correspond to positive ions after the loss of a certain number of PF6− ions. All the peaks give isotopic distributions in agreement with the calculated values.
Fig. 8.
Partial 1H NMR spectra (400 MHz) of the fixed [n]rotaxanes (n = 3, 4, and 5) in CD3SOCD3 (δ = 5.8–9.0 ppm). Signals labeled with a–f are correlated with the resonances of the rings close to the stoppers (RA), and the signals labeled with a′–f′ are assigned to the resonances of other rings RB. The peaks for protons i and j locate at about δ = 4.75 ppm (not shown).
Conclusion
We have developed a highly efficient template-directed, thermodynamically controlled clipping approach to some well defined, homogeneous [n]rotaxanes (with n to 11). The synthesis is based on the formation of two imine bonds in a [24]crown-8 macrocycle from acyclic precursors, a dialdehyde and a diamine, by dynamic covalent chemistry in the presence of secondary dialkylammonium ion templates present in specifically synthesized and well characterized dumbbells. Because this protocol represents one of the most efficient ways to make mechanically interlocked compounds, one might expect that it will also be applied to the template-directed synthesis of even more intricate compounds, including molecular necklaces, dendritic polyrotaxanes, polycatenanes, and so on. Although the extent of [n]rotaxane formation is so far limited to n = 11 (a 21-component self-assembly process) because of solubility constraints, they could be overcome in the future by attaching solubilizing groups to the dumbbell templates as well as to macrocycles. The efficiency of the fixing of dynamic [n]rotaxanes by reduction of the imine bonds shows some dependence on n, that is, it occurs with decreased efficiency as n becomes larger. The fixed [3]-, [4]-, and [5]rotaxanes as pure, well characterized compounds have been successfully prepared.
The importance of being able to synthesize, in high yields, [n]rotaxanes, where n is a double-digit number, cannot be overly stressed. (While this manuscript was being written, Leigh's group described an alternative approach to the synthesis of [n]rotaxanes by using a template-directed clipping methodology. The distinctiveness of their approach lies in the controlled iterative addition of macrocycles onto a single binding site on the rotaxanes' dumbbell precursor. See ref. 50.) Such polyrotaxanes, in particular, when both the dumbbell and ring component can carry (positive) charges and so give the mechanically interlocked polyelectrolyte character, are candidates for studying the dependence of their rheological behavior on pH, on the choice of anions and solvents, and so forth. Also, [n]rotaxanes into which constitutionally different rings have been inserted in a controlled manner hold promise as templates for the production of artificial main-chain polymers containing numerous monomer units whose sequence can be predetermined in a manner reminiscent of many biopolymers.
Materials and Methods
Compound 3 was synthesized according to the procedure reported in ref. 46. All of the other starting materials are commercially available from Aldrich (St. Louis, MO) or VWR (West Chester, PA) and were used as received. All solvents were purified and dried before use. Column chromatography was performed on Silica Gel 60 (Merck, Whitehouse Station, NJ; 40–60 μm, 230–400 mesh). Deuterated solvents (Cambridge Isotope Laboratories, Cambridge, MA) for NMR spectroscopic analysis were used as received. All NMR spectra were recorded on Avance-400 (Bruker, Billerica, MA; at 400 MHz) and Avance-500 (Bruker; at 500 MHz) spectrometers. All chemical shifts are quoted in parts per million relative to tetramethylsilane with the residual solvent peak as a reference standard. Mass spectra were recorded on an Ion Spec 7·OT Ultima FTMS with ESI or MALDI-TOF ion sources. Detailed synthetic procedures and spectroscopic characterizations of all of the new intermediate compounds and the desired dumbbells (DB-Hn·nPF6) are presented in the SI Text. The template-directed thermodynamic clipping reactions and subsequent fixing reactions were performed by using a general protocol summarized as follows.
The dumbbell template, DB-Hn·nPF6 (5–10 mg), compound 2a or 2b (n eq), and compound 3 (n eq) were mixed in a minimum amount of CD3NO2 (0.75–2 ml). A clear golden-yellow solution was obtained in a few minutes (for n = 10 using 2b). 1H NMR spectra and ESI mass spectra of the solution were recorded until they did not register any changes over a 24-h period. A 1 M BH3·THF complex in THF (2 eq per imine bond) was added to the solution. The reduction was complete in 16 h as observed by 1H NMR spectroscopy. The solvents were removed under vacuum, and 2 M NaOH (aq) and CHCl3 were added. The organic layer was washed with H2O and dried (Na2SO4), and the solvent was removed under vacuum. The residue was then purified by preparative TLC on silica gel plates by using different eluents (CHCl3/MeOH = 4:1 for the [3]rotaxane; CHCl3/Et3N = 3:2 for the [4]- and [5]rotaxanes) to give the neutral rotaxanes, which were dissolved in CH2Cl2 before a few drops of TFA were added. The solvents were then removed under vacuum, and the residue was redissolved in a minimum amount of MeOH. Saturated aqueous NH4PF6 was next added to the solution to yield a white precipitate. The mixture was concentrated under vacuum to remove the excess of MeOH and the precipitate was collected, washed with H2O, and dried under vacuum in the presence of P2O5. Pure, fixed [3]-, [4]-, and [5]rotaxanes were isolated in 77%, 74%, and 40% yields, respectively. The fixed higher-order [n]rotaxanes could not be isolated.
Supplementary Material
Acknowledgments
We thank the following organizations for their generous financial support: the National Science Foundation, the Microelectronics Advanced Research Corporation, its focus center on Functional Engineered NanoArchitectonics, the Defense Advanced Research Projects Agency, and the Center for Nanoscale Innovation for Defense.
Abbreviations
- TFA
trifluoroacetic acid
- Boc
tert-butoxycarbonyl
- PCC
pyridinium chlorochromate
- HR-ESI-MS
high-resolution electrospray ionization mass spectra.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705847104/DC1.
References
- 1.Anelli PL, Spencer N, Stoddart JF. J Am Chem Soc. 1991;113:5131–5133. doi: 10.1021/ja00013a096. [DOI] [PubMed] [Google Scholar]
- 2.Bissell RA, Cordova E, Kaifer AE, Stoddart JF. Nature. 1994;369:133–137. [Google Scholar]
- 3.Andersson M, Linke M, Chambron JC, Davidson J, Heitz V, Hammarstrom L, Sauvage JP. J Am Chem Soc. 2002;124:4347–4362. doi: 10.1021/ja0119907. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa M, Brancato G, Fanti M, Leigh DA, Shimizu T, Slawin AMZ, Wong JKY, Zerbetto F, Zhang S. J Am Chem Soc. 2002;124:2939–2950. doi: 10.1021/ja015995f. [DOI] [PubMed] [Google Scholar]
- 5.Iijima T, Vignon SA, Tseng HR, Jarrosson T, Sanders JKM, Marchioni F, Venturi M, Apostoli E, Balzani V, Stoddart JF. Chem Eur J. 2004;10:6375–6392. doi: 10.1002/chem.200400651. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich-Buchecker CO, Sauvage JP, Kern JM. J Am Chem Soc. 1984;106:3043–3045. [Google Scholar]
- 7.Ashton R, Goodnow TT, Kaifer AE, Reddington MV, Slawin AMZ, Spencer N, Stoddart JF, Vicent C, Williams DJ. Angew Chem Int Ed Engl. 1989;28:1396–1399. [Google Scholar]
- 8.Kidd TJ, Leigh DA, Wilson AJ. J Am Chem Soc. 1999;121:1599–1600. [Google Scholar]
- 9.Bäuerle P, Ammann M, Wilde M, Götz G, Steritz EMO, Rang A, Schalley CA. Angew Chem Int Ed. 2007;46:363–368. doi: 10.1002/anie.200602652. [DOI] [PubMed] [Google Scholar]
- 10.Blight BA, Wisner JA, Jennings MC. Angew Chem Int Ed. 2007;46:2835–2838. doi: 10.1002/anie.200604724. [DOI] [PubMed] [Google Scholar]
- 11.Williams AR, Northrop BH, Chang T, Stoddart JF, White AJP, Williams DJ. Angew Chem Int Ed. 2006;45:6665–6669. doi: 10.1002/anie.200602173. [DOI] [PubMed] [Google Scholar]
- 12.Northrop BH, Spruell JM, Stoddart JF. Chem Today. 2007;25(3):4–7. [Google Scholar]
- 13.Dietrich-Buchecker C, Rapenne G, Sauvage JP. Chem Commun. 1997:2053–2054. [Google Scholar]
- 14.Ashton PR, Matthews OA, Menzer S, Raymo FM, Spencer N, Stoddart JF, Williams DJ. Liebigs Ann Chem. 1997:2485–2494. [Google Scholar]
- 15.Adams H, Ashworth E, Breault GA, Guo J, Hunter CA, Mayers PC. Nature. 2001;411:763. doi: 10.1038/35081143. [DOI] [PubMed] [Google Scholar]
- 16.Brüggemann J, Bitter S, Müller S, Müller WM, Müller U, Maier NM, Lindner W, Vögtle F. Angew Chem Int Ed. 2006;46:254–259. doi: 10.1002/anie.200601938. [DOI] [PubMed] [Google Scholar]
- 17.Kelley RF, Tauber MJ, Wasielewski MR. Angew Chem Int Ed. 2006;45:7979–7982. doi: 10.1002/anie.200603046. [DOI] [PubMed] [Google Scholar]
- 18.Chichak KS, Cantrill SJ, Pease AR, Chiu SH, Cave GWV, Atwood JL, Stoddart JF. Science. 2004;304:1308–1312. doi: 10.1126/science.1096914. [DOI] [PubMed] [Google Scholar]
- 19.Pentecost CD, Chichak KS, Peters AJ, Cave GWV, Cantrill SJ, Stoddart JF. Angew Chem Int Ed. 2006;46:218–222. doi: 10.1002/anie.200603521. [DOI] [PubMed] [Google Scholar]
- 20.Pentecost CD, Tangshaivang N, Cantrill SJ, Chichak KS, Peters AJ, Stoddart JF. J Chem Educ. 2007;84:855–859. [Google Scholar]
- 21.Pentecost CD, Chichak KS, Peters AJ, Cave GWV, Cantrill SJ, J Stoddart F. Angew Chem Int Ed. 2007;46:218–222. doi: 10.1002/anie.200603521. [DOI] [PubMed] [Google Scholar]
- 22.Stoddart JF, Tseng HR. Proc Natl Acad Sci USA. 2002;99:4797–4800. doi: 10.1073/pnas.052708999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier CP, Mattersteig G, Wong EW, Luo Y, Beverly K, Sampaio J, Raymo FM, Stoddart JF, Heath JR. Science. 2000;289:1172–1175. doi: 10.1126/science.289.5482.1172. [DOI] [PubMed] [Google Scholar]
- 24.Green JE, Choi JW, Boukai A, Bunimovich Y, Johnston-Halperin E, DeIonno E, Luo Y, Sheriff BA, Xu K, Shin YS, et al. Nature. 2007;445:414–417. doi: 10.1038/nature05462. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Flood AH, Bonvallet PA, Vignon SA, Northrop BH, Tseng HR, Jeppesen JO, Huang TJ, Brough B, Baller M, et al. J Am Chem Soc. 2005;127:9745–9759. doi: 10.1021/ja051088p. [DOI] [PubMed] [Google Scholar]
- 26.Berná J, Leigh DA, Lubomska M, Mendoza SM, Pérez EM, Rudolf P, Teobaldi G, Zerbetto F. Nat Mater. 2005;4:704–710. doi: 10.1038/nmat1455. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TD, Tseng HR, Celestre PC, Flood AH, Liu Y, Stoddart JF, Zink JI. Proc Natl Acad Sci USA. 2005;102:10029–10034. doi: 10.1073/pnas.0504109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen TD, Liu Y, Saha S, Leung KCF, Stoddart JF, Zink JI. J Am Chem Soc. 2007;129:626–634. doi: 10.1021/ja065485r. [DOI] [PubMed] [Google Scholar]
- 29.Saha S, Leung KCF, Nguyen TD, Stoddart JF, Zink JI. Adv Funct Mater. 2007;17:685–693. [Google Scholar]
- 30.Harada A, Li J, Kamachi M. Nature. 1992;356:325–327. [Google Scholar]
- 31.Gong CG, Ji Q, Subramaniam C, Gibson HW. Macromolecules. 1998;31:1814–1818. [Google Scholar]
- 32.Raymo FM, Stoddart JF. Chem Rev. 1999;99:1643–1663. doi: 10.1021/cr970081q. [DOI] [PubMed] [Google Scholar]
- 33.Aricó F, Badjić JD, Cantrill SJ, Flood AH, Leung KCF, Liu Y, Stoddart JF. Top Curr Chem. 2003;249:203–259. [Google Scholar]
- 34.Bravo JA, Raymo FM, Stoddart JF, White AJP, Williams DJ. Eur J Org Chem. 1998:2565–2571. [Google Scholar]
- 35.Seel C, Vögtle F. Chem Eur J. 2000;6:21–24. doi: 10.1002/(sici)1521-3765(20000103)6:1<21::aid-chem21>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Fuller AM, Leigh DA, Lusby PJ, Oswald IDH, Parsons S, Walker DB. Angew Chem Int Ed. 2004;43:3914–3918. doi: 10.1002/anie.200353622. [DOI] [PubMed] [Google Scholar]
- 37.Brady PA, Sanders JKM. Chem Soc Rev. 1997;26:327–336. [Google Scholar]
- 38.Rowan SJ, Cantrill SJ, Graham RL, Cousins RL, Sanders JKM, Stoddart JF. Angew Chem Int Ed. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 39.Mobian P, Kern JM, Sauvage JP. Angew Chem Int Ed. 2004;43:2392–2395. doi: 10.1002/anie.200352522. [DOI] [PubMed] [Google Scholar]
- 40.Badjić JD, Cantrill SJ, Grubbs RH, Guidry EN, Orenes R, Stoddart JF. Angew Chem Int Ed. 2004;43:3273–3278. doi: 10.1002/anie.200453963. [DOI] [PubMed] [Google Scholar]
- 41.Layer WR. Chem Rev. 1963;63:489–510. [Google Scholar]
- 42.Huc I, Lehn JM. Proc Natl Acad Sci USA. 1997;94:2106–2110. doi: 10.1073/pnas.94.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubota Y, Sakamoto SS, Yamaguchi K, Fujita M. Proc Natl Acad Sci USA. 2002;99:4854–4856. doi: 10.1073/pnas.082643499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Myroslav OV, Bogdan A, Bolte M, Böhmer V. Science. 2004;304:1312–1214. doi: 10.1126/science.1096688. [DOI] [PubMed] [Google Scholar]
- 45.Guidry EN, Cantrill SJ, Stoddart JF, Grubbs RH. Org Lett. 2005;7:2129–2132. doi: 10.1021/ol050463f. [DOI] [PubMed] [Google Scholar]
- 46.Glink PT, Oliva AI, Stoddart JF, White AJP, Williams DJ. Angew Chem Int Ed. 2001;40:1870–1875. [PubMed] [Google Scholar]
- 47.Aricó F, Chang T, Cantrill SJ, Khan SI, Stoddart JF. Chem Eur J. 2005;11:4655–4666. doi: 10.1002/chem.200500148. [DOI] [PubMed] [Google Scholar]
- 48.Leung KCF, Aricó F, Cantrill SJ, Stoddart JF. J Am Chem Soc. 2005;127:5808–5810. doi: 10.1021/ja0501363. [DOI] [PubMed] [Google Scholar]
- 49.Leung KCF, Aricó F, Cantrill SJ, Stoddart JF. Macromolecules. 2007;40:3951–3959. [Google Scholar]
- 50.Fuller AML, Leigh DA, Lusby PJ. Angew Chem Int Ed. 2007;46:5015–5019. doi: 10.1002/anie.200700933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.